Abstract
This study was devised to identify potential biomarkers of schizophrenia (SP) using proteomics techniques. We obtained 44 serum specimens from patients with SP, 26 specimens from patients with depression, and 40 specimens from healthy controls. Immobilized metal affinity capture protein chips (IMAC30) and surface-enhanced laser desorption-ionization time-of-flight mass spectrometry were used to isolate and obtain mass spectrometric data of differentially expressed serum proteins. The sequences of the peaks discrepant among the study groups were obtained using matrix-assisted laser desorption/ionization mass spectrometry and proteins identified using Mascot database. In the SP group, there were 91 protein peaks that were different from other study groups at the p value of <0.05 and 54 peaks different at the p value of <0.01. Two protein peaks at the mass-to-charge ratio of 1,207.41 and 1,466.78 were markedly different among the study groups, with the lowest expression in specimens from patients with SP. The amino acid sequences were, respectively, Glu-Gly-Asp-Phe-Leu-Ala-Glu-Gly-Gly-Gly-Val-Arg (EGDFLAEGGGVR) and Asp-Ser-Gly-Glu-Gly-Asp-Phe-Leu-Ala-Glu-Gly-Gly-Gly-Val-Arg (DSGEGDFLAEGGGVR). These proteins were identified as the N-terminal fragments of fibrinogen. In conclusion, these biomarker proteins may be useful for molecular diagnosis of SP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia (SP) is a mental disorder with unknown etiology and unclear pathogenesis [1]. There is a clear need for molecular biomarkers for this disease. Proteomic techniques are becoming increasingly popular for biomarker identification, also in psychiatry [2, 3]. In the present study, we utilized surface-enhanced laser desorption-ionization time-of-flight mass spectrometry (SELDI-TOF-MS) technique to screen serum proteins of SP patients to find potential biomarkers. The proteins of interest were identified by enzymatic digestion and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF-MS).
Materials and Methods
Patients and Healthy Controls
Patients with SP were enrolled in Psychiatry Department of the Affiliated Hospital of Luzhou Medical College between November 2007 and October 2008. The inclusion criteria were: diagnosis of SP made according to the International Diseases Classification and Diagnostic Criteria (10th edition, ICD-10) [4], first onset, no use of antipsychotic drugs after admission, and exclusion of mental disorders caused by cerebral organic or somatic diseases. Forty-four patients met these inclusion criteria. There were 20 male and 24 female patients whose age ranged from 19 to 48 years (mean ± SD age of 33.1 ± 8.4 years).
As control subjects, we enrolled 26 patients with depression. The inclusion criteria in this group were the following: diagnosis of depression according to the Classification and Diagnostic Criteria of Mental Disorders in China (CCMD3) [5], no use of antidepressant drugs after admission, and ruled out SP. There were 11 male and 15 female patients whose age ranged from 17 to 45 years (mean age of 32.5 ± 8.6 years).
We also utilized a healthy control group which comprised 40 healthy individuals (18 male and 22 female individuals, age range of 21–50 years, mean age of 34.0 ± 9.2 years).
Collection of Specimens
Venous blood (approximately 2–3 ml) was collected in the early morning and stored at 4 °C for a maximum of 30 min. Blood specimens were allowed to stand for 1 h to clot and were subsequently centrifuged at 3,000 rpm for 5 min at 4 °C. Serum samples were transferred into microtubes and re-centrifuged at 3,000 rpm for 5 min at 4 °C. Supernatants were separated into three tubes (100 µl/tube) and stored at −80 °C until further use.
Sample Preparation
The frozen serum samples were thawed on ice, centrifuged at 10,000 rpm for 2 min at 4 °C. Ten microliters of serum samples and 90 µl of phosphate buffer were combined in a microtube, and the mixture was mixed well.
Loading and Elution
Protein chip IMAC30 (Ciphergen Biosystems, Fremont, USA) was loaded on the chip holder. The chip was sequentially equilibrated in 50 μl of 0.1 M copper sulfate buffer (10 min), 200 μl of deionized water (1 min), 200 μl of 0.1 M sodium acetate buffer (pH 4.0; 5 min), 200 μl of deionized water again (1 min), and 200 μl of phosphate buffer (5 min). After phosphate buffer was removed, 50 μl of samples prepared as above were added and allowed to bind for 30 min at 4 °C. Then, the sample was removed, and the chip was washed twice with 200 μl of binding buffer (PBS, pH 7.2; 5 min). After the last wash with PBS, the chip was wiped dry and washed twice with 200 μl of HPLC-grade water. The chip was then removed and dried at room temperature.
Preparation of Energy Absorption Molecules (EAM)
Five microliters of trifluoroacetic acid solution (TFA) were added to 495 μl of HPLC-grade water and mixed well. Two-hundred microliters of diluted TFA were combined with 200 μl of acetonitrile solution in a sinapinic acid (SPA) tube (5 μg/tube) and homogenized for 3 min. The mixture was allowed to stand for 5 min and centrifuged for 3 min at 10,000 rpm.
Addition of EAM to the Sample
One microliter of SPA was pipetted onto the surface of the dried protein chip. The protein chip was then air-dried for 15 min and read in the instrument.
Mass Spectrometric Detection of Serum Proteins
Serum proteins attached to the surface of protein chip were detected using PBS II/C protein fingerprint spectrometer, with the optimization range of 1,000–20,000 mass-to-charge ratio (m/z), highest detectable molecular weight of 100,000 m/z, laser intensity of 210, and detection sensitivity of 9. The instrument was calibrated by all-in-one protein standard molecular chip (Ciphergen Biosystems) to confirm that the system’s mass deviation was ≤0.1 %. The Ciphergen Protein chip software was used to automatically collect experimental data and store the original detection spectra. The pre-treatments of the obtained serum protein spectra included reduction of the baseline, homogenization, etc.
Statistical Analysis
The serum protein fingerprint patterns were analyzed by Biomarker Wizard 3.1 software (Ciphergen Biosystems), with the threshold value of occurrence frequency of significant protein peak of 10 %, and signal–noise ratio (S/N) of 5 and 2 for filtering. The t test was performed to compare peak expressions between any two groups. The difference between protein peaks with the p value of <0.05 was considered statistically significant.
Results
Reproducibility of Experimental Results
A randomly selected sample was repeated twice within the same day on the same protein chip, and identical mass spectrometric peaks were obtained (Fig. 1). The same sample was further repeated four times at different days yielding the same mass spectrogram (Fig. 2). These experiments indicated good intra- and inter-assay reproducibility [6, 7].
Detection of Protein Peak Differentially Expressed Among Study Groups
The SELDI-TOF–MS technology was used to detect serum protein fingerprint patterns of 110 samples which included specimens from patients with SP or depression, or healthy individuals. The Ciphergen ProteinChip 3.0 software was utilized for data correction and analysis, and Ciphergen Biomarker Wizard 3.1 software was used for statistical analysis. Compared with the depression and healthy control groups, 91 protein peaks were significantly different in SP group at the p value of <0.05 and 54 protein peaks exhibited significant difference at the p value of <0.01. Fifteen discrepant peaks were identified via secondary mass spectrometry and could be distinguished with the fast classification algorithm (Table 1).
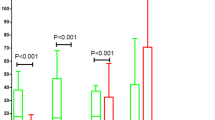
In the SP group, expression of protein peaks with the mass-to-charge ratio (m/z) of 1207.41, 1466.78, 3192.49, 3241.81, 3263.47, 5904.35, and 9287.25 was lower compared with healthy individuals. Further, in this group, protein peaks with m/z of 8932, 8987.59, 8601.29, 8136.86, 4467.69, 4201.54, 2082.83, and 1945.62 were more abundantly expressed (Table 2).
Among the three study groups, we found significant differences in two protein peaks with m/z of 1,207.41 and 1,466.78. Expression of these peaks was the lowest in the SP group, highest in the healthy control group, and intermediate in patients with depression (Table 3). These protein peaks were analyzed by direct sequencing method through MALDI-TOF/TOF tandem mass spectrometry (Figs. 3, 4). By searching in Mascot database, the 1,207.41 and 1,466.78 peaks were identified as the N-terminal fragment of fibrinogen with the following sequence: Glu-Gly-Asp-Phe-Leu-Ala-Glu-Gly-Gly-Gly-Val-Arg (EGDFLAEGGGVR) and Asp-Ser-Gly-Glu-Gly-Asp-Phe-Leu-Ala-Glu-Gly-Gly-Gly-Val-Arg (DSGEGDFLAEGGGVR).
Discussion
In recent years, mass spectrometry techniques have been increasingly used to identify biomarkers [8, 9]. These techniques have been applied to diseases such as leukemia, breast cancer [10] and other cancers (e.g., colon, bladder, prostate, lung, and kidney cancers), as well as neuroblastoma [11]. Attempts have been undertaken to identify the biomarkers for SP. Thus, Mei et al. [12] used SELDI-TOF–MS technique to identify differentially expressed proteins in the dorsolateral prefrontal cortexes of patients with SP or control individuals. Further, Jiang et al. [13] utilized the same technique to screen serum protein biomarkers in patients with SP; these authors also developed an artificial neural network diagnostic model. The model was used to blindly test patients with SP, and exhibited diagnostic sensitivity and specificity of, respectively, 91.7 and 93.8 % [13]. Craddock et al. [14] found that α-defensins were increased in the serum of patients with SP. However, human α-defensins are closely related to the onset and development of many other diseases, such as oral cavity disease, cancer, inflammatory diseases, and HIV [15–17]. Therefore, the sensitivity and specificity of defensin biomarkers do not meet the requirements of differential diagnosis in SP.
Our study reveals higher expression of N-terminal fragment of fibrinogen in serum of patients with SP. Fibrinogen is a synthetic glycoprotein from liver and is abundantly present in blood [18]. However, expression of both 1,207.41 and 1,466.78 peaks was lower in serum of patients with SP in our study. The following may have caused the observed decrease: (1) lower content or reduced activity of fibrinogen in patients with SP, or (2) possible structural changes of fibrinogen in patients with SP that may have led to differential degradation in the serum.
In conclusion, these biomarkers described in our study may be useful for molecular diagnosis of SP. The sequencing of biomarkers enables preparing anti-peptide antibodies to be tested for molecular diagnosis of SP. Studies involving higher numbers of patients should be conducted to ensure reproducibility and reliability of protein biomarkers in the diagnosis of SP.
Abbreviations
- SELDI-TOF–MS:
-
Surface-enhanced laser desorption/ionization, time-of-flight mass spectrometry
- SPA:
-
Sinapinic acid
- MALDI-TOF–MS:
-
Matrix assisted laser desorption/ionization, mass spectrometry
- CV:
-
Coefficient of variance
- m/z :
-
Mass-to-charge ratio
- IMAC30:
-
Immobilized metal affinity surface
- TFA:
-
Trifluoroacetic acid
- EAM:
-
Energy absorption molecules
References
van Os, J., Rutten, B. P., & Poulton, R. (2008). Gene-environment interactions in schizophrenia: Review of epidemiological findings and future directions. Schizophrenia Bulletin, 34, 1066–1082.
Lakhan, S. E. (2006). Schizophrenia proteomics: Biomarkers on the path to laboratory medicine? Diagnostic Pathology, 2006(1), 11.
Zhai, J., Xu, S. Z., & Cui, J. J. (2008). Investigation of clinical value S100B plasma levels in patients with schizophrenia. Journal of Psychiatry, 21, 261–262. in Chinese.
Boyer, L., Murcia, A., Belzeaux, R., Loundou, A., Azorin, J. M., Chabannes, J. M., et al. (2010). Psychometric properties of the Activities Daily Life Scale (ADL). L’Encephale, 36, 408–416. in French.
Sun Xue, L. (2008). Psychiatry. Beijing: Higher Education Press. in Chinese.
Ding, Y. H., Hu, Q. Y., & Liang, S. H. (2009). Using an internal standard calibration method to improve detection repeatability of surface-enhanced laser desorption ionization time-of-flight mass spectrometry. Chinese Journal of Laboratory Medicine, 32, 337–339. in Chinese.
Diamandis, E. P. (2003). Point: Proteomic patterns in biological fluids: Do they represent the future of cancer diagnostics? Clinical Chemistry, 2003(49), 1272–1275.
Jiang, W., & Wang, K. Z. (2006). Application and progress of protein chip technique on clinical research. Laboratory Medicine and Clinical, 2006(3), 308–310(in Chinese).
Lin, Y. W., Lin, C. Y., Lai, H. C., Chiou, J. Y., Chang, C. C., Yu, M. H., & Chu, T. Y. (2006). Plasma proteomic pattern as biomarkers for ovarian cancer. International Journal of Gynecological Cancer, 16(Suppl 1), 139–146.
Ding, Y. H., & Wang, K. Z. (2008). Statistical evaluation of SELDI-TOF-MS in early diagnosis of breast cancer. Letters in Biotechnology, 2008(19), 708–710.
Pham, T. D. (2008). Computational prediction models for cancer classification using mass spectrometry data. International Journal of Data Mining and Bioinformatics, 2008(2), 405–422.
Mei, J., Kolbin, D., Kao, H. T., & Porton, B. (2006). Protein expression profiling of postmortem brain in schizophrenia. Schizophrenia Research, 84, 204–213.
Jiang, W., Wang, K. Z., & Bai, K. Z. (2008). The study on clinical application of serum proteins as biological markers of schizophrenia. Chinese Journal of Nervous and Mental Disease, 34, 27–30. in Chinese.
Craddock, R. M., Huang, J. T., Jackson, E., Harris, N., Torrey, E. F., Herberth, M., & Bahn, S. (2008). Increased alpha-defensins as a blood marker for schizophrenia susceptibility. Molecular and Cellular Proteomics, 7, 1204–1213.
Sfanos, K. S., Wilson, B. A., De Marzo, A. M., & Isaacs, W. B. (2009). Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proceedings of the National Academy of Sciences of the United States of America, 106, 3443–3448.
Ramasundara, M., Leach, S. T., Lemberg, D. A., & Day, A. S. (2009). Defensins and inflammation: The role of defensins in inflammatory bowel disease. Journal of Gastroenterology and Hepatology, 24, 202–208.
Venkataraman, N., Cole, A. L., Ruchala, P., Waring, A. J., Lehrer, R. I., Stuchlik, O., et al. (2009). Reawakening retrocyclins: Ancestral human defensins active against HIV-1. PLoS Biology, 7, e95.
Herrero, A. M., Cambero, M. I., Ordonez, J. A., Castejon, D., Romero de Avila, M. D., & de la Hoz, L. (2007). Magnetic resonance imaging, rheological properties, and physicochemical characteristics of meat systems with fibrinogen and thrombin. Journal of Agriculture and Food Chemistry, 55, 9357–9364.
Acknowledgments
The authors wish to thank the National High Technology Research and Development Program (Program 863, Grant Number 2006AA02090407) for providing funding for the study.
Conflict of interest
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yin-huan Ding and Jian-hong Guo contributed equally to this study and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Ding, Yh., Guo, Jh., Hu, Qy. et al. Protein Biomarkers in Serum of Patients with Schizophrenia. Cell Biochem Biophys 72, 799–805 (2015). https://doi.org/10.1007/s12013-015-0536-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0536-5