Abstract
Human embryonic stem cells (hESCs) are a potential source of material for cell therapy of muscle diseases. To date, it has proven difficult to generate skeletal muscle from hESCs in high yields and within a reasonable timeframe. Further, a hESC-derived Pax3/7-positive skeletal muscle progenitor population has not yet been described. Previous studies have shown that Pax3/7-positive progenitor cells can repopulate the satellite cell niche, indicating the importance of this population for therapy. We sought to optimize the differentiation of hESCs into skeletal muscle in order to characterize myogenesis at a molecular level and shorten the time course. We treated hESCs with retinoic acid (RA) and found an enhancement of skeletal myogenesis, and the expression of the myogenic regulatory factors (MRFs) MyoD and myogenin by day 25. Furthermore, we found that RA treatment expanded the muscle progenitor pool, which occurred as a distinct Pax3+ve population prior to MRF expression. Non-skeletal muscle tissue types were not significantly affected. Therefore, we have identified a differentiation pathway in hESCs that provides a skeletal muscle progenitor population which can undergo myogenesis more efficiently. We propose that RA could fit into a directed culture method for deriving skeletal muscle from hESCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human Embryonic Stem Cells (hESCs) present a promising source for cell therapy of muscle diseases such as muscular dystrophy. While hESC-derived skeletal muscle can be stably engrafted into mice, the most significant barrier for hESC-based therapy is the generation of a desirable amount of muscle in a reasonable timeframe. Successful strategies have included the use of a covalent chemical modifying agent, 5-azacytidine, or derivation of multipotent mesenchymal precursors from hESCs, which give rise to a higher proportion of skeletal myocytes than the parental line [1–3]. The latter approach required a lengthy time course (>45 days), starting with several weeks of low density monolayer culture to generate mesenchymal precursor cells. Furthermore, it did not utilize embryoid body formation, nor did it follow an embryonic pathway involving mesoderm induction followed by the formation of a distinct Pax3/7+ve progenitor population prior to expression of the myogenic regulatory factors (MRFs). Such an embryonic pathway has been shown to occur during skeletal myogenesis in P19 embryonal carcinoma (EC) cells, mouse embryonic stem cells (mESCs), and mouse induced pluripotent stem cells (miPSCs) [4–6]. The formation of a Pax3/7+ve progenitor population would be valuable for therapeutic purposes, as it can replenish the satellite cell niche [7, 8].
Skeletal muscle progenitor cells are marked by the expression of Pax3, Pax7 and Meox1 [9–12]. While Pax3-null mice exhibit a lack of limb and diaphragm muscle, Pax7 is dispensable for normal embryonic myogenesis [13–17]. Compound Pax3/Pax7-null mice however, exhibit a much more severe phenotype characterized by a complete failure of skeletal myogenesis (with the exception of early myotomal muscle), indicating that there is some level of functional redundancy between these two factors [9]. Consistent with this, expression of a dominant-negative Pax3 protein, created by fusion to the repressor domain of engrailed, resulted in a complete inhibition of myogenesis in P19 EC cells and aberrant expression of MyoD and myogenin in cultured satellite cells [12, 18]. These data indicate that Pax activity is necessary for normal myogenesis. Conversely, forced expression of Pax3 is known to induce MRF expression and myogenesis in embryonic tissue and in various cell culture models, including P19 EC cells and mESCs [12, 19, 20]. Furthermore, Pax3 binds directly to a Myf5 enhancer region, activating its expression in the hypaxial somite [21]. Taken together, these data show that Pax3 is required for skeletal myogenesis, upstream of the MRFs.
Our understanding of the role of Meox factors in skeletal myogenesis is complicated by a high degree of redundancy between Meox1 and Meox2. While Meox1-null mice exhibit defects in sclerotomal derivatives, Meox2-null mice have defects in limb muscle development [10, 22]. However, like the Pax3/7 compound mutant, Meox1/Meox2 double knockout mice exhibit a much more severe phenotype characterized by an almost complete abrogation of skeletal myogenesis [10]. Meox activity is also required for myogenesis in P19 EC cells, where expression of a dominant negative Meox1-Engrailed fusion protein disrupts expression of Pax3, Pax7, MyoD and myogenin [11]. In addition to their important roles in myogenesis, the co-expression of Pax3 and Meox1 serves as a good marker for premyogenic mesoderm as the expression domains of these two factors overlap in the paraxial mesoderm, somite, and dermomyotome, while Meox1 is absent from the dorsal neural tube, which has abundant Pax3 expression [23–25]. Therefore, strategies to produce a distinct Pax3+ve/Meox1+ve skeletal muscle progenitor population and enhance skeletal myogenesis in a concise time frame, ideally without genetic or covalent chemical modification, would undoubtedly facilitate the application of hESCs for cell therapy of muscle diseases.
Retinoic acid (RA) is a potent morphogen and is known to direct differentiation of several tissue types in the embryo and in ES cells [26, 27]. RA acts by activating its downstream effectors, the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs), which bind to retinoic acid response elements (RAREs) within regulatory regions of RA-responsive genes. In the presence of RA, RARs and RXRs act by recruiting co-activators that promote gene transcription [28]. We have previously shown that RA enhances skeletal myogenesis in mESCs and in P19 EC cells [6]. In these systems, RA acted early in differentiation to enhance the expression of the precursor genes Pax3 and Meox1 by direct binding of RARs to regulatory regions of these genes [6]. This ultimately resulted in enhanced MRF expression and terminal differentiation.
Although many of the genetic networks controlling pluripotency and differentiation in mESCs are conserved in hESCs, there exist fundamental differences between the two systems [29]. As such, pathways that have previously been elucidated in the murine system bear a re-examination in hESCs for their ability to drive tissue specific differentiation with a view to therapeutic applications. Here, we show that both myogenesis and the expression of skeletal muscle genes were significantly enhanced by RA treatment of hESCs. Importantly, RA treatment resulted in expansion of a Pax3+ve skeletal muscle progenitor population, which appeared prior to MRF expression. Addition of RA had no significant influence on the differentiation of other cell types. Thus, RA might be useful as part of a directed culture system designed to maximize the yield of muscle progenitor cells from hESCs and/or human iPS cells.
Methods
Cell Culture
H9 hESCs were maintained on feeder layers as described with the exception that feeders were mitotically inactivated with 10 μg/ml mitomycin C for 2 h [30]. Briefly, H9 hESCs were maintained in Knock-out DMEM supplemented with 20% Knock-out serum replacement, 1% non-essential amino acids, 1 mM L-glutamine, penicillin-streptomycin (100 U/ml and 100 ug/ml) and 0.1 mM beta-mercaptoethanol. Media was also supplemented with 4 ng/ml basic fibroblast growth factor. Cells were maintained on a mouse embryonic fibroblast layer. Cells were differentiated by generating embryoid bodies in suspension culture for 10 days and then transferred to adherent plates for a further 29 or 15 days. The medium was changed every 2–3 days. Differentiation medium was the same as the propagation medium supplemented with 20% FBS in place of Knock-out serum replacement. All-trans RA (Sigma-Aldrich, Oakville, ON) was added to the culture medium at increasing concentrations from Day 0 to Day 10 of differentiation. Ethanol was used as the vehicle at a final concentration of 0.01%. On day 35 or day 20 of differentiation, the differentiation medium was replaced with D-MEM F12 containing N2 supplement (Invitrogen, Burlington, ON) and Insulin/Transferrin/Selenium (Invitrogen, Burlington, ON), termed N2-ITS medium, until the end point of the experiment. D3 mESCs (ATCC#CRL-1934, Manassas, VA) and P19 EC cells (ATCC#CRL-1825, Manassas VA) were cultured as described [6].
Gene Expression Analysis
Total RNA was harvested from hESCs using RNeasy Micro and Mini kits as per the manufacturer’s instructions (Qiagen, Mississauga, ON). cDNA synthesis was performed using the Quantitect Reverse Transcription Kit (Qiagen, Mississauga, ON) followed by quantitative polymerase chain reaction (QPCR) with either the FastStart SYBR Green Master Mix kit (Roche, Laval, QC) or GoTaq qPCR Master Mix (Promega Madison, WI). QPCR reactions were performed and analyzed on the ABI 7300 or 7500 systems using the SDS analysis software or on the Eppendorf Realplex2 using the Realplex software for analysis. GAPDH was used as a reference for all QPCR reactions and expression changes were quantified relative to Day 0 levels. Unless otherwise stated, error bars represent the standard error of the mean. Statistical analysis was performed using Student’s t test.
Immunofluorescence
For myosin heavy chain (MyHC) and Tuj1 labelling, cells were fixed with methanol and labelled with MF20 (pan-MyHC) or Tuj1-specific antibodies (Sigma-Aldrich, Oakvile, Ontario). MyHC staining was performed as previously described [6]. For alpha smooth muscle actin labelling, cells were fixed with 4% paraformaldehyde and permeabilized for 15 min with PBS containing 0.5% TritonX-100. Cells were then blocked for 1 h with PBS containing 0.1% bovine serum albumin, 0.1% TritonX-100 and 10% donkey serum. The alpha smooth muscle actin antibody was obtained from Abcam (Cambridge, MA). Pax3/MyoD, Pax3/Meox1 and Pax3/7/myogenin labelling was performed as described with the exception that cells were fixed with 4% paraformaldehyde for Pax3/MyoD labelling [31]. Pax3 antibodies were obtained from R&D Systems (Minneapolis, MN) while Meox1 and Pax3/7 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Myogenin labelling was performed with the F5D hybridoma and MyoD antibodies were obtained from BD Biosciences (Franklin Lakes, NJ). Cy3-, Alexa488- and DyLight488-conjugated secondary antibodies were used for detection as appropriate (Jackson ImmunoResearch, West Grove, PA and Invitrogen, Burlington, ON). Confocal images were captured using a Zeiss LSM 510 microscope and processed with Zen software (Zeiss). Epifluorescent images were captured on a Zeiss Axioskop. All images were processed in Canvas11.
Results
Retinoic Acid Enhances Skeletal Myogenesis in hESCs
We have previously shown that treatment with low levels of RA can enhance skeletal myocyte formation in both P19 EC cells and in mESCs [6]. However, since mechanisms are not always conserved between mESCs and hESCs, it is important to determine whether or not RA would enhance skeletal myogenesis in hESCs [29]. Here, we treated differentiating hESCs with increasing concentrations of RA from Day 0 to Day 10 during embryoid body formation, after which the embryoid bodies were plated into tissue culture dishes until day 35. Subsequently, the medium was changed on day 35 to N2-ITS medium and the presence of skeletal myocytes was examined on day 39 by immunofluorescence with an anti-MyHC antibody. We found that treatment with 3nM RA resulted in the presence of abundant hESC-derived skeletal myocytes (Fig. 1A&B). RA-treated hESCs differentiated into skeletal myocytes (Fig. 1A) and multinucleated myotubes (Fig. 1B). Quantification revealed that RA treatment resulted in a significant increase in MyHC-positive nuclei, from approximately 1 to 4% of total cells (Fig. 1C).
Human Embryonic stem cells differentiate into skeletal muscle in response to retinoic acid. H9 hESCs were differentiated for 10 days in the presence of increasing concentrations of RA or vehicle control, followed by growth on adherent plates. Cultures were fixed with methanol for immunofluorescence or harvested for RNA on day 39. A) hESC, P19EC, or mESC aggregates were labelled with a pan-MyHC antibody and Hoechst dye to detect nuclei. P19 cell aggregates were fixed on Day 9, while mESC aggregates were fixed on day 15 (scale bar represents 10 μm). B) hESC derived muscle formed multinucleated myotubes(scale bar represents 10 μm). C) Skeletal myogenesis was quantified by counting MyHC+ve cells with a bipolar morphology. A minimum of 20,000 nuclei were quantified for each treatment across 30 fields of view *p < 0.05. D) RNA was analyzed by QPCR for expression of the genes indicated (n = 4–5; *p < 0.05) and expressed as a percent of maximum for each independent experiment
To compare the myocytes generated from hESCs with those derived from mESCs and P19 EC cells, we differentiated the latter two cell lines and examined their morphology by immunofluorescence. D3 mESCs were differentiated by the formation of embryoid bodies for 7 days, followed by plating onto tissue culture plates and examining cultures on day 15. The embryoid bodies were treated with RA from days 2–7 of differentiation [6]. P19 EC cells were differentiated by aggregation of cells for 4 days in the presence of RA and 1% DMSO, after which cells were plated in tissue culture plates in the absence of drug and examined on day 9 of differentiation [6]. hESCs differentiated into bipolar myocytes with a morphology similar to those derived from either D3 mESCs or P19 EC cells, however, some hESC-derived myocytes appeared thicker and displayed additional cellular projections (Fig. 1A).
To determine if changes in gene expression correlated with the observed changes in cell morphology, cultures were examined for transcript levels of the MRFs myogenin, MyoD, and Myf5 by Q-PCR (Fig. 1D). hESC cultures treated with increasing amounts of RA (from 1nM to 10nM) were differentiated as described above and total RNA was harvested on day 39. Whereas treatment with 1nM RA did not significantly affect the levels of MRF expression relative to vehicle control, treatment with 3nM RA resulted in a significant enhancement of myogenin and MyoD expression levels (Fig. 1D). Notably, treatment of differentiating cells with 10nM RA did not result in increased MRF expression, indicating that RA-mediated enhancement did not follow a dose–response pattern. Unlike MyoD and myogenin, the expression of Myf5 was not significantly upregulated by treatment with RA (Fig. 1D). Although Myf5 expression showed a trend toward upregulation at 1nM RA, this was not statistically significant. Thus, 3nM RA enhanced skeletal myogenesis, upregulated the expression of MyoD and myogenin and was used in our subsequent experiments.
Treatment with RA Results in Expansion of the Skeletal Muscle Progenitor Population
We have previously shown that RA enhances myogenesis in mESCs and P19 EC cells by expanding the premyogenic progenitor population [6]. We sought to determine whether the enhancement of myogenesis by RA in hESCs occurred at the same stage of differentiation. We first analyzed the expression levels of the mesodermal markers BrachyuryT, Mesp1 and Mesp2 in the presence and absence of RA by QPCR at day 3 of embryoid body formation. We found that the expression levels of these three markers remained unaffected by RA treatment, suggesting that RA does not enhance myogenesis through mesoderm induction (Fig. 2A). We next examined control and RA-treated cultures for the expression of premyogenic progenitor markers. The expression levels of Meox1 were analyzed by QPCR on day 8 of embryoid body formation and were found to be increased in RA-treated samples (Fig. 2B). The premyogenic progenitor population is also marked by the expression of Pax3. In order to determine whether RA treatment resulted in an expansion of this Pax3+ve population, we quantified the number of Pax3+ve cells in control and RA-treated cultures. hESCs were differentiated by forming embryoid bodies for 10 days with and without 3nM RA and then plated into tissue culture plates and fixed on day 15. Cultures were labelled with a Pax3-specific antibody and quantified in control and RA-treated cultures. RA treatment increased the number of Pax3+ve cells by approximately 3-fold (Fig. 2C&D). Co-staining with antibodies specific to Pax3 and Meox1 identified a population of Pax3+ve cells that were also positive for Meox1 expression, demonstrating the existence of a Pax3+ve/Meox1+ve muscle progenitor population in hESCs (Fig. 2E). Importantly, co-staining of Pax3 with either MyoD- or myogenin-specific antibodies at Day 15, showed that Pax3+ve cells were negative for myogenin and MyoD expression (data not shown), in agreement with the lack of MyoD or myogenin transcripts present at this time (Fig. 3, see below). These results suggest that RA acts after mesoderm induction to upregulate muscle precursor gene expression, resulting in an expansion of the Pax3+ve muscle progenitor population, leading to enhanced myogenesis.
RA Treatment expands the skeletal muscle progenitor population A) RNA was harvested from Day 3 cultures and analyzed for expression levels of BrachyuryT, Mesp1 and Mesp2. The change in expression is quantified relative to Day 0 levels. Data are from one representative experiment. Error bars represent standard deviation. B) RNA was harvested from Day 8 cultures and analyzed for expression of Meox1 by QPCR (n = 4; *p < 0.05). The change in Meox1 expression levels as a result of RA treatment is expressed relative to vehicle (EtOH) treatment. C) Differentiating cultures were fixed on Day 15 and labelled with an anti-Pax3 antibody and Hoechst dye to detect nuclei (scale bar represents 10 μm). D) The number of Pax3-positive cells was quantified by counting at least 600 cells over 20 fields of view (*p < 0.05). E) Differentiating cultures were fixed on Day 15 and labelled with anti-Pax3 and anti-Meox1 antibodies and stained with Hoechst dye to detect nuclei (scale bar represents 10 μm)
Skeletal muscle genes are temporally regulated during hESC differentiation. RNA was harvested from hESC cultures at the times indicated and analyzed for skeletal muscle gene expression, for the genes indicated, using QPCR (n = 4–5; *p < 0.05). Changes in gene expression levels are expressed relative to undifferentiated cells (Day 0)
Skeletal Muscle Gene Expression is Temporally Regulated During RA-Induced Myogenesis
Studies in mESCs, miPSCs and in P19 EC cells have revealed that skeletal myogenesis in these systems proceeds via a temporal pattern of gene expression that is reminiscent of muscle formation in the embryo [4–6]. The temporal pattern of gene expression was examined by QPCR using a time course of hESC differentiation. hESCs were differentiated in the presence of RA by forming embryoid bodies for 10 days as described above. Total RNA was harvested on days 0, 8, 15, 28, and 39. In RA-treated hESCs, the expression of the premyogenic mesoderm markers Meox1, Pax7 and Pax3 peaked at Day 8 of differentiation (Fig. 3A–C). There was a trend for an increase in Pax3 expression at day 39 but this was not significant. The expression of Myf5, MyoD and myogenin was not significantly upregulated until Day 39 (Fig. 3D–F), 4 days after the switch from serum-containing differentiation medium to N2-ITS medium. Interestingly, there was a trend for increases in Myf-5 expression at earlier stages, coinciding with the premyogenic mesoderm factors, but this was not significant. Thus, myogenesis proceeded via an embryonic pathway, resulting in a progenitor to myoblast transition.
The Differentiation Timecourse of Skeletal Myogenesis in hESCs Can Be Shortened to 25 Days
Given that P19 EC cells and mESCs require 5 and 8 days respectively to form myoblasts from premyogenic mesoderm (Fig. 6), we speculated that our identification of 31 days for hESCs could be shortened. We therefore sought to determine whether skeletal muscle could be derived from hESCs at an earlier timepoint by lowering the serum in the differentiation medium prior to Day 35. Indeed we found that the serum could be lowered as early as Day 20 of RA-directed differentiation and that skeletal myocytes were formed by Day 25, as shown by the presence of MyHC+ve myocytes by immunofluorescence (Fig. 4A). We analyzed the day 25 transcript levels of myogenin by QPCR and found that they were significantly upregulated relative to day 0 levels (Fig. 4B). Therefore, skeletal muscle can be derived from hESCs in a 25 day time course, which is significantly shorter than previously published protocols [1]. To determine if Pax3+ve cells were able to become myoblasts, we also labelled these Day 25 cultures for Pax3 and MyoD. Most MyoD+ve cells at day 25 were negative for Pax3 expression which is likely due to the significant downregulation of Pax3 expression at this timepoint (Fig. 3A). We did however observe some Pax3+ve/MyoD+ve cells, indicating that at least part of the Pax3+ve progenitor population does give rise to myoblasts during hESC differentiation (Fig. 4C). Therefore, hESCs can be differentiated into skeletal muscle via an embryonic pathway that can be as short as 25 days.
The timecourse of skeletal myogenesis in hESCs can be shortened to 25 days. A) Differentiating hESC cultures were fixed with methanol on Day 25 and labelled with antibodies against MyHC and with Hoechst dye to detect nuclei (scale bar represents 10 μm). B) RNA was harvested from Day 0 and Day 25 RA- treated cultures and analyzed for the expression of myogenin. Day 25 expression levels are quantified relative to Day 0. C) Differentiating cultures were fixed on Day 25 and labelled with antibodies against Pax3 and MyoD and with Hoechst dye to detect nuclei. Scale bar represents 10 μm
Low Levels of RA Have Little Effect on the Differentiation of Non-skeletal Muscle Lineages
RA is known to induce or enhance the formation of many cell types during stem cell differentiation, including smooth muscle, bone, cardiac muscle and neurons, although, at least in the case of smooth muscle and neurons, this enhancement occurs at higher (μM) concentrations of RA [32–35]. We therefore examined our RA-treated hESC cultures for differentiation into these other cell types. We assessed cardiomyogenesis by QPCR analysis of the genetic markers Nkx2.5 and Tbx5 and found no significant difference in expression levels of these two genes between control and RA cultures (Fig. 5A). We also quantified the number of MyHC+ve cardiomyocytes in both control and RA-treated conditions, and found that these cells accounted for approximately 8% of total cells in each context (Fig. 5E&G). We analyzed expression levels of the smooth muscle markers VEGFR2 and MyHC11 and found that RA treatment did not significantly enhance the expression of these two genes, nor did it result in an increase in the number of alpha-smooth muscle actin (alpha-SMA)+ve smooth muscle cells (SMCs), which accounted for approximately 6% of total cells in both control and RA-treated cultures (Fig. 5D, F&H). We also examined the expression of neurogenic (NeuroD1, Neurogenin1/Ngn1) and osteogenic (Osteopontin/OSP and Osteocalcin/OSC) markers and we did not observe a significant increase in the expression of these genes in RA-treated cultures (Fig. 5B&C). We were able to visualize very few Tuj1+ neurons in our differentiated cultures and thus the extent of terminal neurogenesis was negligible (<1% of total cells, data not shown), despite the presence of neurogenic transcripts. We were unable to detect any mature osteoblasts by Alizarin Red staining (data not shown). The majority of the cells in our cultures appeared to have a mesenchyme-like morphology (Fig. 5I), which is difficult to identify by specific genetic markers [36]. Therefore, the effects of RA were limited to the myogenic lineage and had no significant impact on the differentiation of the other cell types examined here.
Retinoic Acid does not affect the differentiation of non-skeletal muscle lineages. A–D) RNA was harvested from Day 39 hESC cultures and analyzed by QPCR for the markers indicated (n = 5–7; *p < 0.05). Changes in gene expression are expressed relative to undifferentiated cells (Day 0). E&F) Differentiated hESCs were fixed on Day 25 (αSMA, Panel F) or on Day 39 (MyHC, Panel E) and labelled with the antibodies indicated. Hoechst dye was used to detect nuclei. Scale bar represents 10 um. G&H) The number of MyHC+ve cardiomyocytes (G) and the number of alpha-SMA+ve smooth muscle cells (H) were quantified by counting a minimum of 800 nuclei across 20 fields of view for control and RA-treated cultures. I) Differentiated hESCs were visualized on Day 39 and most cells exhibited a mesenchymal-like morphology
Discussion
hESCs represent a promising source of material for cell replacement therapy of muscle diseases. In this study, we have shown that the treatment of differentiating hESCs with RA resulted in the enhancement of skeletal myogenesis and the upregulation of expression of the MRFs MyoD and myogenin (Fig. 1). Furthermore, RA functioned after mesoderm induction by expanding the muscle progenitor population, shown by the upregulation of Meox1 transcript levels and an increase in the proportion of cells expressing Pax3 (Fig. 2). The identification of muscle progenitor cells expressing both Pax3 and Meox1, and not MyoD or myogenin demonstrated that hESCs can be induced to form muscle via an embryonic pathway. The muscle progenitor cells could differentiate into skeletal myoblasts, as shown by the presence of Pax3+ve/MyoD+ve cells on day 25 of differentiation. Thus, this study represents the first clear demonstration of skeletal myogenesis from hESCs that utilizes an embryonic pathway, involving the formation of a Pax3+ve/Meox1+ve skeletal muscle progenitor population generated prior to MyoD and myogenin expression. Finally, we have shown that our method does not significantly affect the differentiation of non-muscle tissue types that are known to be enhanced by RA.
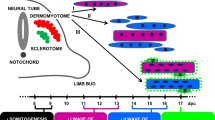
The identification of an embryonic pathway for skeletal myogenesis in hESCs allows for a comparison of the timeline of expression of mesodermal, premyogenic, and myogenic genes during skeletal myogenesis in P19 EC, mESC, and hESCs (Fig. 6). In P19 EC cells, the expression of the mesodermal marker BrachyuryT is initiated on Day 1 of differentiation and maintained until Day 3 while in mESCs it is expressed from Day 2 to Day 4 [11, 37]. This is followed by the expression of the premyogenic mesoderm markers Meox1 and Pax3 on Days 4 and 7 in P19 EC cells and mESCs, respectively [6, 11]. While P19 EC cells begin to express MRF genes on days 7–9 of differentiation and undergo terminal differentiation by day 9, mESCs do not form skeletal myocytes until Day 15 of differentiation, in response to low-serum conditions [6, 11]. Interestingly, hESCs follow a similar, albeit lengthened, pattern of expression during early differentiation (Fig. 6). The mesoderm markers BrachyuryT, Mesp1 and Mesp2 are expressed on Day 3 of differentiation (Figs. 2A and 6), followed by the expression of Pax3 and Meox1 on Day 8 (Figs. 3 and 6) [38]. Finally, MRF expression is initiated at Day 25, in response to low serum conditions. Thus, the three systems utilize a comparable embryonic pathway to achieve skeletal myogenesis and show a similar enhancement by RA of premyogenic mesoderm, leading to augmentation of the myoblast or myocyte populations [6].
A timeline for myogenic differentiation. P19 cells, mESCs and hESCs share common pathways during myogenic differentiation, although the timelines are distinct. Pluripotent cells are specified to the mesodermal lineage and express Brachyury T. These cells are committed to premyogenic progenitor cells expressing Meox1, Pax3 and Pax7 which undergo terminal differentiation into skeletal muscle, expressing the MRFs MyoD and myogenin
The consistency observed between P19 EC cells, mESCs and hESCs supports the validity of these first two systems as models for the study of molecular mechanisms that contribute to tissue-specific differentiation of hESCs. This comparison illustrates the conservation of the pattern of gene expression during differentiation in all of these systems. In addition, it clearly shows that the similarity to embryonic myogenesis can be exploited to identify future methods to enhance myogenesis in ES systems. Finally, the differences in the timing can be explained in part by differences in the embryonic stages represented by each stem cell and are reflective of the different times of gestation between mouse and human [39–41]. Future studies will determine if lowering the serum earlier than day 20 will result in a further reduction in the time course of myogenesis from hESCs.
Interestingly, the enhancement of myogenesis in hESCs by RA did not show a standard dose–response pattern (Fig. 1D). This has been observed previously during osteogenesis from mesenchymal stem cells and myogenesis from mESCs [42, 43]. This property of RA is poorly understood but may represent a negative feedback mechanism that is activated at high RA concentrations. Alternatively, it may indicate the activation of different subsets of differentiation pathways for each concentration of RA, changing the presence of various progenitor populations. This is supported by the morphogenic nature of RA signalling in the embryo. In the developing embryo, RA is present in an anterior-posterior gradient which provides positional information that contributes to cell fate specification [44]. Therefore it is not surprising that specific RA concentrations exert differential effects during stem cell differentiation.
The ability of RA to expand a Pax3+ve muscle progenitor population is particularly important with regard to the use of hESCs for cell therapy, as it may facilitate purification of progenitor cells with the capacity to repopulate the satellite cell niche [7, 8, 45]. It appears that as MRF expression is increased, the ability to replenish the stem cell niche decreases [8]. Previous studies by others have not shown the formation of a Pax3+ve or Pax7+ve population formed prior to expression during skeletal myogenesis in hESCs [1].
We showed that low levels of RA did not greatly affect the development of other cell types examined in this system, including cardiac and smooth muscle, bone and neurons. Although the differentiation of these cell types is known to be enhanced by RA treatment in various systems, it is not surprising that we did not observe an increase, since RA-directed differentiation is heavily dependent on concentration as well as on timing of addition and withdrawal [46]. For example, neurogenic differentiation in hESCs requires RA concentrations in the range of 100nM to 1 μM, while we are treating cultures at 3nM [35]. Enhancement of smooth muscle differentiation by RA requires treatment of monolayer hESCs with a concentration of 10 μM, which is orders of magnitude higher than the levels used here [32]. The low RA concentrations used to induce skeletal myogenesis can also promote cardiomyogenesis in mESCs, however, the timing of treatment required is different [42]. It was shown previously that while skeletal muscle differentiation is enhanced by early exposure of ES cells to RA at the onset of embryoid body formation (Day 0), cardiomyogenesis is inhibited by early exposure to RA and enhanced by later treatment (Day 5–7) [42]. As we were treating our cultures from embryoid body formation at Day 0, we did not expect to see any enhancement. However, we did not observe inhibition of cardiomyogenesis by RA, which may be due to fundamental differences in the timing of origin between mESCs and hESCs [39].
In conclusion, we have devised a method that employs RA to enhance the formation of myogenic progenitor cells, and consequently, of skeletal muscle from hESCs without genetic or covalent chemical modification. This method has little effect on other lineages known to be enhanced by RA. Furthermore, the time required is shorter than that previously reported and the ability to distinguish embryonic stages is unique. We propose that RA can promote skeletal myogenesis for the purposes of cell therapy.
Abbreviations
- (hESCs):
-
human embryonic stem cells
- (mESCs):
-
mouse embryonic stem cells
- (EC) cells:
-
P19 embryonal carcinoma
- (RA):
-
retinoic acid
- (iPS) cells:
-
induced pluripotent stem
- (MyHC):
-
myosin heavy hhain
References
Barberi, T., Bradbury, M., Dincer, Z., Panagiotakos, G., Socci, N. D., & Studer, L. (2007). Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nature Medicine, 13, 642–648.
Barberi, T., Willis, L. M., Socci, N. D., & Studer, L. (2005). Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Medicine, 2, e161.
Zheng, J. K., Wang, Y., Karandikar, A., et al. (2006). Skeletal myogenesis by human embryonic stem cells. Cell Research, 16, 713–722.
Mizuno, Y., Chang, H., Umeda, K., et al. (2010). Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB Journal, 24, 2245–53.
Petropoulos, H., & Skerjanc, I. S. (2002). Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. Journal of Biological Chemistry, 277, 15393–15399.
Kennedy, K. A., Porter, T., Mehta, V., et al. (2009). Retinoic acid enhances skeletal muscle progenitor formation and bypasses inhibition by bone morphogenetic protein 4 but not dominant negative beta-catenin. BMC Biology, 7, 67.
Montarras, D., Morgan, J., Collins, C., et al. (2005). Direct isolation of satellite cells for skeletal muscle regeneration. Science, 309, 2064–2067.
Kuang, S., Kuroda, K., Le Grand, F., & Rudnicki, M. A. (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell, 129, 999–1010.
Relaix, F., Rocancourt, D., Mansouri, A., & Buckingham, M. (2005). A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature, 435, 948–953.
Mankoo, B. S., Skuntz, S., Harrigan, I., et al. (2003). The concerted action of Meox homeobox genes is required upstream of genetic pathways essential for the formation, patterning and differentiation of somites. Development, 130, 4655–4664.
Petropoulos, H., Gianakopoulos, P. J., Ridgeway, A. G., & Skerjanc, I. S. (2004). Disruption of Meox or Gli activity ablates skeletal myogenesis in P19 cells. Journal of Biological Chemistry, 279, 23874–23881.
Ridgeway, A. G., & Skerjanc, I. S. (2001). Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. Journal of Biological Chemistry, 276, 19033–19039.
Bober, E., Franz, T., Arnold, H. H., Gruss, P., & Tremblay, P. (1994). Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development, 120, 603–612.
Tremblay, P., Dietrich, S., Mericskay, M., Schubert, F. R., Li, Z., & Paulin, D. (1998). A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Developmental Biology, 203, 49–61.
Li, J., Liu, K. C., Jin, F., Lu, M. M., & Epstein, J. A. (1999). Transgenic rescue of congenital heart disease and spina bifida in Splotch mice. Development, 126, 2495–2503.
Jostes, B., Walther, C., & Gruss, P. (1990). The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mechanisms of Development, 33, 27–37.
Mansouri, A., Stoykova, A., Torres, M., & Gruss, P. (1996). Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development, 122, 831–838.
Relaix, F., Montarras, D., Zaffran, S., et al. (2006). Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. The Journal of Cell Biology, 172, 91–102.
Maroto, M., Reshef, R., Munsterberg, A. E., Koester, S., Goulding, M., & Lassar, A. B. (1997). Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell, 89, 139–148.
Darabi, R., Gehlbach, K., Bachoo, R. M., et al. (2008). Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nature Medicine, 14, 134–143.
Bajard, L., Relaix, F., Lagha, M., Rocancourt, D., Daubas, P., & Buckingham, M. E. (2006). A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes & Development, 20, 2450–2464.
Mankoo, B. S., Collins, N. S., Ashby, P., et al. (1999). Mox2 is a component of the genetic hierarchy controlling limb muscle development. Nature, 400, 69–73.
Williams, B. A., & Ordahl, C. P. (1994). Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development, 120, 785–796.
Reijntjes, S., Stricker, S., & Mankoo, B. S. (2007). A comparative analysis of Meox1 and Meox2 in the developing somites and limbs of the chick embryo. International Journal of Developmental Biology, 51, 753–759.
Candia, A. F., Hu, J., Crosby, J., et al. (1992). Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development, 116, 1123–1136.
Ross, S. A., McCaffery, P. J., Drager, U. C., & De Luca, L. M. (2000). Retinoids in embryonal development. Physiological Reviews, 80, 1021–1054.
Soprano, D. R., Teets, B. W., & Soprano, K. J. (2007). Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitamins and Hormones, 75, 69–95.
Marletaz, F., Holland, L. Z., Laudet, V., & Schubert, M. (2006). Retinoic acid signaling and the evolution of chordates. International Journal of Biological Sciences, 2, 38–47.
Schnerch, A., Cerdan, C., & Bhatia, M. (2010). Distinguishing between mouse and human pluripotent stem cell regulation: the best laid plans of mice and men. Stem Cells, 28, 419–30.
Xu, C., Inokuma, M. S., Denham, J., et al. (2001). Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnology, 19, 971–974.
Savage, J., Conley, A. J., Blais, A., & Skerjanc, I. S. (2009). SOX15 and SOX7 differentially regulate the myogenic program in P19 cells. Stem Cells, 27, 1231–1243.
Huang, H., Zhao, X., Chen, L., et al. (2006). Differentiation of human embryonic stem cells into smooth muscle cells in adherent monolayer culture. Biochemical and Biophysical Research Communications, 351, 321–327.
Yamashita, A., Takada, T., Narita, J., Yamamoto, G., & Torii, R. (2005). Osteoblastic differentiation of monkey embryonic stem cells in vitro. Cloning and Stem Cells, 7, 232–237.
Niebruegge, S., Nehring, A., Bar, H., Schroeder, M., Zweigerdt, R., & Lehmann, J. (2008). Cardiomyocyte production in mass suspension culture: embryonic stem cells as a source for great amounts of functional cardiomyocytes. Tissue Engineering. Part A, 14, 1591–1601.
Schuldiner, M., Eiges, R., Eden, A., et al. (2001). Induced neuronal differentiation of human embryonic stem cells. Brain Research, 913, 201–205.
Kalluri, R., & Zeisberg, M. (2006). Fibroblasts in cancer. Nature Reviews. Cancer, 6, 392–401.
Willey, S., Ayuso-Sacido, A., Zhang, H., et al. (2006). Acceleration of mesoderm development and expansion of hematopoietic progenitors in differentiating ES cells by the mouse Mix-like homeodomain transcription factor. Blood, 107, 3122–3130.
Sumi, T., Tsuneyoshi, N., Nakatsuji, N., & Suemori, H. (2008). Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development, 135, 2969–2979.
Rossant, J. (2008). Stem cells and early lineage development. Cell, 132, 527–531.
Brons, I. G., Smithers, L. E., Trotter, M. W., et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature, 448, 191–195.
Tesar, P. J., Chenoweth, J. G., Brook, F. A., et al. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature, 448, 196–199.
Wobus, A. M., Rohwedel, J., Maltsev, V., & Hescheler, J. (1994). In vitro differentiation of embryonic stem cells into cardiomyocytes or skeletal muscle cells is specifically modulated by retinoic acid. Roux’s Archives of Developmental Biology, 204, 36–45.
Zhang, W., Deng, Z. L., Chen, L., et al. (2010). Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One, 5, e11917.
White, R. J., Nie, Q., Lander, A. D., & Schilling, T. F. (2007). Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biology, 5, e304.
Darabi, R., Santos, F. N., Filareto, A., et al. (2011). Assessment of the myogenic stem cell compartment following transplantation of pax3/pax7-induced embryonic stem cell-derived progenitors. Stem Cells, 29, 777–790.
Rohwedel, J., Guan, K., & Wobus, A. M. (1999). Induction of cellular differentiation by retinoic acid in vitro. Cells, Tissues, Organs, 165, 190–202.
Acknowledgements
We thank Anastassia Voronova for helpful comments and reading the manuscript. T.R. was supported by an OGSST award. This work was supported by a grant to I.S.S. from the Muscular Dystrophy Association (113716).
Conflicts of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Support: This work was supported by funding from the United States Muscular Dystrophy Association (113716)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1
QPCR Primer sequences (DOC 43 kb)
Rights and permissions
About this article
Cite this article
Ryan, T., Liu, J., Chu, A. et al. Retinoic Acid Enhances Skeletal Myogenesis in Human Embryonic Stem Cells by Expanding the Premyogenic Progenitor Population. Stem Cell Rev and Rep 8, 482–493 (2012). https://doi.org/10.1007/s12015-011-9284-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9284-0