Abstract
Recent progress in cancer biology indicates that eradication of cancer stem cells (CSCs) is essential for more effective cancer therapy. Unfortunately, cancer stem cells such as glioma stem-like cells (GSLCs) are often resistant to either radio- or chemotherapy. Therefore, screening and development for novel therapeutic modalities against CSCs has been an important emerging field in cancer research. In this study, we report that a synthetic dl-nordihydroguaiaretic acid compound (dl-NDGA or “Nordy”), inhibited self-renewal and induced differentiation of GSLCs in vitro and in vivo. We found that Nordy inhibited an enzyme known to be involved in leukemia stem cell and leukemia progression, Alox-5, and attenuated the growth of GSLCs in vitro. Nordy reduced the GSLC pool through a decrease in the CD133+ population and abrogated clonogenicity. Nordy appeared to exert its effect via astrocytic differentiation by up-regulation of GFAP and down-regulation of stemness related genes, rather than by inducing apoptosis of GSLCs. The growth inhibition of xenografted glioma by Nordy was more long-lasting compared with that of the akylating agent BCNU, which exhibited significant relapse on drug discontinuation resulting from an enrichment of GSLCs. Meanwhile, transient exposure to Nordy reduced tumorigenecity of GSLCs and induced differentiation of the xenografts. Taken together, we have identified Alox-5 as a novel target in GSLCs and its inhibition with Nordy exhibits therapeutic implications through inducing GSLC differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One recent conceptual shift in the biology of solid tumors is the notion that cancer stem cells (CSCs), which resemble their normal counterparts in partial aspects of biological properties such as self-renewal and multipotency of differentiation, initiate the tumorigenesis process and contribute to heterogeneity of cancer cell population within a tumor mass. Using markers of somatic stem cells, CSCs have been tentatively identified in a series of solid tumors including cancers of the breast, brain, lung, stomach and colon, as well as melanoma [1]. CSCs are highly tumorigenic and are functionally designated as tumor-initiating cells [2]. Given to their indefinite self-renewing and exclusive tumor propagating capabilities, CSCs are not only believed to fuel tumor progression and but also be the possible “seeds” of recurrence after conventional therapies for most types of malignant tumors, such as glioblastoma [1]. Indeed, the research from glioblastoma, leukemia and carcinomas of the liver, colon and breast have demonstrated that CSCs are capable of surviving from conventional chemotherapy or ionizing radiation by mechanisms that were inherent from their transformed precursors or reacquired in the latter phase of clonal evolution, highlighting the significance of targeting this unique population of cancer cells for improved therapeutic efficiency [1].
Until now, several possible approaches to therapeutics for CSCs have been suggested, such as directly targeting CSCs by specific markers, disruption of the vascular niche that maintains the CSCs, and titration of the developmental program operative in CSCs to enforce their differentiation because of the enhanced sensitivity of their mature progenies to conventional therapy [3]. However, owing to heterogeneity of CSCs among individual glioma samples [1], efficiently targeting CSCs remains quite difficult due to the lack of specific markers even when CD133 was considered [4, 5].
Nordy (dl-nordihydroguaiaretic acid, dl-NDGA) is a synthetic compound which has demonstrated anti-tumor activity [6, 7]. Furthermore, the anti-tumor effect of Nordy was found to be mediated by its regulatory roles in differentiation induction and growth inhibition of glioma cells both in vitro and in vivo using a proteomic approach [7]. However, a distinct cellular target for Nordy has yet to be identified. Recently, the Alox-5 enzyme has been implicated in the progression of CML and the function of the CML stem cell [8]. In this study, we found up-regulated expression of Alox-5 in GSLCs, and thus we tested the effect of Nordy on Alox-5 enzymatic activity, and its potential anti-tumor effects on this subpopulation of malignant glioma cells both in vitro and in vivo. We found that Nordy triggered a differentiation program on GSLCs towards an astrocytic phenotype that reduced GSLCs frequency and inhibited the growth of xenografted glioma, providing novel insight into the molecular anti-cancer mechanism of Nordy and its therapeutic potential as an anti-glioma agent.
Materials and Methods
Cell Culture and Treatment
The glioblastoma cell line U87 was obtained from ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal bovine serum (FBS) (Gibco, Australia), 100 U/ml penicillin G and 100 μg/ml streptomycin (Gibco, Australia). GSLCs from xenografts formed by U87 cells (U87-xenograft cells) were cultured as previously described [9, 10]. Briefly, xenografted glioma tissues after serial passages in nude mice were minced, digested with collagenase type IV, released by gentle pipetting and filtrated through a 70 μm cell strainer. Adherent culture of U87-xenograft cells were performed by plating the cells in a gelatin-coated plastic flask in DMEM for 24 h and washed with PBS to remove red blood cells and cell debris. Then the tumor cells were collected and seeded in serum-free neural stem cell medium at clonal density of 10 cells per μl for 5 to 7 days to obtain floating tumorospheres, which were tentatively defined as GSLCs [9–12]. Adherent non-tumorospheres forming U87-xenograft cells were defined as non-GSLCs [9–12].
Sphere Formation Assay
To evaluate effects of Nordy on GSLCs population, freshly dissociated U87-xenograft cells were subjected to tumorospheres formation in neural stem cell medium. Sphere formation assays in Fig. 2b and 5c were carried out by seeding of single U87-xenograft cells into 96-well plates by serial dilution and wells with single cell were checked under microscopy. The cells were cultured for 14 days and tumorospheres with more than 50 cells were counted. U87-xenograft cells or cells pretreated with Nordy (10 μM or 50 μM), or 50 μM Nordy plus LTB4 (0.1 μM or 0.4 μM), or vehicle (ethanol) for 2 days in neural stem cell medium were subjected to culture by single cell per well for another 14 days. Alternatively, single freshly isolated U87-xenograft cells were directly maintained in neural stem cell medium containing Nordy, LTB4, or vehicle (ethanol) as indicated above for 14 days. Culture medium and drugs were refreshed every 3 days. Sphere forming efficiency (SFE) was calculated as number of tumorospheres/number of single cell well.
Cell Differentiation Assay
To examine the effect of Nordy on differentiation of GSLCs, tumorospheres were dissociated mechanically by gentle pipetting and attached to gelatin-coated coverslips in DMEM medium containing 10% FBS for 4 h. Then the serum-containing medium was aspirated, washed with phosphate-buffered saline (PBS) three times and replaced with defined neural stem cell medium. Then the culture medium was added with Nordy (10 μM or 50 μM) for 24 h, 48 h, or 72 h and GSLCs culture in neural stem cell medium with same period served as medium control. For analyzing changes in cell cycle and transcripts of stem cell associated gene during GSLCs differentiation, GSLCs were cultured at clonal intensity in neural stem cell medium containing 10 μM Nordy for 2 or 4 days in gelatin-coated plastic flask. GSLCs maintained in neural stem cell medium served as medium control.
Cell Proliferation Assay
To test effect of Nordy treatment on propagation of GSLCs, tumorospheres were dissociated as described in [11] and seeded into 96-well plate at 1,000 cell per well in 100 μl neural stem cell medium added with Nordy (10 μM or 50 μM), or 10 μM Nordy plus 0.4 μM LTB4, or vehicle (ethanol) for 5 days. Then culture medium was incubated with 10 μl CCK-8 solution (Dojindo, Japan) for 2 h and absorbance was measured at 450 nm in a plate reader (μQuant, Bio-TEK) everyday. Wells with appropriate amount of culture medium, drugs, and CCK-8 solution without cells served as blank control.
Colony Formation Assay
Anchorage independent colony formation assay was carried out by suspending 200 GSLCs in DMEM plus 10% FBS medium containing 0.3% noble agar (Difco, BD) and Nordy (10 μM or 50 μM), or 10 μM Nordy plus 0.4 μM LTB4, or vehicle (ethanol) on top of a 0.6% noble agar layer containing DMEM plus 10% FBS medium using 3.5 cm diameter plates. Cells were incubated for 14 days and colonies were visualized using an image analysis system (Alpha Ease FC). Colonies with more than 50 cells were counted. Each treatment was set up in triplicate and three independent assays were performed.
Lipoxygenase Inhibitor Screening
Lipoxygenase inhibitory activity was tested using lipoxygenase inhibitor screening assay Kit (Cayman chemical, Ann Arbor, Michigan) and Alox-5 enzyme (Cayman chemical, Ann Arbor, Michigan) following the manufacturer’s protocol, which measured specific inhibitory effect of the compounds NDGA, meso-NDGA, and Nordy on Alox-5. Briefly, Nordy was dissolved in DMSO and added into the assay system, which was initiated by adding of substrate arachidonic acid and terminated by Chromogen. The wells added with assay DMSO, and 15-Lipoxygenase standard served as vehicle control and positive control respectively. Absorbance was determined at 500 nm using a plate reader (μQuant, Bio-TEK), which is correlated with lipoxygenase activity.
Immunofluorescence and Laser Confocal Scanning Microscopy
For immunostaining of GSLCs, Nordy treated cells were washed with PBS three times and then fixed in 4% paraformaldehyde for 20 min at room temperature. For immunohistochemical staining of patient or xenograft glioblastomas, the specimens were embedded in Tissue-Tek OCT (optimal cutting temperature) compound at −20°C for cryostat sections (6 μm), which were mounted on poly-l-lysine-coated coverslips and fixed in acetone for 20 min at 4°C. Both cultured cells and tissue slides were blocked with unimmunized goat serum for 30 min at 37°C, incubated with primary antibodies (rabbit anti-human Alox-5, mouse anti-human CD133, mouse anti-human nestin, mouse anti-human GFAP, or mouse anti-human Ki-67) (all from Abcam except Alox-5 antibody from Santa Cruz, USA) overnight at 4°C, followed by appropriate goat anti-rabbit or anti-mouse IG antibodies conjugated with Cy3 or FITC (abcam, USA) for 30 min at 37°C. The cell number of positive cells in at least 10 randomly selected microscopic fields was normalized to the total number identified by counterstaining with hochest 33258 to demonstrate the nuclei and observed using laser confocal scanning microscope (Leica TCS-SP5, Germany).
Real Time RT-PCR and Semi-quantitative RT-PCR
Total RNA was extracted from GSLCs treated with or without Nordy using RNAiso reagent (TaKaRa, Japan), according to manufacturer’s instructions. Then reverse transcription and Real time-PCR was carried out to detect the expression of Alox-5 mRNA in GSLCs and non-GSLCs using SYBR primescript RT-PCR kit (TaKaRa, Japan) on a Rotor-Gene 6000 real-time genetic analyzer (Corbett Life Science, USA) according to manufacturer’s instructions. Primers of Target genes were listed in Table 1, with indicated amplicon length and annealing temperature.
Semi-quantitative RT-PCR was performed to examine stemness-associated transcription factors transcripts and stem cell markers using TaKaRa RNA PCR kit 3.0 (TaKaRa, Japan). The housekeeping gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control. Primers of target genes were listed in Table 1, with indicated amplicon length and annealing temperature. Amplification of cDNA was performed for 29 cycles. Aliquots of each PCR product were subjected to electrophoresis in 1.5% agarose gel containing 0.5 μg /ml ethidium bromide. Relative expression level of target genes was quantified using a Quantity One system (AlphaEase FC).
Western Blot
To detect expression of Alox-5 protein, Whole-cell lysates of GSLCs and non-GSLCs were prepared using M-PER mammalian protein extraction reagent (Pierce) and equal amounts of protein were loaded into 10% SDS-PAGE gels for electrophoresis, transferred to a nitrocellulose membrane (Amersham, UK), and probed with goat polyclonal Alox-5 antibody (1:200, Santa Cruz) and rabbit polyclonal GAPDH antibody (1:5000, Abcam, USA). Horseradish peroxidase–conjugated secondary antibodies to goat or rabbit immunoglobulin G was used (1:5000, Invitrogen). Antibodies were diluted in blocking buffer (5% w/v BSA and 0.1% v/v Tween 20 dissolved in PBS). All other buffers used during Western blotting were prepared following protein electrophoresis protocol. Protein expression was visualized using Super Signal West Femto kit (Pierce) by enhanced chemiluminescence detection system (Amersham) scanning of the membranes.
Flow Cytometry
Flow cytometry was performed in a FACSCalibur (BD Biosciences). For analysis of CD133 expression, 1 × 106 U87-xenograft cells of different treatment groups (Fig. 5b) or U87-xenograft cells seeded in serum-free neural stem cell medium containing either Nordy(10 μM or 50 μM), or Nordy 50 μM plus (LTB4 0.1 μM or LTB4 0.4 μM) , or vehicle only (ethanol) for 5 days were harvested, washed twice with PBS. Then the cells were suspended in 100 μL assay buffer (PBS, 0.5% BSA, 2 mM EDTA, pH 7.2) and 10 μl of the phycoerythrin-conjugated anti-CD133 antibody (Miltenyi Biotec, Germany) was added. The incubations were performed in the dark at 4°C for 30 min and terminated by washing with 1 ml of assay buffer twice and centrifuge at 300 g for 10 min. The cell pellet was resuspended in PBS for analysis.
Cell cycle was analyzed for GSLCs. GSLCs were incubated with 10 μM Nordy for 2 days or 4 days and GSLCs maintained in neural stem cell medium served as control. Then harvested cells were washed with PBS twice, subjected to treatment using solvent (50% methanol/50% H2O) for 36 h, and resuspended in 500 μL hypotonic fluorochrome solution (50 μg/ml propidium iodide, 0.1% mmol/L sodium citrate, and 0.1% Triton X-100 in PBS). DNA content analysis was performed with twenty thousand events for each sample.
Xenografts
To explore effect of transient exposure to Nordy ex vivo on GSLCs-initiated gliomagenesis in vivo, GSLCs were maintained in serum free neural stem cell medium containing Nordy (50 μM or 100 μM), or no drug (medium control) for 2 days. Cell viability was determined by a trypan blue test and equal number of viable cells (2 × 104) were dissociated in 50 μL PBS and implanted subcutaneously into the flanks of 4 week-old female nude mice (5 mice in each group). Five weeks later, animals were sacrificed and xenograft tumors were measured and subjected to immunofluorescent analysis.
For in vivo treatment of Nordy, xenograft gliomas were established by inoculation of GSLCs subcutaneously (2 × 104 cells per mouse, 8 mice in each group). On the 10th day of GSLC injection, the tumor-bearing mice were injected intraperitoneally with Nordy (13.5 mg/kg or 27 mg/kg) every other day (8 times in total) or with 20 mg/kg of BCNU (Jinyao Amino Acid Co., Tianjin) every 4 days (4 times in total) during a treatment period of 16 days. Control mice were injected with a corresponding volume of vehicle (PBS). Drug administration was discontinued on day 26. Three mice were randomly selected, euthanized, and the tumors were harvested. To mimic tumor relapse after chemotherapy in clinical settings, prolonged observation was conducted on the remaining xenografts until the 34th day. Tumor growth was measured using an external caliper every fourth day and tumor volume was calculated as (length/2) × width2. All animal experimental protocols used in this study were in accordance with institutional Guidelines for Animal Experiments and nude mice were maintained at The Center for Experimental Animals of Third Military Medical University.
Statistical Analysis
All the experiments were presented with representative data from at least three independent replicates. Student’s t test or ANOVA were performed when applicable, which were analyzed by SPSS10.0 statistical software and shown as means±SD.
Results
Alox-5 is Preferentially Expressed by GSLCs and is Inhibited by Nordy
Given the significant role of Alox-5 in maintaining chronic myeloid leukemia stem cells [8], and its implications in the malignant progression of brain tumors [13–17], we first examined the expression pattern of Alox-5 and the putative GSLC and neural progenitor marker Nestin in human glioblastoma [18]. Immunostaining demonstrated high expression of Alox-5 in the cytoplasm and perinuclear area of glioma cells (Fig. 1a). Alox-5 appeared to stain cells that were Nestin positive (Fig. 1a, arrow), a known GSLC marker, indicating Alox-5 expression in a subpopulation of GSLCs. Moreover, we confirmed the expression of Alox-5 in GSLCs and their differentiated counterparts by real-time RT-PCR and western blot. Expression of Alox-5 transcripts and protein in GSLCs was significantly higher than in non-GSLCs (Fig. 1b and c), suggesting a heterogeneous expression pattern of Alox-5 in human glioblastoma cells and its preferential expression in a GSLC population.
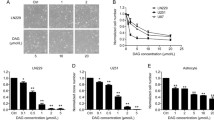
ALOX-5 mediated growth inhibition of GSLCs by Nordy. a Immunofluorescence imaging showing co-expression of Nestin (green) and ALOX-5 (red) in a subset of GBM (Glioblastoma-090116DP) cells. Scale bars = 20 μm. b and c Quantitative reverse transcription-polymerase chain reaction (q-RT-PCR) analysis (b) and Western-blotting (c) showing expression of ALOX-5 in GSLCs. # P < 0.05. d Dose-dependent inhibition rate of ALOX-5 by Nordy, meso-NDGA, and NDGA with a lipoxygenase inhibitor screening assay Kit; e Growth curves of GSLCs treated with different concentration of Nordy, LTB4, or vehicle (ethanol) and representative images of GSLCs cultured for 5 days; f Shown are representative agar plates of anchorage-independent clone formation of GSLCs. Data are shown as the mean±standard error of the mean. Abbreviations: NDGA, Nordihydroguaiaretic acid; meso-NDGA, meso-Nordihydroguaiaretic acid
Alox-5 exerts its biological function mainly by acting as rate-limiting enzyme in leukotriene synthesis in the metabolism process of arachidonic acid. In our previous study, we revealed inhibition of glioma growth by Nordy treatment [7], but the mechanistic basis of such anti-glioma activity is largely unknown. In light of enhanced expression of Alox-5 in GSLCs, we tested whether Nordy, the racemate of a lipoxygenase inhibitor NDGA, could inhibit the enzymatic activity of Alox-5 in an in vitro assay system. Indeed, Nordy as well as its precursor compound NDGA and chiral counterpart meso-NDGA inhibited Alox-5 activity in a dose-dependent manner. As is shown in Fig. 1d, IC50 value for NDGA, meso-NDGA, and Nordy are 19 μM, 25 μM, and 36 μM respectively. Although less potent compared with NDGA these results demonstrate that Nordy indeed possesses the ability to inhibit Alox-5.
Nordy Inhibited Self-Renewal of GSLCs and This Effect was Rescued by a Downstream Product of the Alox-5 Enzyme
Then tumorosphere culture assay was carried out to determine whether Nordy was involved in regulating propagation of GSLCs in vitro. Exposure of tumorospheres to Nordy in neural stem cell medium reduced proliferation of GSLCs in a dose-dependent manner, as is shown in Fig. 1e. Incubation of 10 μM Nordy for 5 days reduced tumorosphere growth by 53% (P < 0.01), while 50 μM Nordy achieved a 67% of inhibitory rate. However, growth inhibition of GSLCs was rescued by LTB4, a downstream product of Alox-5 oxidized eicosanoids. Indeed, 0.4 μM LTB4 reduced inhibitory effect of 10 μM Nordy by 60% (P < 0.01). And Nordy incubation for 5 days in GSLCs culture resulted in much smaller tumorospheres, which was reversed by LTB4 (Fig. 1e).
In addition, growth inhibition of GSLCs by Nordy was further examined by soft agar clone formation assay. Nordy treatment resulted in a dose-dependent loss of clonogenicity of GSLCs. Similar to the tumorosphere results, the clone formation of GSLCs was rescued by the addition of LTB4, which restored clone formation two-fold (P < 0.01) compared with GSLCs treated with 10 μM Nordy alone (Fig. 1f). Taken together, these results suggest that growth inhibition of glioblastoma tumorospheres by Nordy was, at least in part, due to its inhibitory activity on Alox-5.
In our previous studies, we found Nordy treatment repressed growth of glioma cells in vitro and in vivo [6, 7], but the present findings suggest growth inhibition of GSLCs could be occurring. We then asked whether Nordy treatment might target glioma cells as a whole or target GSLCs preferentially. To this end, U87-xenograft cells were subject to Nordy treatment, and flow cytometry analysis revealed that CD133+ cells, the putative GSLC population, decreased significantly upon Nordy incubation (Fig. 2a). We found that 6.2 ± 0.7% of U87-xenograft cells cultured in stem cell medium were positive for CD133 (Fig. 2a), while only 0.5% of long term serum cultured U87 cells were CD133 positive (data not shown). Treatment with 10 μM Nordy is efficient to reduce CD133 positive rate to 2.9 ± 0.3%, which is about a half of U87-xenograft cells, while 50 μM Nordy treatment doubled this effect. However, LTB4 incubation alleviated the reduction of CD133+ cells by Nordy. Up to 1.8 ± 0.3% and 7.4 ± 0.6% cells were CD133 positive when treated with 0.1 μM or 0.4 μM LTB4 in combination with 50 μM Nordy, indicating a clear rise in CD133+ cells compared with 50 μM Nordy alone.
Nordy depletes U87-xenograft cells of a GSLC subpopulation as demonstrated by CD133+ cell counts and sphere forming efficiency. a CD133 expression of U87-xenograft cells treated with vehicle only (control), or Nordy, Nordy plus LTB4 was determined by flow cytometry analysis. Data are shown as the mean±standard error of the mean. b and c Tumorosphere formation efficiency of single U87-xenograft cells pretreated (b) or treated with drugs of indicated dosages (c). Abbreviations: SFE, sphere forming efficiency
Other research suggested that GSLCs may also reside in CD133 negative cells [4, 5], thus we adopted surrogate measures of GSLCs, namely sphere-formation of single cells (sphere forming efficiency, SFE), as an indicator of tumor stem cell properties [9, 11]. Spheres formed for 7.3 ± 1.0% of control U87-xenograft cells. Pre-incubation (Fig. 2b) or continuous treatment (Fig. 2c) with Nordy depleted U87-xenograft cells of sphere forming ability in a dose dependent manner, with higher Nordy doses leading to lower SFE. With Nordy incubation for 14 days, only 3.4 ± 0.6% (10 μM) and 0.8 ± 0.2% (50 μM) of cells were capable of forming tumorospheres. SFE was restored by LTB4 in a dose dependent manner (Fig. 2b and c), indicating that Alox-5 inhibition by Nordy is a plausible mechanism to account for its depletion of GSLCs in U87-xenograft cells in vitro.
Nordy Induced Differentiation of GSLCs
The decrease in the GSLC pool of glioma cells and in GSLC propagation by Nordy treatment may be attributed to inhibition of cell proliferation, induction of cell death, or promotion of stem cell differentiation. Nordy-treated cells did not show an appreciable difference in apoptosis compared to controls (Fig. 3c). To explore the differentiation possibility, we analyzed differentiation markers associated GSLCs upon Nordy treatment. Under the control neural stem cell culture condition, GSLCs rarely expressed GFAP (astrocytic marker) (Fig. 3a), but highly expressed CD133 and Nestin [11, 12], markers associated with stem cell phenotype. However, expression levels of the differentiation marker (GFAP) increased substantially in a time-dependent manner (Fig. 3a) when the cells are exposed to Nordy. At 72 hrs, up to 24 ± 4.7% (10 μM Nordy) and 46.9 ± 7.5% of GSLC cells (50 μM Nordy) expressed GFAP (Fig. 3a), indicating the triggering of an aberrant differentiation program of GSLCs by Nordy leading to an astrocytic phenotype.
Nordy promotes astroglial differentiation of GSLCs in vitro. a Immunofluorescence showing time- and concentration-dependent expression of GFAP protein (green) in GSLCs elicited by Nordy treatment. Representative images are shown, Scale bars = 50 μm. b Expression changes in stemness-related gene transcripts in GSLCs treated with stem cell medium (medium control, for 4 days), or 10 μM Nordy for 2 days or 4 days by reverse transcription PCR analysis. c Flow cytometry analysis of cell cycle distribution dynamics of GSLCs incubated with medium control or 10 μM Nordy of indicated period. Abbreviations: Melk, maternal embryonic leucine zipper kinase
Specific transcription factors are associated with stem cell state [19–21], thus we examined expression changes of stemness-related gene transcripts in GSLCs upon Nordy treatment. As is shown in Fig. 3b, GSLCs maintained in neural stem cell medium expressed stemness transcription factors (Nanog, OCT-4, and SOX-2) and stem cell markers (CD133, Nestin). Treating GSLCs with 10 μM Nordy reduced mRNA expression of these genes in a time-dependent manner. When cultured in neural stem cell medium with 10 μM Nordy for 4 days, expression of these genes in GSLCs was barely detectable. Thus transcription of stem cell-associated genes was attenuated in the process of enforced astrocytic differentiation of GSLCs by Nordy.
Differentiation of stem cells involves cell cycle changes [22]. We found that stem cell medium cultured GSLCs resided predominantly (more than 60%) in quiescent stage, as is shown in (Fig. 3c), consistent with the notion that GSLCs are heterogeneous with a subpopulation of slow cycling cells. Incubation of GSLCs with Nordy leads to a transient reduction of cells in G0/G1 stage (8%), concomitant with a corresponding portion of cells entering G2/M + S stage (Fig. 3c). In contrast, prolonged treatment resulted in reversion of such cell cycle change (Fig. 3c), leading to 10% of cells re-entering quiescence. However, no substantial increase was observed in sub-G0/G1 phase, indicating that Nordy treatment did not induce apparent apoptosis of GSLCs in our experimental condition. In light of the differentiating effect of Nordy on GSLCs, this cell cycle change pattern, characteristic of primitive stem cell differentiation [22], indicates that Nordy treatment may activate cells in quiescence and trigger the intrinsic differentiation machinery of GSLCs.
Nordy pre-treatment Attenuates the Tumorigenicity of GSLCs and Induces Differentiation of Their Xenografts
To examine the effect of in vitro pretreatment of GSLCs with Nordy on tumorigenic potential, we injected nude mice with either control or Nordy treated GSLCs. Animals receiving control GSLCs developed tumors on the 10th day, while animals receiving Nordy-treated GSLCs did not develop tumors until the 14th (50 μM Nordy) or the 19th day (100 μM Nordy), demonstrating prolonged tumorigenic latency of GSLCs with Nordy treatment. Furthermore, there was also a difference in tumor volume upon harvest of xenograft tumor from control and Nordy treated groups. As is shown in (Fig. 4b), pretreating GSLCs with 50 μM and 100 μM Nordy resulted in tumor volume of 940 ± 150 mm3 and 90 ± 20 mm3 respectively, compared with a much larger volume of 2,250 ± 460 mm3 in control tumors. Moreover, immunofluorescence analysis of randomly selected microscopic fields revealed less CD133+ cells (1.4 ± 0.6%) and increased expression of GFAP protein (38.2 ± 2.4%) in tumors formed by Nordy-treated GSLCs compared with that of control (Fig. 4a). These results suggest that transient exposure of GSLCs to Nordy ex vivo was capable of inhibiting tumorigenecity in nude mice, through a reduction of the GSLC population and an increase in astroglial differentiation.
In vitro pretreatment of GSLCs by Nordy leads to attenuation of tumorigenecity in nude mice. a Shown are laser confocal scanning microscopic analysis of CD133 (red) and GFAP (red) protein expression, revealing in vitro exposure of GSLCs to Nordy leads to enhanced differentiation phenotype of GSLC-initiated tumors. Representative images are shown, Scale bars = 50 μm. B, Tumor volume as shown in histogram demonstrates attenuated tumorigenic capability of GSLCs in vivo by Nordy incubation in vitro
In Vivo Administration of Nordy Reduced GSLCs Frequency in Xenograft Tumors
Because previous studies have shown that Nordy attenuated the growth of gliomas formed by U87 cells [6, 7], we presumed that xenograft tumors initiated by stem cell counterparts could also be inhibited by Nordy. To measure the tumor propagation, we established a subcutaneous tumor model in nude mice [6] and also harvested xenograft tumors for flow cytometric analysis and tumor sphere culture. We found that subcutaneous injection of 2 × 104 GSLCs formed tumors nodules that were palpable after 10 days in all nude mice. As is shown in (Fig. 5a), administration of both Nordy and the cancer chemotherapeutic BCNU inhibited tumor growth. On the 26th day of tumor establishment (drug discontinuation), xenograft tumors regressed to 50 ± 10 mm3 in BCNU group and reached 380 ± 80 mm3 and 290 ± 50 mm3 in 13.5 mg/kg and 27 mg/kg Nordy group respectively, compared with 650 ± 70 mm3 in vehicle control group. This shows that during treatment period, growth inhibition by BCNU was greater than that induced by Nordy. However, prolonged observation revealed that xenograft tumors in BCNU group expanded in a much quicker rate than Nordy group after drug withdrawal (Fig. 5a). On the 34th day, tumor volumes in BCNU group soared to 1,500 ± 110 mm3, which was in contrast to 930 ± 50 mm3 in the 27 mg/kg Nordy group.
Nordy treatment inhibits GSLCs-initiated gliomagenesis and depletes xenograft tumors of their GSLC population. a Growth curves of tumors treated with indicated dosages of Nordy and BCNU and representative dissected tumors in each group on drug discontinuation (day 26) and 8 days thereafter (day 34), showing inhibition of tumor growth by both BCNU and Nordy with prolonged inhibitory activity of Nordy compared to BCNU. b Analysis of CD133 + cell population upon drug withdrawal by flow cytometry, demonstrates a significant enrichment of CD133 + cells by BCNU, but depletion of CD133 + cells by Nordy treatment. Typical graphs are shown in. c Tumorosphere formation efficiencies in each xenograft tumor on drug withdrawal were examined as SFE%, which revealed enhanced sphere formation capability of tumor cells treated with BCNU and abrogation of sphere formation by Nordy in vivo. Abbreviations: SFE, sphere forming efficiency; BCNU, Bischloro-nitrosourea
Although tumor volume in both of the two treatment groups was smaller than that of in vehicle control group (2,140 ± 170 mm3), growth inhibition in Nordy group was more long-lived than in BCNU group after drug withdrawal (Fig. 5a). And it is important to note that body weight of nude mice treated with BCNU was dramatically less, while that of the Nordy group was comparable to the vehicle control, suggesting lower systemic toxicity of Nordy compared to BCNU.
To elucidate cellular mechanisms underlying the differential inhibitory effect of Nordy and BCNU, tumor volumes, CD133+ cell population, and sphere forming capacity of xenograft tumors were quantified upon drug discontinuation. Eight days after drug discontinuation, the BCNU treated mice had larger tumor volumes in comparison to those treated with Nordy (Fig. 5a). The CD133+ cell fraction reached 32.9 ± 3.3% in BCNU treated xenograft cells, while the control group had only 32.9 ± 3.3% (Fig. 5b). SFE reached 26.4 ± 4.2% in BCNU treated xenograft cells, compared to 9.3 ± 1.0% in the vehicle control group (Fig. 5c). In contrast to BCNU, the Nordy-treated cells showed less positive staining for CD133 (3.7% for 13.5 mg/kg and 0.9% for 27 mg/kg) and did not form as many tumorospheres (3.1 ± 0.6% in 13.5 mg/kg and 1.3 ± 0.6% in 27 mg/kg Nordy) (Fig. 5b, c). These results suggest enrichment of GSLCs with BCNU administration in contrast to depletion of GSLCs by Nordy treatment in vivo.
Further Immunofluorescent staining of xenograft tumor tissues demonstrated that 27 mg/kg Nordy administration reduced the number of cells positive for the GSLC marker CD133 (1.5 ± 1.3%) and Nestin (7 ± 3.2%) and led to increased GFAP positive cells (66.6 ± 7.7%) (Fig. 6a and b). In response to BCNU, more of the cells were CD133 (32.3 ± 4.5%) and Nestin positive (36.6 ± 4.7%), but GFAP positive rate was reduced to 14.8 ± 2.8% (Fig. 6b). Both Nordy and BCNU treatment repressed ki-67 expression in vivo (4.8 ± 10.9% and 6.8 ± 2.2% respectively, compared with 35.8 ± 3.4% in control group) (Fig. 6b), which may account for decreased growth rate seen by the drug treatments.
Astroglial differentiation phenotype of GSLC-initiated tumors elicited by Nordy. a Immunofluorescent detection of GSLCs marker CD133 (red) and Nestin (green), growth index marker Ki67 (green) and astroglial differentiation marker GFAP (green) in xenograft tumors on drug discontinuation, showing immature phenotype in BCNU group and differentiated phenotype in Nordy group, while growth index decreased in both condition. Scale bars = 50 μm. b Quantitation of immunofluorescence data by the counting of positively stained cells in multiple (10) fields. Abbreviations: BCNU, Bischloro-nitrosourea
Discussion
The Alox-5 pathway of arachidonic acid metabolism plays an important role in carcinogenesis, modulating several hallmarks of cancers including robust proliferation, resistance to apoptosis, promotion of angiogenesis, and tissue invasion [23–26]. Moreover, overexpression of Alox-5 was correlated with a poor prognosis in patients with colorectal cancer [27], and Alox-5 inhibitors were shown to be beneficial for chemotherapy and chemoprevention [24, 28–30]. In the context of glioblastoma, increased expression of Alox-5 has been reported [13–17] but its exact role in gliomagenesis was unknown. We show for the first time that proliferation of a critical subpopulation of glioblastoma cells, GSLCs, was regulated by Alox-5, as its inhibition by Nordy restrained tumorosphere growth and soft agar clone formation, which is consistent with its previously identified effect of inhibiting neoplastic growth [25, 31]. Furthermore, inhibition of Alox-5 by Nordy resulted in depletion of CD133+ self-renewing GSLC and promoted astrocytic differentiation, demonstrating a novel mechanism by which Alox-5 could be involved in maintaining tumor stem cells in glioma. The discovery of such a target in GSLC could be important for the eradication of glioblastoma. Indeed, treating of nude mice with Nordy attenuated xenograft tumor growth and this correlated with a decrease in GSLC population. Although BCNU exhibited stronger growth inhibition of glioblastoma compared with Nordy during the administration period, its withdrawal resulted in rapid outgrowth of the xenograft tumor, reminiscent of disease recurrence of malignant tumors in a clinical setting. This was due to preferential enrichment of GSLCs with BCNU treatment [32]. In contrast, growth inhibition by Nordy was more long-lasting, highlighting the importance of targeted depletion of glioma stem cells with agents like Nordy to improve glioma therapy.
Self-renewal state ascribed to an embryonic stem cell-like gene expression module is a characteristic of malignant tumors and neoplastic stem cells in myeloid leukemia and solid tumors [20, 21]. Indeed, key transcription factors including OCT-4 [33] and SOX-2 [34] play fundamental roles in maintaining an undifferentiated state of tumor stem cells. In this regard, we found that compared with differentiated glioma cells, GSLCs expressed relatively high levels of transcripts of embryonic stem cell specific transcription factors (Nanog, OCT-4), neural stem cell, NSC, regulator (SOX-2) and markers (CD133, Nestin, Melk). Among them, Nanog, OCT-4, and SOX-2 are associated with reprogramming of mature cells to a pluripotent stem cell state [35]. This demonstrates that apart from inherent similarities between GSLCs and NSCs, the molecular circuitry that maintains self-renewal of GSLCs is, to some extent, distinct from NSC. The GSLCs are in a more immature state, which might be acquired in the evolution process and thus present a novel anti-glioma target. Therefore, our finding that expression of these stemness related regulators in GSLCs is modulated by Nordy further demonstrates that induced differentiation is mediated by reversion of an intrinsic developmental program. The miRNA precursor processing enzyme Dicer was found to contain an Alox-5 binding domain (5LObd) and Alox-5 directly interacted with 5LObd and regulated pre-miRNA processing of Dicer in human cells [36]. Owing to the essential role of miRNA in modulating biology of tumor stem cells [37, 38], and more specifically the significance of aberrant miRNA processing in transformation and malignant progression [39], it is tempting to speculate that inhibition of Alox-5 by Nordy may regulate miRNA maturation and expression in GSLCs, thus resulting in a loss of stemness.
CSCs are notoriously resistant to conventional chemotherapy and radiotherapy [1–3, 11, 32], which might be one of the most important causes of disease recurrence and progression for cancer patients in clinical settings. In the context of gliomas, the survival advantage of CSCs to radiation was mediated in part by enhanced PI3K/Akt activity [40] and activating phosphorylation of DNA damage checkpoint proteins [40, 41]. In addition, activation of PTEN/ PI3K/Akt pathway was shown to promote pumping activity of ABCG2 transporter, which is closely related with a side population phenotype in GSLCs and accordingly, chemoresistance [42]. In light of this property, successful cancer treatment might be achieved through reversion or abrogation of CSC resistance. One promising strategy, especially in leukemia, is differentiation therapy [43, 44]. In chronic myeloid leukemia, enforced differentiation of CSCs relies on disruption of the PML protein by arsenic trioxide (As2O3), which led to the eradication of leukemia CSCs [22]. However, screening of compound that selectively target CSC in solid tumors has not been realized until recently salinomycin was found to drive epithelial differentiation and loss of CD44+/CD24- breast CSC [45]. We have shown that Nordy treatment was effective to inhibit self-renewal of GSLCs and induce astrocytic differentiation. Based on these findings, we anticipate that combination of Nordy and chemotherapeutic drugs for glioblastoma such as BCNU or temozolomide (TMZ) might provide additional therapeutic benefit by two mechanisms: (1) simultaneous targeting of GSLCs with Nordy and non-GSLCs with cytotoxic drugs; (2) differentiation induced by Nordy might sensitize GSLCs to cytotoxicity of BCNU or TMZ. This approach could be applicable to targeting of CSC in other tissues as well. A promising future direction in cancer stem cell research rests in integrating differentiation strategies with conventional therapy to better eliminate CSCs, thus opening up a novel avenue for cancer therapy.
References
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer, 8, 755–68.
Clarke, M. F., Dick, J. E., Dirks, P. B., et al. (2006). Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res, 66, 9339–44.
Lee, J., Son, M. J., Woolard, K., et al. (2008). Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell, 13, 69–80.
Wang, J., Sakariassen, P. O., Tsinkalovsky, O., et al. (2008). CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer, 122, 761–8.
Beier, D., Hau, P., Proescholdt, M., et al. (2007). CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res, 67, 4010–5.
Chen, J. H., Bian, X. W., Yao, X. H., et al. (2006). Nordy, a synthetic lipoxygenase inhibitor, inhibits the expression of formylpeptide receptor and induces differentiation of malignant glioma cells. Biochem Biophys Res Commun, 342, 1368–74.
Bian, X. W., Xu, J. P., Ping, Y. F., et al. (2008). Unique proteomic features induced by a potential antiglioma agent, Nordy (dl-nordihydroguaiaretic acid), in glioma cells. Proteomics, 8, 484–94.
Chen, Y., Hu, Y., Zhang, H., Peng, C., & Li, S. (2009). Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet, 41, 783–92.
Singh, S. K., Clarke, I. D., Terasaki, M., et al. (2003). Identification of a cancer stem cell in human brain tumors. Cancer Res, 63, 5821–8.
Bao, S. D., Wu, Q. L., Sathornsumetee, S., et al. (2006). Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res, 66, 7843–8.
Yu, S. C., Ping, Y. F., Yi, L., et al. (2008). Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett, 265, 124–34.
Yi, L., Zhou, Z. H., Ping, Y. F., et al. (2007). Isolation and characterization of stem cell-like precursor cells from primary human anaplastic oligoastrocytoma. Mod Pathol, 20, 1061–8.
Nathoo, N., Prayson, R. A., Bondar, J., et al. (2006). Increased expression of 5-lipoxygenase in high-grade astrocytomas. Neurosurgery, 58, 347–54.
Lim JY, Oh JH, Jung JR, et al. MK886-induced apoptosis depends on the 5-LO expression level in human malignant glioma cells. J Neurooncol 2009.
Ishii, K., Zaitsu, M., Yonemitsu, N., Kan, Y., Hamasaki, Y., & Matsuo, M. (2009). 5-lipoxygenase pathway promotes cell proliferation in human glioma cell lines. Clin Neuropathol, 28, 445–52.
Zhang, L., Zhang, W. P., Hu, H., et al. (2006). Expression patterns of 5-lipoxygenase in human brain with traumatic injury and astrocytoma. Neuropathology, 26, 99–106.
Rajaraman, P., Brenner, A. V., Butler, M. A., et al. (2009). Common variation in genes related to innate immunity and risk of adult glioma. Cancer Epidemiol Biomark Prev, 18, 1651–8.
Aguado, T., Carracedo, A., Julien, B., et al. (2007). Cannabinoids induce glioma stem-like cell differentiation and inhibit gliomagenesis. J Biol Chem, 282, 6854–62.
Ben-Porath, I., Thomson, M. W., Carey, V. J., et al. (2008). An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet, 40, 499–507.
Somervaille, T. C. P., Matheny, C. J., Spencer, G. J., et al. (2009). Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell, 4, 129–40.
Wong, D. J., Liu, H., Ridky, T. W., Cassarino, D., Segal, E., & Chang, H. Y. (2008). Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell, 2, 333–44.
Ito, K., Bernardi, R., Morotti, A., et al. (2008). PML targeting eradicates quiescent leukaemia-initiating cells. Nature, 453, 1072–8.
Furstenberger, G., Krieg, P., Muller-Decker, K., & Habenicht, A. J. (2006). What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis? Int J Cancer, 119, 2247–54.
Hyde, C. A., & Missailidis, S. (2009). Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol, 9, 701–15.
Peters-Golden, M., & Henderson, W. R., Jr. (2007). Leukotrienes. N Engl J Med, 357, 1841–54.
Pidgeon, G. P., Lysaght, J., Krishnamoorthy, S., et al. (2007). Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev, 26, 503–24.
Öhd, J. F., Nielsen, C. K., Campbell, J., Landberg, G., Löfberg, H., & Sjölander, A. (2003). Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomas. Gastroenterology, 124, 57–70.
Sun, Z., Sood, S., Li, N., et al. (2006). Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7, 12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis, 27, 1902–8.
Melstrom, L. G., Bentrem, D. J., Salabat, M. R., et al. (2008). Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res, 14, 6525–30.
Cianchi, F., Cortesini, C., Magnelli, L., et al. (2006). Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol Cancer Ther, 5, 2716–26.
Hammamieh, R., Sumaida, D., Zhang, X., Das, R., & Jett, M. (2007). Control of the growth of human breast cancer cells in culture by manipulation of arachidonate metabolism. BMC Cancer, 7, 138.
Kang, M. K., & Kang, S. K. (2007). Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev, 16, 837–47.
Chen, Y. C., Hsu, H. S., Chen, Y. W., et al. (2008). Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS ONE, 3, e2637.
Gangemi, R. M., Griffero, F., Marubbi, D., et al. (2009). SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells, 27, 40–8.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–76.
Dincbas-Renqvist, V., Pepin, G., Rakonjac, M., et al. (2009). Human Dicer C-terminus functions as a 5-lipoxygenase binding domain. Biochim Biophys Acta, 1789, 99–108.
Godlewski, J., Nowicki, M. O., Bronisz, A., et al. (2008). Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res, 68, 9125–30.
Wellner, U., Schubert, J., Burk, U. C., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol, 11, 1487–95.
Viswanathan, S. R., Powers, J. T., Einhorn, W., et al. (2009). Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet, 41, 843–8.
Hambardzumyan, D., Becher, O. J., Rosenblum, M. K., Pandolfi, P. P., Manova-Todorova, K., & Holland, E. C. (2008). PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev, 22, 436–48.
Bao, S., Wu, Q., McLendon, R. E., et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature, 444, 756–60.
Bleau, A. M., Hambardzumyan, D., Ozawa, T., et al. (2009). PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell, 4, 226–35.
Sell, S. (2006). Cancer stem cells and differentiation therapy. Tumour Biol, 27, 59–70.
Massard, C., Deutsch, E., & Soria, J. C. (2006). Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol, 17, 1620–4.
Gupta, P. B., Onder, T. T., Jiang, G., et al. (2009). Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell, 138, 645–59.
Acknowledgements
We are indebted to Dr. Wei Sun and Dr. Li-ting Wang (Central Laboratory, Third Military Medical University, Chongqing, China) for expertise in immunofluorescence imaging. This work was supported by grants from the National Basic Research Program of China (973 Program, No.2010CB529403) and the national Natural Science Foundation of China (NSFC Nos. 30725035 and 30930103).
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by grants from the National Basic Research Program of China (973 Program, No.2010CB529403) and the national Natural Science Foundation of China (NSFC Nos. 30725035 and 30930103).
Rights and permissions
About this article
Cite this article
Wang, B., Yu, Sc., Jiang, Jy. et al. An Inhibitor of Arachidonate 5-Lipoxygenase, Nordy, Induces Differentiation and Inhibits Self-Renewal of Glioma Stem-Like Cells. Stem Cell Rev and Rep 7, 458–470 (2011). https://doi.org/10.1007/s12015-010-9175-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-010-9175-9