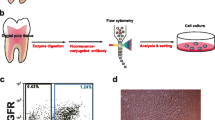
Abstract
Dental pulp stem cells (DPSCs), originating from neural crests, can be found within dental pulp. Up to now, it has been demonstrated that these cells are capable of producing bone tissue, both in vitro and in vivo and differentiate into adipocytes, endotheliocytes, melanocytes, neurons, glial cells, and can be easily cryopreserved and stored. Moreover, recent attention has been focused on tissue engineering and on the properties of these cells. In addition, adult bone tissue with good vascularisation has been obtained in grafts. The latest use in clinical trials for bone repair enforces the notion that DPSCs can be used successfully in patients. Therefore, their isolation, selection, differentiation and banking is of great importance. The isolation and detection techniques used in most laboratories are based on the use of antibodies revealed by flow-cytometers with cell sorter termed FACS (fluorescent activated cell sorter). In this report, we focus our attention on the main procedures used in the selection of DPSCs by flow cytometry, cell culture, freezing/thawing, cell cycle evaluation, histochemistry/immunofluorescence and differentiation of DPSCs. In addition, new methods/protocols to select and isolate stem cells without staining by fluorescent markers for implementation in biomedical/clinical laboratories are discuss. We emphasize that the new methods must address simplicity and short times of preparation and use of samples, complete sterility of cells, the potential disposable, low cost and complete maintenance of the viability and integrity of the cells with real-time response for subsequent applications in the biomedical/clinical/surgical fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cells are cells delegated to maintain the structural and functional integrity of tissues, through the replacement of mature damage cells. Multipotent adult stem cells, as mesenchymal stem cells (MSC), are present in all mature tissues of the human body and are thought to reside in a specific area of each tissue where they may remain quiescent (non-dividing) for many years until they are activated by disease or tissue injury. A stem cell is self-renewable and capable of differentiating into at least two distinctive cell types [1]. These two properties must both be satisfied for a cell to be defined as a stem cell. Self-renewal denotes that undifferentiated daughter cells are a precise replica and can further replicate many generations without losing their original characteristics [2, 3]. Cells of an immortalized cell line can replicate many generations, but are generally incapable of multi-lineage differentiation. Thus, cell lines are not stem cells. Multi-lineage differentiation refers to the capacity of a single population of stem cells to differentiate into at least two distinctively different cell types. For example, a single population of MSCs can differentiate into both osteoblasts and chondrocytes. Pre-osteoblasts can differentiate into osteoblasts, but are incapable of differentiating into other mesenchymal lineages, such as chondrocytes or adipocytes, at least not without undergoing de-differentiation back toward MSCs. In the adult, MSCs maintain physiologically necessary tissue turnover and, upon injury or disease, differentiate to launch tissue regeneration. MSCs have been experimentally differentiated into all mesenchymal or connective tissue lineages [2, 4] and, in many cases, have been used to tissue engineering. Regeneration of tissue structures from stem cells is an insurmountable effort until advances from several seemingly unrelated disciplines—such as cell and molecular biology, polymer chemistry, molecular genetics, materials science, and mechanical engineering—converged into the self-assembling field of tissue engineering [5, 6]. To engineer a functional biological structure, cells must be instructed to differentiate and receive positional cues, and to synthesize the appropriate extracellular matrix molecules in the overall shape and dimensions of the diseased or missing tissues/organs. Biomimetic scaffolds are frequently needed to enable cell growth and differentiation to occur in an environment that has been previously unfamiliar to either biologists or engineers. Large-scale tissue engineering research began to take place in the early 1990s and has grown exponentially ever since. Numerous niches of stem cells within the human body have been studied up to now and include as main sources for tissue repair/regeneration: Dental stem cells, adipose tissue, mesenchymal and connective (MSCs), Muscle Stem Cells (MyoSC), Cartilage stem cells, Bone-marrow MSCs forming bone. For example, dental pulp stem cells (DPSCs) have been engineered for bone tissue building and repair. In fact, these cells easily differentiate and proliferate into osteoblasts producing, already in vitro a woven bone which then, after transplantation into immunosuppressed rats give rise to a complete, vascularised lamellar bone [7–9]. Those findings are of high interest in bone tissue repair due to their origin, their differentiative abilities, proliferation rate and log lifespan [10]. In a broader overview, the adult stem cells are an excellent resource in a tissue engineering and regenerative medicine as they can be used as an effective therapeutic strategy in demolition pathologies.
Embryonic Origin of Dental Pulp
Embryonic cells migrate from the neural crests to reinforce head and neck mesenchyme strongly determining the development of this area of the human body. During the sixth week of embryogenesis, ectoderm covering the stomodeum begins to proliferate, giving rise to the dental laminae. Reciprocal interactions between ectoderm and mesoderm layers lead to placode formation. One of these thick, ovoid ectodermal structures develops into tooth germs, where cells, belonging to the neural crest, will differentiate into the dental germ, containing both dental papilla and follicle. Therefore, dental pulp is made of ecto-mesenchymal components, containing neural crest-derived cells, which display plasticity and multipotential capabilities [11].
Pulp is externally separated from dentin by odontoblasts and by Höhl’s subodontoblastic cells, that are pre-odontoblasts [12]. Adjacent to this layer the pulp is rich in collagen fibres and poor in cells. Then, another, more internal layer, contains progenitor cells and undifferentiated cells, some of which are considered stem cells [13]. From this layer, undifferentiated cells migrate to various districts where they can differentiate under different stimuli and make new differentiated cells and tissues. The final, inner-most layer is the core of the pulp; this area is comprised of the vascular plexus and nerves. Up to the more recent discoveries [9, 14], researchers hypothesized that DPSCs were present in this layer [15]. Actually, only undifferentiated perivascular cells can be found in it.
The third molar tooth germ begins development around the sixth year of life. Until this time, embryonic tissues of dental lamina remain quiescent and undifferentiated within the jaw of the child. Although crown mineralization begins during the eighth year of life, often third molar roots are still incomplete at the age of 18. This means that the structure of those teeth is still immature at this age and a conspicuous pool of undifferentiated cells, resident within the “cell rich zone” of the dental germ pulp, are needed for development.
Techniques for Dental Pulp Stem Cell Isolation
The stem population, isolated and cultured in vitro, includes a mosaicism of different cell types with different stages of differentiation. Numerous laboratories use the same techniques of detection and isolation of stem cells (SC): tissue surgical removal under sterile conditions, digestion in collagenase/dispase, detection and selection by selective markers. For example, SC isolated from dental pulp express CD34 antigen and they are mesenchymal stem cells [7].
The SC isolation technique used in most laboratories is based on the use of flow-cytometers with cell sorter termed FACS (fluorescent activated cell sorter). This methodology offers many advantages: high purity; good separation of the populations using multiple parameters on the basis of fluorescent intensity; limited cell death; separation also on the basis of intracellular staining (for examples DNA, expression of cytokines, GFP); from two to six populations sorted simultaneously; large number of cells examined. This technique, however, presents some important limitations: 1. the separation can generate suffering stem cells due to electric charge applied to the cell; 2. semi-sterility of sample; 3. high cost of cytometer maintenance; 4. high cost of reagents, such as fluorescent antibodies and, finally, 5. long time in the preparation protocols and selection of stem cells. Moreover, the sorters are mainly and only used for high end cellular research. Therefore, new methods/protocols should be proposed to select and isolate stem cells without staining by fluorescent markers or use of magnetic beads. These new procedures should be based on biophysical differences among the different cell populations in order to obtain interesting peculiarities for implementation in biomedical/clinical laboratories, such as simplicity and short times of preparation and use of samples, complete sterility of stem cells, the potential disposable, low cost and complete maintenance of the vitality and integrity of the cells with real-time response for subsequent applications in the biomedical/clinical/surgical fields.
Materials
Listing of all buffers, reagents solutions and equipment needed for carrying out the protocols:
Reagents and Solutions for Cell Cultures, Immunofluorescence, Histochemistry and Electronic Microscopy
-
1.
Chlorexidin gel (Forhans, N.Y., USA), stored at room temperature until exposure date.
-
2.
I Type Collagenase (GIBCO, Invitrogen, San Giuliano Milanese, Milan, Italy), stored at 4°C until exposure date.
-
3.
Dispase (GIBCO, Invitrogen, San Giuliano Milanese, Milan, Italy), stored at 4°C until exposure date.
-
4.
Gentamicin solution (Sigma, Milan, Italy), stored at room temperature until exposure date.
-
5.
PBS w/o Ca++, Mg++, (Lonza, Milan, Italy), stored at 4°C until exposure date.
-
6.
70 μm Falcon strainers (Becton & Dickinson, Buccinasco, Milan, Italy), stored at room temperature.
-
7.
MegaCell culture medium (Sigma, Milan, Italy), stored at 4°C until exposure date.
-
8.
Fetal Bovine Serum,South American Origin, (GIBCO, Invitrogen, San Giuliano Milanese, Milan, Italy), stored at −20°C until exposure date.
-
9.
L-Ascorbic acid (Sigma, Milan, Italy), stored at room temperature until exposure date.
-
10.
L-glutamine (Lonza, Milan, Italy), stored at −20°C until exposure date.
-
11.
Penicillin and streptomycin, Pen/Strep, (Lonza, Milan, Italy), stored at −20°C until exposure date.
-
12.
α-MEM culture medium (Invitrogen, San Giuliano Milanese, Milan, Italy), stored at 4°C until exposure date.
-
13.
EDTA (Sigma, Milan, Italy), stored at room temperature until exposure date.
-
14.
Bovine Serum Albumin (BSA) fraction V (Sigma, Milan, Italy), stored at room temperature until exposure date.
-
15.
Toluidine blue (Sigma, Milan, Italy), stored at room temperature.
-
16.
Paraformaldehyde (Sigma, Milan, Italy), stored at room temperature.
-
17.
TGF-β1 (Abcam, Cambridge, UK), stored at −20°C until exposure date.
-
18.
Trypsin-EDTA solution (Lonza, Milan, Italy), stored at 4°C until exposure date.
-
19.
Neurobasal A medium (1X) (Invitrogen, San Giuliano Milanese, Milan, Italy), stored at 4°C until exposure date and to use within 1 month after its opening.
-
20.
B27 (Invitrogen, San Giuliano Milanese, Milan, Italy), stored at 4°C until exposure date.
-
21.
Basic Fibroblast Growth Factor, bFGF, (Sigma, Milan, Italy), stored at −20°C until exposure date.
-
22.
Epidermal Growth Factor, EGF, (Sigma, Milan, Italy), stored at −20°C until exposure date.
-
23.
Dexamethasone (Sigma, Milan, Italy), stored at +4°C until exposure date.
-
24.
Recombinant human insulin (Sigma, Milan, Italy), stored at +4°C until exposure date.
-
25.
Indomethacin (Sigma, Milan, Italy), stored at room temperature until exposure date.
-
26.
3-isobutyl-1-methyl-xantina, IBMX, (Sigma, Milan, Italy), stored at −20°C until exposure date.
-
27.
Oil red-O (Sigma, Milan, Italy), stored at room temperature until exposure date.
-
28.
BMPurple solution (Roche, Segrate, Milan, Italy), stored at room temperature until exposure date.
-
29.
Calcein-AM (Dojindo Molecular Technologies, Gaithersburg, MD, USA), stored at −20°C until exposure date.
-
30.
Gluteraldehyde (EM grade) (Sigma, Milan, Italy), stored at room temperature, until exposure date.
-
31.
Alkaline phosphatase, ALP, (Sigma, Milan, Italy), stored at room temperature, until exposure date.
-
32.
Dimethyl sulfoxide, DMSO, (Sigma, Milan, Italy), stored at room temperature, until exposure date.
-
33.
Osmium tetroxide, OsO4, (Sigma, Milan, Italy), stored at room temperature.
-
34.
Triton X100 (Sigma, Milan, Italy), stored at room temperature.
-
35.
Fix&Perm Kit (Invitrogen, San Giuliano Milanese, Milan, Italy), stored at room temperature, until exposure date.
-
36.
Epoxy Resins Epon 812 (Sigma, Milan, Italy), stored at room temperature.
Antibodies
-
1.
CD31 (PECAM-1) (Becton & Dickinson, Buccinasco, Milan, Italy)
-
2.
CD34 PE (Milteniy Biotec, Calderara di Reno, Bologna, Italy)
-
3.
CD44 (MBL, Woburn, MA, USA)
-
4.
CD45 CY (Becton & Dickinson, Buccinasco, Milan, Italy)
-
5.
CD54, (MBL, Woburn, MA, USA)
-
6.
CD90 FITC (Becton & Dickinson, Buccinasco, Milan, Italy)
-
7.
CD117 FITC (c-kit) (Santa Cruz, CA, USA),
-
8.
CD133 PE (Milteniy Biotec, Calderara di Reno, Bologna, Italy)
-
9.
Flk-1 (Santa Cruz, CA, USA)
-
10.
STRO-1 (Dr. Torok-Storb through DBA, Segrate, Milan, Italy)
-
11.
Osteocalcin (Santa Cruz, CA, USA)
-
12.
RUNX-2 (Santa Cruz, CA, USA)
-
13.
Von-Willebrand (domain 1 and 2) (Santa Cruz, CA, USA)
-
14.
Angiotensin-converting-enzyme, ACE, (Santa Cruz, CA, USA)
All antibodies are stored at 4°C in the dark until exposure date as indicated from manufacturer.
Reagents for Cell Cycle Analyses
-
1.
Iodide propide solution (Sigma, Milan, Italy), stored at 4°C in the dark until exposure date.
-
2.
Sodium citrate (Sigma, Milan, Italy), stored at room temperature until exposure.
-
3.
RNAse A (Sigma, Milan, Italy), stored at −20°C in the dark until exposure date.
Equipments
-
1.
BD FACSAria II Near UV Upgrade kit for 3 laser system (Becton Dickinson, Franklin Lakes, NJ, USA).
-
2.
Electron Microscope (Philips 400S, Eindhoven, The Netherlands).
-
3.
Fluorescence Microscope (Nikon TE 2000-S, Milan, Italy).
Methods
The procedures that are preferably and mainly used in the laboratories in order to detect, isolate, proliferate and differentiate DPSCs include the following steps.
Dental Pulp Extraction and Digestion
-
1.
Human dental pulp are extracted from teeth of healthy adults. Prior to extraction, each subject was checked for systemic and oral infection or diseases. Only disease-free subjects must be selected. Each subject, which is usually a patient that must undergo a third molar extraction, follows a pre-treatment week with professional dental hygiene. Before extraction, the dental crown is covered with 0.3% chlorexidin gel (Forhans, N.Y., USA) for 2 min and then pulp is extracted with a dentinal excavator or a Gracey curette.
-
2.
Once removed, the pulp is immersed in a digestive solution (3 mg/ml I type collagenase (GIBCO, Invitrogen, San Giuliano Milanese, Milan, Italy) plus 4 mg/ml dispase (GIBCO, Invitrogen, San Giuliano Milanese, Milan, Italy) and 5 μl/ml gentamicin (40 μg/ml) (Sigma, Milan, Italy) for 1 h at 37°C.
-
3.
The solution was then filtered with 70 μm Falcon strainers (Becton & Dickinson, Buccinasco, Milan, Italy) to remove the tissue pieces not digested.
Cell Culture
-
1.
After filtration, cells are immersed in MegaCell culture medium (Sigma, Milan, Italy) supplemented with 10% FBS (GIBCO, Invitrogen, San Giuliano Milanese, Milan, Italy), 100 μM 2P-ascorbic acid (Sigma, Milan, Italy), 2 mM L-glutamine (Lonza, Milan, Italy), 100 U/ml penicillin (Lonza, Milan, Italy), 100 μg/ml streptomycin (Lonza, Milan, Italy) and placed in 75 cm2 flasks with filtered valves.
-
2.
Flasks were incubated at 37°C and 5% CO2 and the medium changed twice a week. Just before cells become confluent, they can be subdivided into new flasks. The number of passages must be reduced to the minimum and performed avoiding cell stress and senescence. This because the interactions between cells are of paramount importance to obtain the deposition of the extracellular matrix and its mineralization.
-
3.
Stem cells are sorted (see below) only when their number reaches at least 1,000,000 per flask. This number is achieved around day 22, when they are still undifferentiated.
-
4.
Differentiated cells are obtained from sorted stem cells cultured for at least 30 days in α-MEM culture medium with 20% FBS (all purchased from Invitrogen, San Giuliano Milanese, Milan, Italy); in fact, FBS exerts a differentiation activity favouring osteoblastic differentiation when used in high percentage [7, 17].
Fluorescence Activated Cell Analysis and Sorting
-
1.
Cells are detached using 0.02% EDTA solution in PBS and collected (10 min at 1,000 rpm)
-
2.
Cells are washed in 0.1% BSA in PBS, then
-
3.
incubated in a solution of antibody (1 μg/μl).
-
4.
After incubation, cells are then washed in the same solution (see above) and are ready for observation. Antibodies used for sorting are the following mouse anti-human antibodies: CD117 FITC (c-kit) (Santa Cruz, CA, USA), CD34 PE (Milteniy Biotec, Calderara di Reno, Bologna, Italy), flk-1 (Santa Cruz, CA, USA) and STRO-1 (Dr. Torok-Storb through DBA, Segrate, Milan, Italy). We sorted using both morphological (high side scatter and low forward scatter) and antigenic criteria (first with CD117 and CD34 and then, serially for STRO-1 and flk-1).
-
5.
Only cells that expressed all these markers are selected, in order to obtain a homogeneous population.
-
6.
After sorting, with the same procedures, the cells can be analyzed with CD45 CY (Becton & Dickinson, Buccinasco, Milan, Italy), CD90 FITC (Becton Dickinson) and anti-CD133 PE (Miltenyi Biotec).
-
7.
After differentiation, cells are examined for the following antibodies, other than the previous antibodies for stem cell markers, in order to assess osteogenic differentiation: CD54, CD44 (MBL, Woburn, MA, USA), Osteocalcin (Santa Cruz, CA, USA) and the transcription factor RUNX-2 (Santa Cruz, CA, USA). For RUNX-2 intracellular analysis, Fix&Perm Kit (Invitrogen, San Giuliano Milanese, Milan, Italy) was used according to guidelines.
-
8.
To asses endothelial differentiation the following antibodies are used: CD31 (PECAM-1) (Santa Cruz, CA, USA), von-Willebrand (domain 1 and 2) (Santa Cruz, CA, USA); Angiotensin-converting-enzyme (ACE) (Santa Cruz, CA, USA), and flk-1 (Santa Cruz, CA, USA).
Colony Efficiency Assays and Proliferation Potential
-
1.
To evaluate colony efficiency and proliferation potential of sorted stem cells, single cells obtained by limiting dilutions are plated. Numbers of clones and cells are evaluated.
-
2.
After 3 weeks of culture, cells are stained with 0.1% (v/v) toluidine blue (Sigma, Milan, Italy) in 1% paraformaldehyde (Sigma, Milan, Italy).
-
3.
The number of clones (>50 cells) are counted.
Differentiation
Sorted cells can be challenged to assess their multipotency.
-
1.
For smooth muscle differentiation, cells are cultured in the presence of 2% FBS and 10 ng/ml TGF-β (Abcam, Cambridge, UK) for 4–6 days.
-
2.
For neural differentiation, cells are cultured at high densities, spontaneously forming spherical clumps of cells that were isolated with 0.25% trypsin solution (Lonza, Milan, Italy). Free-floating neurospheres that are released from the cell culture surface into the culture media were also collected.
-
3.
The spheres of cells are transferred to a Petri dish and cultured in Neurobasal medium (Invitrogen, San Giuliano Milanese, Milan, Italy) supplemented with B27 (Invitrogen, San Giuliano Milanese, Milan, Italy), 20 ng/ml bFGF (Sigma, Milan, Italy), and 20 ng/ml EGF (Sigma, Milan, Italy) for 4–7 days.
-
4.
The culture density of the spheroid bodies is maintained at 10–20 cells/cm2 to prevent self aggregation.
-
5.
For adipocyte differentiation, the culture medium must be supplemented with 10% FBS and 1 μM dexamethasone, 10 μM recombinant human insulin, 200 μM indomethacin and 0,5 mM 3-isobutyl-1-methyl-xantina (IBMX) (all purchased from Sigma, Milan, Italy) twice a week for 2 weeks.
-
6.
Oil red-O (Sigma, Milan, Italy) staining is used to identify lipid-laden fat cells.
Cell Cycle Analysis
Cell cycle is analyzed by flow cytometry.
-
1.
Cells are harvested in phosphate-buffered saline (PBS) containing 2 mM EDTA (Sigma, Milan, Italy),
-
2.
They are washed once with PBS and
-
3.
stained with 50 ug/ml iodide propide (Sigma, Milan, Italy) in sodium citrate (Sigma, Milan, Italy) buffer 0,1% plus 1 mg/ml RNAse A (Sigma, Milan, Italy) for 2 h at room temperature in the dark.
-
4.
Stained nuclei are analyzed with a fluorescence-activated cell sorter (FACS) ARIAII (Becton-Dickinson) and the data analyzed using a Mod-Fit cell cycle analysis program (Becton-Dickinson).
-
5.
Analyses are performed in four’s for each sample and time from day 15 up to the 45th day of culture.
Histochemistry and Immunofluorescence Analyses
Alkaline Phosphatase (ALP) Staining
-
1.
Cells and tissue samples are washed in PBS.
-
2.
Fixed in 4% paraformaldehyde in PBS, with 0.2% Triton X100 (Sigma, Milan, Italy) for 30 min at 4°C.
-
3.
Washed twice in 0.1% BSA in PBS at room temperature for 10 min each.
-
4.
Analyses are performed by light microscope.
Alkaline Phosphatase (ALP) Activity Assay
-
1.
ALP activity is measured using 100,000 cells/sample, detached with PBS/EDTA 0.02% and centrifuged for 10 min at 140 g.
-
2.
The pellet is incubated with 1 ml of BMPurple solution (Roche, Segrate, Milan, Italy) for 8 h in the dark.
-
3.
Supernatant is read in a spectrophotometer at 615 nm.
-
4.
As control, c-kit−/STRO-1−/CD34− cells were used.
-
5.
The values are expressed as the ratio of sample and BMPurple stock solution. BMPurple solvent was used as blank.
Calcein Staining
-
1.
A solution of 50 μM calcein-AM in PBS (Dojindo Molecular Technologies, Gaithersburg, MD, USA), is added to culture medium (1/10 v/v) for 30 min, at 37°C with 5% CO2.
-
2.
After incubation, cells are washed twice with PBS.
-
3.
The observation is performed using a fluorescence microscope (Nikon TE 2000-S, Milan, Italy) with 490 nm excitation and 512 nm emission filters.
Electronic Microscope
-
1.
Cells and chips of the new-formed woven bone were fixed in 2.5% gluteraldehyde (EM grade) (Sigma, Milan, Italy) in a phosphate buffer,
-
2.
post-fixed in 0.1% OsO4 (Sigma, Milan, Italy) in the same buffered solution for 1 h,
-
3.
then dehydrated and embedded in epoxy resins (Sigma, Milan, Italy).
-
4.
Counterstained (uranyl acetate and lead citrate) ultrathin sections were observed under an electron microscope (Philips 400S, Eindhoven, The Netherlands).
Cryopreservation
If a long-term storage is needed during the study, it is possible to safety cryopreserve both stem and differentiated pulp cells using the following method.
-
1.
Cells (stocks of 250,000 for stem cells and of 500,000 for differentiated cells), are frozen in 10% dimethyl sulfoxide (DMSO) (Sigma, Milan, Italy) in MegaCell at 10% FBS in a cryotube (Lonza, Milan, Italy).
-
2.
They are immersed in liquid nitrogen.
-
3.
Storage can be prolonged for up to 2 years.
-
4.
At the end of the freezing period, cells can be quickly thawed by the addition of 1 ml of medium at 10% FBS at 37°C and
-
5.
then added to 10 ml of the same medium.
-
6.
Cells are pelleted at 1,500 rpm for 10 min.
-
7.
The supernatant removed .
-
8.
Fresh medium is added to the tube.
-
9.
Cells are then placed in flasks and cultured at 37°C in a 5% CO2 atmosphere. They are vital and the amount of apoptotic cells is less than 10%.
Note
Cryopreservation of DPSCs
Cryopreservation of cells and tissue, mainly of the reproductive system, has been significantly improved recently, but to date prevailingly haematopoietic stem cells have been cryopreserved and then successfully utilized for transplantation. Moreover, to date there are no reports on the ability of either stem cells or already differentiated cells to re-start proliferation, differentiation, and new tissue formation for therapeutic use .
After long-term cryopreservation (2 years), osteoblasts differentiated from SBP-DPSCs, are still capable of quickly re-starting proliferation and the production of mineralized matrix, in a manner similar to what we have already demonstrated for fresh cells [7, 8, 16]. The differences in percentages regarding STRO-1 and flk-1 with respect to the percentages observed for the other stem cell antigens are due to the fact that we performed multi-parametric cell sorting using both morphological and antigenic criteria, and sorted first for CD117 and CD34 together and then sequentially for STRO-1 and flk-1 antigens, before and after cryopreservation [8]. Thus, pre-endothelial cells, such as pericytes positive for both CD117 and CD34, could have altered the overall percentages. These cells would be responsible for the differentiation of endothelium which occurs in parallel with osteoblast differentiation, as demonstrated in the embryo during the ossification process. Moreover, both osteoblasts and endotheliocytes express the VEGF-2 receptor (flk-1).
Furthermore, after thawing, no apoptotic death was observed, and cells retained their differentiation multipotency, all which is of interest when assessing the suitability of stem cells for use after cryopreservation. Moreover, osteoblasts produced a large-scale woven bone, which was observed in at least 100 25 cm2 flasks. Samples of this bone, when transplanted into immunosuppressed rats, were remodelled into lamellar bone, further demonstrating their vitality.
Ultrastructurally, osteoblasts were cuboidal in shape, forming a layer along the border of the extracellular matrix, as observed in vivo during osteogenesis. These differentiated cells contained an extremely diffuse RER as well as matrix membrane vesicles, containing crystal-like structures. These ultrastructural observations confirmed that cells were unaltered [8].
Oppositely, no stem cells can be obtained from whole pulp cryopreservation. Several experiments and efforts were carried out in order to overtake this problem, but without positive results. In fact, when the whole pulp is cryopreserved stem cells are lost in all cases. Therefore, this technique cannot be followed.
New Required Procedures
The stem population, isolated and cultured in vitro, includes a mosaicism of different cell types with different stages of differentiation. Numerous laboratories use the same techniques of detection and isolation of stem cells: tissue surgical removal under sterile conditions, digestion in collagenase/dispase, detection and selection by selective markers. For example, stem cells isolated from dental pulp express CD34 antigen and they are mesenchymal stem cells.
Techniques for cell harvest, culture, expansion and enrichment of stem cells are standardised. Many laboratories use the same techniques based on tissue surgical removal under sterile conditions, transport to a laboratory in a physiological solution, digestion in collagenase/dispase, centrifugation and, then, use of selective markers (depending on cells and origin) using fluorescence activated cell sorters (FACS) or magnetic beads. The traditional techniques of selection and enrichment of stem cells are based essentially on the use of flow-cytometry with cell sorter [17–19]. Flow cytometry is a technology that simultaneously measures and then analyzes multiple physical characteristics of single cells, which flow in a fluid stream through a beam of light. The cell characteristic analysed include cell’s relative size, granularity or internal complexity, and relative fluorescence intensity [20]. In recent years, the flow-cytometry has achieved a remarkable expansion, both in laboratories and in clinical research laboratories [21–23]. The references to “flow cytometry” in the Medline data base were zero in 1970, 113 in 1980, 2286 in 1990, and 4893 in the year 2000. Factors that have contributed significantly to this development are: the possibility of using different laser emissions, which can be used for multiparametric analyses; availability of MoAb conjugated with a wide range of fluorochromes and direct against a wide variety of membrane/intracellular markers; quantifying several parameters for each individual cell; detection and isolation of cells by specific marker expression due to evolution of flow cytometers equipped with sorting. Up to now, cell sorting is crucial in the isolation of stem cells starting from heterogeneous cell populations. In a sorting cytometer, cells flow through the analysis point where they are illuminated and their scatter and fluorescence signals detected as in a nonsorting instrument. They then continue to flow downstream where, as the stream breaks up into drops, they become enclosed in individual drops. The flow operator will have drawn sort regions around cells “of interest” according to their flow parameters. In the case of denatl pulp stem cells, specific stem markers is CD34 antigen [7, 9]. If a cell in the analysis point has been determined to be a cell of interest according to the sort regions, the drop containing that cell will be charged positively or negatively so that it will be deflected either to the left or right as it passes the positive and negative deflection plates. Modern cytometers have the ability to charge drops in four ways (strongly or weakly positive and strongly or weakly negative), so that four sort regions can be designated and four subpopulations of cells can be isolated from the original population. Once the stem cell has been isolated from the original population by sorting, it is put in culture to test its differentiation potential, clonogenic and proliferative capacity and self-renewal.
On the basis of these considerations, it is important to develop new protocols to identify and isolate stem cells from tissues. An example could be that to realize biosensors, using acoustic resonators, such as thickness-shear mode (TSM) resonator, as transducer [24, 25]. In particular, the quartz crystal microbalance (QCM) immunosensor is a popular TSM-based transducer among immunosensing methods [26–28]. Because of its simplicity, convenience, low cost, and real-time response, this method can be important for detection of biomolecules in the field of cell biology, for the detection and direct differentiation of stem cells to specific cell types.
Conclusions
In conclusion: 1) dental pulp is a remarkable site of stem cells; 2) collecting stem cells from dental pulp is a non-invasive practice that can be performed in the adult during life and in the young after surgical extraction of wisdom teeth, a common surgical practice; 3) tissue sacrifice is very low when collecting dental pulp stem cells; 4) several cytotypes can be obtained from dental pulp stem cells due to their multipotency; 5) transplantation of new-formed bone tissue obtained from dental pulp stem cells lead to formation of vascularized adult bone and integration between of the graft with the surrounding host blood supply; 6) dental pulp stem cells can be cryopreserved and stored for long periods; 7) dental pulp is ideal for tissue engineering and for clinical use in several pathologies requiring bone tissue growth and repair; 8) their use in clinical trials has demonstrated their ability to repair bone defects; 9) new easy and better methods are necessary for their isolation, expansion and use in therapy.
Patent
“Stem cells obtained from pulp of deciduous or permanent teeth and of dental germ, able to produce human bone tissue” PCT/EP2005/0081; WO 2006/010600
References
Parker, G. C., Anastassova-Kristeva, M., Broxmeyer, H. E., et al. (2004). Stem cells: shibboleths of development. Stem Cells and Development, 13(6), 579–584.
Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9(5), 641–650.
Alhadlaq, A., & Mao, J. J. (2004). Mesenchymal stem cells: isolation and therapeutics. Stem Cells and Development, 13(4), 436–448. Review.
Pittenger, M. F., Mackay, A. M., Beck, S. C., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Nerem, R. M. (1992). Tissue engineering in the USA. Medical & Biological Engineering & Computing, 30(4), CE8-12. Review.
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920–926. Review.
Laino, G., d’Aquino, R., Graziano, A., et al. (2005). A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). Journal of Bone and Mineral Research, 20(8), 1394–1402.
Papaccio, G., Graziano, A., d’Aquino, R., et al. (2006). Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. Journal of Cellular Physiology, 208(2), 319–325.
d’Aquino, R., Graziano, A., Sampaolesi, M., Laino, G., Pirozzi, G., De Rosa, A., et al. (2007). Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death and Differentiation, 14(6), 1162–1171.
Graziano, A., d’Aquino, R., Laino, G., & Papaccio, G. (2008). Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Reviews, 4(1), 21–26. Review.
Paino, F., Ricci, G., De Rosa, A., et al. (2010). Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. European Cells and Materials, 20, 295–305.
Sinanan, A. C., Hunt, N. P., & Lewis, M. P. (2004). Human adult craniofacial muscle-derived cells: neural-cell adhesion-molecule (NCAM; CD56)-expressing cells appear to contain multipotential stem cells. Biotechnology and Applied Biochemistry, 40, 25–34.
Goldberg, M., & Smith, A. J. (2004). Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Critical Reviews in Oral Biology and Medicine, 15, 13–27.
Jo, Y. Y., Lee, H. J., Kook, S. Y., et al. (2007). Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Engineering, 13, 767–773.
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97, 13625–13630.
Laino, G., Graziano, A., d’Aquino, R., et al. (2006). An approachable human adult stem cell source for hard-tissue engineering. Journal of Cellular Physiology, 206, 693–701.
Battye, F. L., Light, A., & Tarlinton, D. M. (2000). Single cell sorting and cloning. Journal of Immunological Methods, 243(1–2), 25–32. Review.
Battye, F. L., & Shortman, K. (1991). Flow cytometry and cell-separation procedures. Current Opinion in Immunology, 3(2), 238–241. Review.
Tucker, H. A., & Bunnell, B. A. (2011). Characterization of human adipose-derived stem cells using flow cytometry. Methods in Molecular Biology, 702, 121–131.
Wang, L., Gaigalas, A. K., Marti, G. E., Abbasi, F., & Hoffman, R. A. (2008). Toward quantitative fluorescence measurements with multicolor flow cytometry. Cytometry. Part A, 73A, 279–288.
Kern, W., Schnittger, S., Voskova, D., Hiddemann, W., Schoch, C., & Haferlach, T. (2004). Prognostication in acute myeloid leukemia: applicability and role of multi-parametric flow cytometry to quantify minimal residual disease in comparison to established prognostic parameters. Cytometry, Part A59A, p. 59.
Weir, E. G., & Borowitz, M. J. (2001). Flow cytometry in the diagnosis of acute leukemia. Seminars in Hematology, 38, 124.
Wood, B. L. (2007). Myeloid malignancies: myelodysplastic syndromes, myeloproliferative disorders, and acute myeloid leukemia. Clinics in Laboratory Medicine, 27, 551. vii.
Wong, Y. Y., Ng, S. P., Ng, M. H., Si, S. H., Yao, S. Z., & Fung, Y. S. (2002). Immunosensor for the differentiation and detection of Salmonella species based on a quartz crystal microbalance. Biosensors & Bioelectronics, 17, 676.
Bovenizer, J. S., Jacobs, M. B., O’Sullivan, C., & Guilbault, G. G. (1998). The detection of pseudomonas aeruginosa using the quartz crystal microbalance. Analytical Letters, 31, 1287.
Battaglia, R., Palomba, E., Palumbo, P., Colangeli, L., & della Corte, V. (2004). Development of a micro-balance system for dust and water vapor detection in the Mars atmosphere. Advances in Space Research, 33(12), 2258–2262.
Muramatsu, H., Dicks, J. M., Tamiya, E., & Karube, I. (1987). Piezoelectric crystal biosensor modified with protein A for determination of immunoglobulins. Analytical Chemistry, 59, 2760–2763.
Lasky, S. J., & Buttry, D. A. (1989). Sensors based on biomolecules immobilized on the piezoelectric quartz crystal microbalance. Chemical Sensors and Microinstrumentation, 403, 237–251.
Acknowledgments
This study was supported by Grants FIRB_06/10 to GP.
Conflicts of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tirino, V., Paino, F., d’Aquino, R. et al. Methods for the Identification, Characterization and Banking of Human DPSCs: Current Strategies and Perspectives. Stem Cell Rev and Rep 7, 608–615 (2011). https://doi.org/10.1007/s12015-011-9235-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9235-9