Abstract
Mouse embryonic fibroblasts have been utilized as a surrogate stem cell model for the postnatal bone marrow-derived stromal stem cells (BMSC) to study mesoderm-type cell differentiation e.g. osteoblasts, adipocytes and chondrocytes. However, no formal characterization of MEF phenotype has been reported. Utilizing standard in vitro and in vivo assays we performed a side-by-side comparison of MEF and BMSC to determine their ability to differentiate into mesoderm-type cells. BMSC were isolated from 8–10 weeks old mouse bone marrow by plastic adherence. MEF were established by trypsin/EDTA digestion from E13.5 embryos after removing heads and viscera, followed by plastic adherence. Compared to BMSC, MEF exhibited telomerase activity and improved cell proliferation as assessed by q-PCR based TRAP assay and cell number quantification, respectively. FACS analysis revealed that MEF exhibited surface markers characteristic of the BMSC: Sca-1+, CD73+, CD105+, CD29+, CD44+, CD106+, CD11b−, and CD45−. In contrast to BMSC, ex vivo osteoblast (OB) differentiation of MEF exhibited a less mature osteoblastic phenotype (less alkaline phosphatase, collagen type I and osteocalcin) as assessed by real-time PCR analysis. Compared to BMSC, MEF exhibited a more enhanced differentiation into adipocyte and chondrocyte lineages. Interestingly, both MEF and BMSC formed the same amount of heterotopic bone and bone marrow elements upon in vivo subcutaneous implantation with hydroxyapatite/tricalcium phosphate, in immune deficient mice. In conclusion, MEF contain a population of stem cells that behave in ex vivo and in vivo assays, similar but not identical, to BMSC. Due to their enhanced cell growth, they may represent a good alternative for BMSC in studying molecular mechanisms of stem cell commitment and differentiation to osteoblasts, adipocytes and chondrocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone marrow stromal stem cells (also known as mesenchymal stem cells) (BMSC) are clonogenic cells present in bone marrow stroma and have the ability to differentiate into mesoderm-like lineages e.g. osteoblasts, adipocytes and chondrocytes [1]. There has been an enormous interest during the recent years in studying the biological characteristics of BMSC in order to identify cellular and molecular mechanisms controlling differentiation into a specific lineage [2–5]. BMSC cultures are typically established from bone marrow mononuclear cells through their ability to adhere to plastic surfaces. However, the number of BMSC obtained is small and estimated to represent only 0.001 to 0.01% of the total bone marrow cell population [6]. Moreover, in vitro expansion of BMSC is limited due to in vitro replicative-senescence and thus it is difficult to obtain a large number of cells needed for mechanistic studies [7, 8]. In addition, mouse BMSC exhibited enormous variation in their yield, growth, surface marker profiles and differentiation capacities between different mouse strains [9, 10]. Finally, establishing mouse BMSC cultures needs special culture techniques, serum requirement and additional supplements [11].
To overcome these limitations, alternatives for BMSC have commonly been employed for osteogenic differentiation using, for example, preosteoblastic osteosarcoma cell lines (e.g. MC3T3) or spontaneously immortalized embryonic cell lines e.g. C2C12 or C3H10T1/2 which have the disadvantage of transformed phenotypes and abnormal chromosomes.
Mouse embryonic fibroblasts have also been utilized as a surrogate stem cell model for BMSC to study mesoderm-type cell differentiation. Mouse embryonic fibroblasts (MEF) are derived from 13.5 day old embryos after removing head and viscera, thus mesodermal tissues are the major contributor of MEF [12]. MEF have been employed for many years in numerous studies related to fibroblast biology, cancer and aging and have also been used as a feeder layer for culture of embryonic stem (ES) cells in vitro [13]. MEF cells provide several advantages as a model system for mesoderm-type cell differentiation compared with the above mentioned cell models. MEF cultures are relatively easy to establish and maintain [14] and can provide a valuable system for studying the cellular phenotype of genetically modified mice, especially where gene disruption leads to embryonic lethality [14–16]. In addition, MEFs can readily be genetically modified to express ectopic genes by plasmid transfection or infection with viral vectors and are thus a suitable cell model for the analysis of gene function in ex vivo cultures [14]. MEF cultures have been employed in a large number of studies related to mesodermal cells such as cell cycle regulation, transformation, immortalization, senescence and apoptosis [14, 17–20], but few studies have examined the commitment and differentiation of MEF into the mesenchymal lineage: e.g. differentiation into adipocytes [21, 22], chondrogenic lineage in micormass culture [18] or osteoblastic cells [23]. However, all these studies have implied that MEF are equivalent to other mesodermal cell models including BMSC. Nevertheless, no formal investigations have been conducted to compare, side-by-side, bona fida BMSC with MEF.
In this report, we performed a comprehensive side-by-side comparison of cell surface markers, growth rates, ability to differentiate into osteoblasts, adipocytes and chondrocytes utilizing standard ex vivo and in vivo assays.
Material and Methods
Cell Culture
Primary Murine Bone Marrow Stromal Stem Cells (BMSC)
Bone marrow cells were harvested according to the protocol described by Peister et al [24], with some in house modifications [25]. Briefly, cells were isolated from 8–10 weeks old mice by centrifugation and plated in IMDM (GIBCO) supplemented with 12% batch-tested fetal bovine serum (FBS) (GIBCO) and 100 U/ml penicillin (GIBCO), 100 μg/ml streptomycin (1% PS; GIBCO) at a density of 1 × 106 cells/cm2. After 48 h of culture, non-adherent cells were removed, adherent cells were washed with PBS and fresh medium was added. Media was changed every 3rd day and after 1–2 weeks cells were dissociated using Trypsin/EDTA (GIBCO) for 4 min at 37°C and plated according to the experimental setup.
Primary Mouse Embryonic Fibroblast (MEF)
Primary mouse embryonic fibroblasts (MEF) were harvested, according to the standard protocol [26, 27]. Briefly, uteri were obtained at E13.5 days of pregnancy and embryos were isolated. Heads and viscera were removed and the remaining bodies were washed in PBS, transferred to 35 mm petri-dishes, minced with pair of scissors and digested with 0.25% trypsin/EDTA 1 mM (GIBCO) for 5–10 min at 37°C. Following digestion, 1–3 ml DMEM, supplemented with 10% FBS and 1% P/S, was added and the tissue pipetted up and down to get a single cell suspension. MEF were cultured in T–75 flasks until 80–85% confluent and were passaged at a 1:2 ratio. In this study we used, MEF at passage 3.
Cell Proliferation Study
Short-term proliferation was assessed by Nucleo-counter® (Chemo-Metec, Denmark) at day 1, 3, 6 & 9. Long-term cell growth in vitro was determined by calculating the cumulative population doubling level (PDL). When cells were confluent, cells were trypsinized by incubation with trypsin/EDTA for 2 min at 37°C, and counted using a haemocytometer. At each passage, cells at confluence were counted and the population doubling (PD) was calculated by using the formula: log N / log 2, where N is the number of cells at confluence divided by the initial cell number. PDL is the sum of PD at each passage.
β-galactosidase Staining for Senescence
Cellular senescence was assessed by using a β–galactosidase associated staining as described previously [28]. Senescent cells appear greenish blue and were identified by light microscopy.
Surface Marker Characterization
Flow Cytometry (FACS) Analysis
Cells were trypsinised to a single cell suspension, incubated with a panel of non-conjugated mouse BMSC markers (Sca-1, CD44, CD73, CD29, CD105, CD106, CD45 and CD11b) (R&D system, UK, Catalog number: SC018) for 40 min on ice, followed by incubation with secondary anti-rat IgG conjugated to a fluorochrome (DAKO, Cytomation, A/S, Glostrup, Denmark) for 30 min on ice. After washing, cells were re-suspended in 500ul of 0.5% BSA in PBS and analyzed by FACScan linked with Cell-Quest 3.1 software (Becton Dickinson, Biosciences, Brøndby, Denmark). Similarly, the alkaline phosphatase positive fraction was determined by ALPL anti-body specific against mouse (R&D systems, UK). As a control, cells in separate tubes were treated with isotype controls (R&D system, UK). Data were analyzed using WinMdi (http://www.cyto.purdue.edu/flowcyt/software/Winmdi.htm).
Immunocytochemical Staining
Cells were cultured on chamber slides until 80% confluent. Cells were fixed with 4% para formaldehyde for 15 min, washed with PBS and fixed with 0.3% H2O2 in methanol for 15 min. Staining was performed using DAKO EnVision + for CD29 (Biogenex), CD45 (1:100; DakoCytomation A/S, Glostrup, Denmark) and Sca-1 (1:100; BD-Bioscience). Cells were incubated with primary antibody diluted in ChemMate antibody diluent (Dako) for 1 h at RT. For Envision+, cells were incubated with secondary anti-rabbit Ig/HRP-conjugated polymer. After post-blocking (DPVO + 500Post; Immunovision Technologies Co.) cells were incubated with poly-HRP rabbit anti-mouse IgG polymer (DPVO + 500HRP; Immunovision Technologies Co.). Secondary antibodies were visualized with DAB chromogen (Zymed) and nuclei were counterstained with haematoxylin. Negative control cells were performed under the same conditions without primary antibodies.
Differentiation Assays
Osteoblast Differentiation
BMSC
Cells were plated at a density of 20 × 103 cells/cm2 in the presence of complete expansion media (CEM): Iscove modified Dulbecco medium (IMDM; GIBCO) containing 12% FBS, 1% P/S and 12 μM L-glutamine (GIBCO). At 70% confluence cells were incubated with CEM supplemented with osteoblastic differentiation mixture containing 10nM dexamethasone (Sigma), 10 mM β—glycerol-phosphate (Sigma) and 50 μg/ml L-ascorbate-2-phosphate (Sigma). Media were changed every 3rd day for 15 days.
MEF
Cells were plated at a density of 8 × 103 cells/cm2 in the presence of DMEM, 10% FBS and 1% P/S. At 70% confluent, cells were induced with 20 ng/ml BMP-2 (R&D), 100 μg/ml Vitamin C (Sigma) and 10 mM β—glycerol-phosphate (Sigma). Media were changed for every 2nd day for 15 days.
Adipocyte Differentiation
BMSC
Cells were plated at high densities 25 × 103 cells/cm2 in 24 well plates in CEM. The cells were incubated with CEM supplemented with 5 μg/ml Insulin (Sigma), 10 nM dexamethasone (Sigma) and 50 μM Indomethacin (Sigma). Media were changed every 3rd day for 15 days.
MEF
Cells were plated at 8 × 103 cells/cm2 in 24 well plates in DMEM media containing 10% FBS (Gibco), 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco). When cells reached 95% confluence, media was changed to adipocyte induction medium (AIM) containing: 5 μg/ml Insulin (Sigma), 1 × 10−6 M dexamethasone, and 0.5 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma). Medium was changed every 2nd day for 15 days.
Chondrocytic Differentiation
BMSC
Cells were induced into chondrocytic cells using a previously described pellet culture method [29]. Briefly, 2 × 105 cells were centrifuged in 15 ml polypropylene tube at 500 × g for 5 min. Pellets were cultured in 500 μl chondrogenic medium consisting of high-glucose DMEM (Gibco Invitrogen) supplemented with 10 ng/ml of TGF-β3 (R&D Systems, UK), 100 nM dexamethasone, 50 μg/ml of L-ascorbate-2-phosphate, 40 μg /ml of L-proline (Sigma-Aldrich Corp), 100 μg/ml of sodium pyruvate (Sigma-Aldrich Corp.), 50 mg/ml of ITS + Premix (BD Biosciences and 500 ng/ml of BMP-2 (Peprotek)). Pellets were maintained for 7, 14 and 21 days with medium change every 3–4 days.
MEF
Cells were induced to the chondogenic lineage using micromass method and pellet culture method. Micromass method has been described for MEF previously [18]. Briefly, 1 × 105 cells were incubated in 10 μl drop for 2 h at 37°C in 24 well plates. After 2 h 500 μl medium (D-MEM + 15% FBS + 100 ng/ml BMP-2) was added. Medium was changed every 3–4 days for 21 days. MEF were also induced to chondrogenic lineage in the pellet culture method described above. For MEF, chondrogenic medium was supplemented with 100 ng/ml BMP-2.
Cytochemical Staining
Alkaline Phosphatase Staining
Cells were fixed with acetone/citrate buffer pH 4.2 (11/2:1) for 5 min at room temperature and stained with Napthol-AS-TR-phosphate solution for 1 h at room temperature. Napthol-AS-TR-phosphate solution consists of Napthol-AS-TR-phosphate (Sigma) diluted 1:5 in H2O and Fast Red TR (Sigma) diluted 1:1.2 in 0.1 M Tris buffer (OUH pharmacy), pH 9.0, subsequently both solutions were mixed 1:1. Cells were counterstained with Mayers-Hematoxylin for 5 min at room temperature.
Alizarin Red Staining for Mineralized Matrix
Cells were fixed with 70% ice-cold ethanol for 1 h at −20°C, and stained with 40 mM alizarin red S (AR-S; Sigma), pH 4.2 for 10 min at room temperature.
Oil Red O Staining
Cells were fixed with 4% paraformaldehyde for 10 min at room temperature, rinsed with 3% isopropanol solution, and stained with Oil Red O (Sigma) solution for 1 h at room temperature. Cells were counterstained with Mayers-Hematoxylin for 5 min at room temperature.
Alcian Blue Staining
Alcian Blue staining was performed for demonstration of proteoglycans. Micromass cultures were fixed with 4% paraformaldehyde for 15 min at RT and incubated with 1% Alcian Blue, pH 3.8 overnight at RT.
Immunocytochemistry
Pellets were fixed with 4% paraformaldehyde for 15 min at RT. Samples were embedded in paraffin and cut into 5 μm sections. Production of sulfated proteoglycans was demonstrated with alcian blue staining. Type II collagen production was detected by immunohistochemistry. Rehydrated sections were digested with bovine testicular hyaluronidase (2000 U/ml) in PBS (pH 5.5) for 60 min at +37°C to facilitate antibody access. Nonspecific antibody binding was blocked by 3% BSA (Sigma-Aldrich Corp.)/PBS. Sections were incubated with an anti-collagen type II monoclonal antibody (6B3 Chemicon Millipore, USA) for 1 h at RT. Samples were incubated with “Ready to use” Post Blocking for 20 min, washed with TNT buffer and incubated with Poly-HRP anti-mouse/rabbit IgG (PV-HRP) for 30 min. DAB chromogen was used for detection of the primary antibody. Sections were counterstained with hematoxylin. In control sections, the primary antibody was replaced with normal rabbit serum.
In Vivo Cell Implantation
Cells (1 × 106), mixed with hydroxyl-apatite/tricalcium phosphate ceramic powder (HA/TCP, 40 mg: Zimmer Scandinavia, Denmark), were implanted subcutaneously in the dorsal surface of 2 months old female NOD/SCID mice. MEF cells were cultured in osteoblast induction media for 5 days before implantation. 8-wk implants were fixed in 4% paraformaldehyde (Bie & Berntsen), decalcified in formic acid (Local Pharmacy, Odense University Hospital), and embedded in paraffin. Implants were sectioned (7.5 μm thick) and stained with eosin/hematoxylin. The amount of bone formed per implant was quantified as described previously [30].
For the remaining methods: RNA isolation, cDNA synthesis and qRT-PCR, Telomerase Repeat Amplification Protocol (TRAP), Alkaline phosphatase activity assay as well as the list of primers used in real time PCR (mouse) (Table S1) are described are included supplementary Material and Methods.
Results
MEF and BMSCs Exhibit Comparable CD Surface Marker Profiles
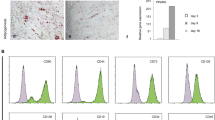
Using FACS analysis, cell surface markers of both MEF and BMSC were compared. MEF expressed cell surface markers known to be expressed by BMSC [31–33]: Sca-1++, CD106++, CD105++, CD73++ (>60%) CD29+++, CD44+++ (>90%) and negative for CD45−, CD11b−. No major differences were observed in the CD markers expression between MEF and BMSC (Fig. 1 b & c). While, only a small portion of a population was ALP+ (BMSC: ~11% and MEF: ~13.5%) in both cell types (Fig. 1 b & c). Moreover, compared to MEF, BMSC were largely negative for CD45-- and CD11b-- (>60%) (Fig. 1 b). Immunocytochemical staining further confirmed FACS data that both BMSC and MEF are Sca-1+, CD29+ and CD45- (Fig 1 S).
Flow cytometric (FACS) analysis of cell surface marker of mouse embryonic fibroblast (MEF) and bone marrow-derived stromal stem cells (BMSC). a Micrographs of confluent cultures of BMSC and MEF at passage 3. b BMSC and MEF were stained for cell surface markers as described in the method section. Both BMSC and MEF were largely positive for Sca-1, CD44, CD29, CD105, CD106 and CD73 (60–90%) and negative for CD45 and Cd11b (50–90%), while only a small fraction of cell population was positive for ALP in both (10–15%). Bar: (A) 100 μm
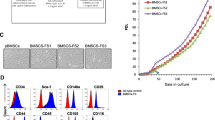
MEF Displayed Telomerase Activity and Increased Proliferation Rate Compared to BMSCs
MEF exhibited higher levels of telomerase activity, compared with BMSC as evidenced by TRAP assay (Fig. 2 a). Additionally, MEF exhibited a higher cell proliferation rate in short-term cultures (Fig. 2 b), a higher population doubling rate and a shorter population doubling time (3.43 days in MEF vs 11.6 days in BMSC) (Fig. 2 c) and fewer senescent cells stained positive for β-galactosidase (Fig. 2 d) compared with BMSC.
Comparative analysis of mouse embryonic fibroblast (MEF) and bone marrow-derived stromal stem cells (BMSC). a Telomerase activity was measured by telomerase repeat amplification protocol (TRAP) as described in the materials and methods. Human BMSC immortalized by telomerase were used for generating a standard curve. 0.5 μg of protein extract was used for both MEF and BMSC. b Short term proliferation of BMSC and MEF at day 1, 3, 6 and 9. c Long-term proliferation of BMSC and MEF. Population doubling time (PDT): MEF PDT of 3.43 days and BMSC PDT of 11.6 days. d β–galactosidase (β–gal) staining for BMSC and MEF. β—gal+ cells in BMSC cultures. Data are represented of means ± SD of three independent experiments. *p ≤ 0.05, ** p ≤ 0.005. Bar: (D) 100 μm
Ex Vivo Osteoblast Differentiation
Both MEF and BMSC differentiated into osteoblastic cells as evidenced by positive staining for ALP and formation of a mineralized matrix that stained positive with Alizarin red (Fig. 3 a–b). Treatment of MEF, but not BMSC, with BMP-2 was needed for induction of osteoblastic differentiation. Moreover, some phenotypic differences were observed between the two cell populations. At baseline, ALP activity was higher in BMSC compared to MEF, and BMSC-derived OB cells showed higher ALP levels compared with MEF-derived OB at day 15 of the differentiation induction (Fig. 3 c). Additionally, BMSC-derived OB exhibited higher levels of gene expression of Runx2 (runt-related transcription factor-2), Col1a1 (collagen type 1), Sparc (secreted protein, acidic, cysteine rich) and OC (osteocalcin) compared with MEF-derived OB (Fig. 3 d). Conversely, Osx (osterix), Ibsp (bone sialoprotein) and Opn (osteopontin) were significantly higher in MEF-derived OB cultures (Fig. 3 d).
Ex vivo and in vivo osteoblastic differentiation and bone formation of mouse embryonic fibroblast (MEF) and bone marrow-derived stromal stem cells (BMSC). BMSC and MEF cells were cultured and induced to osteoblastic cells as described in Material and Methods. Both control and differentiated cells (Induced) were examined at day 15. a alkaline phosphatase (ALP) histochemical staining. b Alizarin red staining for mineralized matrix. c ALP activity measurements corrected for protein content of cell layer. d Real-time qPCR analysis of steady state gene expression of osteoblastic genes, (Runx2, Osterix, Alkaline phosphatase, Collagen type 1 alpha1, Bone sialoprotein, Osteopontin, Osteonectin, and Osteocalcin). Expression of each target gene was calculated as a relative expression to beta-actin and represented as fold induction over control non-induced cells. Data are represented as mean ± SD of 3 independent experiments. *p ≤ 0.05. e In vivo heterotopic bone formation. Cells were mixed with hydroxyapatite/tricalcium phosphate (HA/TCP) and implanted subcutaneously into immune deficient (NOD-SCID) mice. Control represents HA/TCP implants without cells. Bone formed was quantitated as described in the Method section and expressed as bone volume (BV)/total volume (TV) %. Bar: 10 μm. H = HA/TCP, B = bone, A = adipocytes
Ex Vivo Adipocyte Differentiation
MEF and BMSC differentiated readily into adipocytes. MEF showed an increase in the number of mature adipocytes containing lipid droplets compared to BMSC, as demonstrated by Oil red O staining (Fig. 4 a). The improved adipogenesis in MEF cultures was also accompanied by significantly higher gene expression levels of known adipogenic markers: Pparγ (peroxisome proliferator-activated receptor gamma), Cebp (CCAAT/enhancer binding protein), Adipoq (adiponectin) and aP2 (adipocyte lipid binding protein) compared to BMSC cultures (Fig. 4 b).
Ex vivo adipocyte differentiation of mouse embryonic fibroblast (MEF) and bone marrow-derived stromal stem cells (BMSC). Cells were cultured and induced to adipocytes as described in the Methods. Both control and differentiated cells (Induced) were examined at day 15. a Oil red O staining for intracellular lipid. Bar 100 μm b Real-time qPCR analysis of steady state gene expression of adipocytic genes (Peroxisome proliferator-activated receptor gamma), Cebp (CCAAT/enhancer binding protein), Adipoq (Adiponectin) and aP2 (Adipocyte lipid binding protein). Expression of each target gene was calculated as a relative expression to beta-actin and represented as fold induction over control non-induced cells. Data are represented as mean ± SD of 3 independent experiments. *p ≤ 0.05
Ex Vivo Chondrogenic Differentiation
MEF were able to differentiate into chondrocytic cells in micromass cultures, as evidenced by expression of chondrocyte marker proteins (Fig. 5 a). Moreover, both MEF and BMSC differentiated to chondrocytic cells in pellet cultures as evidenced by positive staining for alcian blue and Col II as well as gene expression of chondrocytic markers: Sox9 (SRY-box containing gene 9), Col2a1(collagen type 2), Acan (Aggrecan) and Col10a1(Collagen type 10) (Fig. 5 b & c). Cyotchemical and immunocytochemical analysis revealed enhanced proteoglycan production (Fig. 5 b) and a higher number of type II collagen positive cells with chondrocyte-like morphology in induced MEF cultures compared to BMSC cultures (Fig. 5 b). Additionally, gene expression levels of chondrocytic markers: Sox9, Col2a1, Acan and Col10a1 were higher in MEF-differentiated chondrocytes compared to BMSC-differentiated chondrocytes at day 21 (Fig. 5 c).
Ex vivo chondrocyte differentiation of mouse embryonic fibroblast (MEF) and bone marrow-derived stromal stem cells (BMSC). a MEFs were induced into chondrogenesis in micromass system as described in methods. Left panels are staining of chondrocytic markers, right panel is real time qPCR of chondrocytic genes Bar 100 μm. b BMSC and MEF were differentiated in pellet cultures as described in the Methods. Staining for Alcian blue and type II collagen were performed at day 7, 14 & 21. Bar 50 μm. c Real time qPCR analysis of steady state chondrocytic genes. For real time qPCR, expression of each target gene was calculated as a relative expression to beta-actin and represented as fold induction over control non-induced cells. Sox9 (SRY-box containing gene 9), Col2a1 (collagen 2 alpha 1), Acan (Aggrecan) and Col10a1(Collagen type 10). Data are mean ± SD of 3 independent experiments. *p ≤ 0.05, ** p ≤ 0.005
In Vivo Heterotopic Bone Formation
Both MEF and BMSC were able to form characteristic heterotopic bone and bone marrow containing adipocytes and hematopoietic cells when mixed with hydroxyapatite/tricalcium phosphate (HA/TCP) and implanted subcutaneously for 8 weeks in NOD-SCID mice (Fig. 3 e). However, BMP-2 treatment prior to implantation was required for MEF but not for BMSC. The quantity of bone formed by MEF and BMSC were comparable (Fig. 3 f). We did not find any evidence of teratoma or tumor formation or formation of other tissues in implants containing MEF.
Discussion
In the present study, we demonstrated that MEF and BMSC exhibit a similar CD surface marker profile and capacity for ex vivo differentiation into osteoblastic, adipocytic and chondrocytic cells. Additionally, both cell types could form heterotopic bone and bone marrow when implanted in vivo in immune deficient mouse. However, important differences were also observed in proliferation and differentiation responses.
We found that MEF exhibit higher telomerase activity in contrast to BMSC. The presence of telomerase activity and consequently long telomeres have previously been reported in MEF cultures [34, 35]. Lack of telomerase activity and telomere shortening is an important factor mediating the in vitro replicative senescence phenotype of BMSC and other cultured diploid cells [8, 36] and the mechanism explaining the accumulation of senescent β-gal+ positive cells, the reduced cell proliferation rate and longer population doubling time that we observed in BMSC cultures compared to MEF [37].
No previous studies have compared CD surface marker profiles of murine BMSC and MEF. Like human BMSC, we observed that murine BMSC were largely negative for CD45 and CD11b. Interestingly, MEF cultures contained a small population that was positive for CD45 and CD11b suggesting the presence of cells with hematopoietic potential and reflecting the cellular heterogeneity of MEF cells. It is also plausible that MEF contain a population of primitive mesodermal cells that are, in addition to being CD45+and CD11b+, CD34+ and CD133+. However, further studies are needed to verify this hypothesis through single cell cloning studies.
Conversely, MEF were not distinguishable from BMSC based on typical BMSC CD marker expression: CD106, CD105, CD73, CD44, CD29 and Sca-1 [31–33]. Moreover, FACS analysis revealed similar numbers of ALP+ cells. Our data suggest that surface marker expression is not predictive for the functional phenotype of the cells. Similar to our findings, Wagner et al [38], employing 22 CD markers including known markers of BMSC, were unable to distinguish between human fibroblast cell lines (HS68 & NHDF), devoid of differentiation potential, and the multipotent BMSC derived from bone marrow, adipose tissue or umbilical cord. Additionally, our group has reported that CD markers defining human BMSC were not able to distinguish bone-forming (and thus true osteoprogenitor) from non-bone forming clonal human BMSC [39].
MEF cells differentiated readily into osteoblastic cells and our data corroborate previous findings on the ability of MEF for osteoblastic differentiation [23]. However, the osteoblastic differentiation of MEF required BMP-2 treatment suggesting MEF are not equivalent to BMSC in their differentiation stage. This is also supported by our results showing that, in vivo heterotopic bone formation by MEF required prior treatment with BMP-2 which was not the case with BMSC. BMP-2 is known to induce an osteoblastic phenotype in a number of cellular models including fibroblastic cells [40, 41]. Additionally, the presence of higher levels of Runx2 in BMSC compared to MEF suggest a more “osteoblastic” commitment or enrichment of “preosteoblastic cells” [4, 18].
Both BMSC and MEF were able to differentiate to adipocytic and chondrocytic cells thus corroborating previous reports [21, 22, 42, 43]. However, we observed more enhanced adipocytic and chondrocytic differentiation in MEF compared to BMSC cultures. These differences may be related to culture conditions. For example, the pellet culture system is commonly used for inducing chondrogenesis in BMSC but has not been previously reported for MEF. This model permits cell-cell interactions and may allow recapitulation of the prechondrogenic condensation stage of embryonic development [29, 44] and thus provides better ex vivo conditions for chondrocytic differentiation of MEF. In support for this hypothesis, we observed increased expression of chondrocytic transcription factors Sox9 in pellet culture compared to cells cultured in micromass cultures. Sox9 is essential for the commitment of mesenchymal stem cells into chondrogenesis and plays an important role in the expression of Col2a1 and other cartilage specific proteins such as Aggrecan [45, 46].
While we observed differences between MEF and BMSC in their response to differentiation signals, MEF, in general, performed comparably with BMSC. Whether all MEF behave like BMSC or only a small fraction needs further investigation. Although analysis of CD marker expression demonstrates comparable differentiation ability between MEF and BMSC, in vitro differentiation data suggests there are differences in the cellular response to differentiation signals, thus BMP-2 is required for induction of the osteoprogenitor phenotype in MEF. Another explanation for the differences noted between MEF and BMSC is the presence of a higher degree of cellular heterogeneity in MEF cultures compared to BMSC where only a subset of MEF cells are BMSC-like. This may be expected since MEF are derived from different connective tissues, including subcutaneous fat and cartilage compared to BMSC. It is plausible that this BMSC-like cell population is selected by culture conditions including BMP-2 treatment, or by an inherent growth advantage over other fibroblastic cells present in the crude preparation of MEF. Thus, clonal analysis of MEF should be carried out to test this hypothesis. However, establishing clonal MEF lines may compromise the use of MEF as “primary”, “non-transformed” cells as establishment of clonal strains can cause murine cells to spontaneously immortalize during extensive passaging.
In conclusion, our findings demonstrate that MEF cultures are heterogeneous and less differentiated compared to BMSC cultures. Thus, differentiation studies employing MEF complement, but do not substitute for BMSC. The availability of primary MEF from an increasing number of gene-modified and disease models provides a novel approach for studying early cellular and molecular regulation of osteoblast, adipocyte and chondrocyte commitment and differentiation during prenatal development.
References
Bianco, P., Kuznetsov, S. A., Riminucci, M., & Gehron, R. P. (2006). Postnatal skeletal stem cells. Methods in Enzymology, 419, 117–148.
Kassem, M. (2004). Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning and Stem Cells, 6(4), 369–374.
Kuroda, Y., Kitada, M., Wakao, S., et al. (2010). Unique multipotent cells in adult human mesenchymal cell populations. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8639–8643.
Post, S., Abdallah, B. M., Bentzon, J. F., & Kassem, M. (2008). Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone, 43(1), 32–39.
Zhou, S. (2011). TGF-beta regulates beta-catenin signaling and osteoblast differentiation in human mesenchymal stem cells. J Cell Biochem.
Pittenger, M. F., Mackay, A. M., Beck, S. C., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Saeed, H., Abdallah, B. M., Ditzel, N., et al. (2011). Telomerase-deficient mice exhibit bone loss due to defects in osteoblasts and increased osteoclastogenesis by inflammatory microenvironment. Journal of Bone and Mineral Research.
Simonsen, J. L., Rosada, C., Serakinci, N., et al. (2002). Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nature Biotechnology, 20(6), 592–596.
Peister, A., Mellad, J. A., Larson, B. L., Hall, B. M., Gibson, L. F., & Prockop, D. J. (2004). Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood, 103(5), 1662–1668.
Phinney, D. G., Kopen, G., Isaacson, R. L., & Prockop, D. J. (1999). Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. Journal of Cellular Biochemistry, 72(4), 570–585.
Kuznetsov, S. A., Mankani, M. H., Gronthos, S., Satomura, K., Bianco, P., & Robey, P. G. (2001). Circulating skeletal stem cells. The Journal of Cell Biology, 153(5), 1133–1140.
Rossi, O., Barbieri, O., & Frosina, G. (2003). Time-course of spontaneous transformation of CD-1 mouse embryonic fibroblasts. Anticancer Research, 23(2B), 1373–1377.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Sun, H., Gulbagci, N. T., & Taneja, R. (2007). Analysis of growth properties and cell cycle regulation using mouse embryonic fibroblast cells. Methods in Molecular Biology (383), 311–319.
Ciruna, B. G., Schwartz, L., Harpal, K., Yamaguchi, T. P., & Rossant, J. (1997). Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development, 124(14), 2829–2841.
Deng, C. X., Wynshaw-Boris, A., Shen, M. M., Daugherty, C., Ornitz, D. M., & Leder, P. (1994). Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes & Development, 8(24), 3045–3057.
Kamijo, T., Zindy, F., Roussel, M. F., et al. (1997). Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell, 91(5), 649–659.
Lengner, C. J., Lepper, C., van Wijnen, A. J., Stein, J. L., Stein, G. S., & Lian, J. B. (2004). Primary mouse embryonic fibroblasts: a model of mesenchymal cartilage formation. Journal of Cellular Physiology, 200(3), 327–333.
Lowe, S. W., Jacks, T., Housman, D. E., & Ruley, H. E. (1994). Abrogation of oncogene-associated apoptosis allows transformation of p53-deficient cells. Proceedings of the National Academy of Sciences of the United States of America, 91(6), 2026–2030.
Steinman, H. A., Sluss, H. K., Sands, A. T., Pihan, G., & Jones, S. N. (2004). Absence of p21 partially rescues Mdm4 loss and uncovers an antiproliferative effect of Mdm4 on cell growth. Oncogene, 23(1), 303–306.
Alexander, D. L., Ganem, L. G., Fernandez-Salguero, P., Gonzalez, F., & Jefcoate, C. R. (1998). Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. Journal of Cell Science, 111(Pt 22), 3311–3322.
Yun, Z., Maecker, H. L., Johnson, R. S., & Giaccia, A. J. (2002). Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Developmental Cell, 2(3), 331–341.
Garreta, E., Genove, E., Borros, S., & Semino, C. E. (2006). Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Engineering, 12(8), 2215–2227.
Peister, A., Mellad, J. A., Larson, B. L., Hall, B. M., Gibson, L. F., & Prockop, D. J. (2004). Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood, 103(5), 1662–1668.
Post, S., Abdallah, B. M., Bentzon, J. F., & Kassem, M. (2008). Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone, 43(1), 32–39.
Zhang, Y., Zhao, L., Wang, C., & Lei, B. (2003). Isolation and culture of mouse embryonic fibroblast. Sichuan Da Xue Xue Bao. Yi Xue Ban, 34(2), 344–346.
Hogan, B., Beddington, R., Costantini, F., & Lacy, E. (1994). Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor Press.
Dimri, G. P., Lee, X., Basile, G., et al. (1995). A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences of the United States of America, 92(20), 9363–9367.
Johnstone, B., Hering, T. M., Caplan, A. I., Goldberg, V. M., & Yoo, J. U. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research, 238(1), 265–272.
Abdallah, B. M., Ditzel, N., & Kassem, M. (2008). Assessment of bone formation capacity using in vivo transplantation assays: procedure and tissue analysis. Methods in Molecular Biology, 455, 89–100.
Abdallah, B. M., Haack-Sorensen, M., Burns, J. S., et al. (2005). Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochemical and Biophysical Research Communications, 326(3), 527–538.
Baddoo, M., Hill, K., Wilkinson, R., et al. (2003). Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. Journal of Cellular Biochemistry, 89(6), 1235–1249.
Foster, L. J., Zeemann, P. A., Li, C., Mann, M., Jensen, O. N., & Kassem, M. (2005). Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells, 23(9), 1367–1377.
Blasco, M. A., Lee, H. W., Hande, M. P., et al. (1997). Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell, 91(1), 25–34.
Itahana, K., Campisi, J., & Dimri, G. P. (2004). Mechanisms of cellular senescence in human and mouse cells. Biogerontology, 5(1), 1–10.
Stenderup, K., Justesen, J., Clausen, C., & Kassem, M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone, 33(6), 919–926.
Campisi, J. (2011). Cellular senescence: putting the paradoxes in perspective. Current Opinion in Genetics & Development, 21(1), 107–112.
Wagner, W., Wein, F., Seckinger, A., et al. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental Hematology, 33(11), 1402–1416.
Larsen, K. H., Frederiksen, C. M., Burns, J. S., Abdallah, B. M., & Kassem, M. (2010). Identifying a molecular phenotype for bone marrow stromal cells with in vivo bone-forming capacity. Journal of Bone and Mineral Research, 25(4), 796–808.
Rutherford, R. B., Moalli, M., Franceschi, R. T., Wang, D., Gu, K., & Krebsbach, P. H. (2002). Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Engineering, 8(3), 441–452.
Wang, E. A., Israel, D. I., Kelly, S., & Luxenberg, D. P. (1993). Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors, 9(1), 57–71.
Abdallah, B. M., & Kassem, M. (2008). Human mesenchymal stem cells: from basic biology to clinical applications. Gene Therapy, 15(2), 109–116.
Bianco, P., Riminucci, M., Gronthos, S., & Robey, P. G. (2001). Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells, 19(3), 180–192.
Barry, F., Boynton, R. E., Liu, B., & Murphy, J. M. (2001). Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Experimental Cell Research, 268(2), 189–200.
Goldring, M. B., Tsuchimochi, K., & Ijiri, K. (2006). The control of chondrogenesis. Journal of Cellular Biochemistry, 97(1), 33–44.
Lefebvre, V., & Smits, P. (2005). Transcriptional control of chondrocyte fate and differentiation. Birth Defects Research. Part C, Embryo Today, 75(3), 200–212.
Acknowledgements
The authors are grateful to Lone Christiansen for excellent technical assistance. The study was supported by grants from the Lundbeck foundation, the NovoNordisk Foundation, the Karen Elise Jensen’s foundation, Denmark and King Abdulaziz City for Science and Technology (09-BIO740-20), KSA. H. Saeed has received a PhD fellowship from the NovoNordisk Foundation, Denmark.
Conflict of interests
Authors have no conflicting interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.65 MB)
Rights and permissions
About this article
Cite this article
Saeed, H., Taipaleenmäki, H., Aldahmash, A.M. et al. Mouse Embryonic Fibroblasts (MEF) Exhibit a Similar but not Identical Phenotype to Bone Marrow Stromal Stem Cells (BMSC). Stem Cell Rev and Rep 8, 318–328 (2012). https://doi.org/10.1007/s12015-011-9315-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9315-x