Abstract
The aim of the study was to investigate the role of genistein in alleviating radiation-induced pneumonitis (RIP) through down-regulating levels of the inflammatory cytokines by inhibiting the expression of apurinic/apyrimidinic endonuclease 1/redox factor-1 (Ape1/Ref-1). Fifty female C57BL/6J mice (8 weeks old) were randomly divided into a control group, a pure irradiation (IR) group and a genistein + IR group. At the four time points after IR, hematoxylin, and Masson’s trichrome stainings were used to examine the pathological changes and collagen fiber deposition. Flow cytometry was used to detect reactive oxygen system (ROS) changes, EMSA was used to estimate the nuclear factor kappa B (NF-κB) transcriptional activities and an ELISA assay was used to measure the levels of TGF-β1, IL-1β, TNF-α, and IL-6 in the serum and bronchoalveolar lavage fluid (BALF) 2 weeks after IR. The pathological detection results showed acute inflammatory/fibrinoid exudation of the thoracic tissue after IR, which was significantly alleviated with genistein. The IR-induced an APE1 protein expression increase and NF-κB was effectively suppressed by genistein (P < 0.05). The induction of the inflammatory cytokines TGF-β1, IL-1β, TNF-α, and IL-6 by IR were in turn inhibited in the serum and BALF of the genistein-pretreated mice (P < 0.05). In addition, the ROS production was significantly boosted in the A549 cells after IR, which could be down-regulated by the pretreatment of genistein. The results demonstrate that genistein alleviates RIP by attenuating the inflammatory response in the initiation of RIP. A possible target of genistein is the Ape1/ref-1, which regulates key inflammatory cytokines by activating the NF-κB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy is one of the main treatments for thoracic tumor. However, serious complications in radiotherapy of the thoracic tumor can occur such as the radiation-induced thoracic injury, including radiation-induced pneumonitis (RIP), and radiation-induced fibrosis (RIF). Radiotherapy still lacks effective protection strategies and is considered as a dose-limiting factor [1]. Respiration is the key physiological activity, thus the initiation of RIP has significant impact on the respiratory capacity which limits the dose applied to lung cancer radiotherapy. Currently, it is widely recognized that the oxidative stress-induced inflammatory cytokine cascade mediates and amplifies the RIP [2–5]. It has been proved that nuclear factor kappa B (NF-κB), which is a key responsive transcription factor under oxidative stress, plays a crucial role in the expression of various inflammatory cytokines. Inhibiting NF-κB can effectively suppress the inflammatory response [6, 7]. A valid signal pathway ionizing radiation-activated NF-κB is important for selecting molecular targets to inhibit the NF-κB activity. Multifunctional protein apurinic/apyrimidinic endonuclease 1/redox factor-1 (Ape1/Ref-1) has been reported to be the redox activator of NF-κB. Inhibition of Ape1/Ref-1 may down-regulate the NF-κB DNA binding activity, whereas overexpression of Ape1/Ref-1 could activate NF-κB [8, 9]. Our previous studies have proven that 5,7,4/-genistein is a promising radiation protector which is worthy of further study [10].
Previous studies have demonstrated that genistein can enhance the effect of the radiotherapy for prostate cancer, by restraining the NF-κB activity by down-regulating the Ape1/Ref-1 expression [9]. However, the effect of possible tissue injury has not been reported. The cascading effect of the inflammatory cytokines mediates and amplifies the RIP, when the NF-κB activity is closely related to the release of the inflammatory cytokines. However, it remains unclear whether genistein exerts a protective effect on the RIP through decreasing NF-κB activity by down-regulating the Ape1/Ref-1 expression. We hypothesized that genistein exerts a protective effect on the RIP by the pathway: expression decrease of APE1/Ref-1 → down-regulation of NF-кB transcriptional activity → down-regulation of inflammatory factors. Thus, in this study, we detected APE1/Ref-1 expression at different stages after the RIP and after the administration of genistein. In the meantime, the changes in inflammatory factors in the mice serum and bronchoalveolar lavage fluid (BALF) were detected to analyze the protective effect of genistein on the RIP via the abovementioned pathway.
Materials and Methods
Animals and Cell Strains
A total of 50 female C57BL/6J mice (Experimental Animal Center of Daping Hospital, Third Military Medical University, Chongqing) aged 8 weeks and weighing (20 ± 2) g were used in the experiments. The mice were housed in facilities accredited at Experimental Animal Center of Daping Hospital, Third Military Medical University and were treated in accordance with approved protocols. In the main experiment, we used computer-generated random numbers to divide the mice into three experimental groups: control group (n = 10), pure irradiation (IR) group (n = 10) and genistein + IR group (n = 30). The genistein (200 mg/kg, purity ≥98 %, normal saline as the solvent, Sigma Inc. USA) was injected subcutaneously at 8, 16, and 24 h before IR [11, 12]. The mice were housed in a standard laminar flow animal room. Human A549 thoracic adenocarcinoma cell line (ATCC sequence number: CCL-185) was kindly presented by the Cancer Institute of Xinqiao Hospital, Third Military Medical University and cultured routinely.
Radiation
The mice in the control group received sham IR (0 Gy) of the thorax under the same conditions, and the mice in the other groups were treated with whole thorax IR of (12 ± 0.2) Gy. The mice were anesthetized intramuscularly with sodium pentobarbital (50 mg/kg) and fixed with Perspex jigs. All mice in groups B and C received a single dose of 8 MV X-ray IR by digital linear accelerator (ELEKTA Company) to the whole thorax, with a dose of 12 Gy (approximately 0.5 Gy/min) and SSD = 100 cm [13].
Pathological Observation of the Thoracic Tissues
The mice were killed and the right thoracic tissues were taken and placed in 10 % formaldehyde for fixation. The thorax was then embedded in paraffin, and sliced into 5 μm sections for hematoxylin (HE) and Masson’s trichrome stainings. The pathological tissue structure change was observed under microscope and the histopathological scores evaluated, i.e., the hamster mycoplasma pneumoniae pneumonia histopathology score system [14].
Detection of ROS Content With a Flow Cytometry
The 2.0 × 105/well of A549 cells at logarithmic growth phase were inoculated in a 24-well plate, with 0.5 ml per well. After adherence, the cells were divided into six groups: control group, pure IR with 5 Gy group (IR 5 Gy group), pure IR with 10 Gy group (IR 10 Gy group), pure genistein group, genistein + IR 5 Gy group, and genistein + IR 10 Gy group. Wherein, genistein with 30 μmol/l of final concentration was added 24 h before IR; IR with 5 or 10 Gy, respectively, was done with 8 MV X-ray, with absorption dose rate of 0.5 Gy/min. After IR, the cells were continued to be cultured for 24 h. A quantity of 1.7 mg DCF (Sigma Inc) was sufficiently dissolved with 500 μl of DMSO. Then, 50 mmol/l of NaOH solution was prepared, and 500 μl was added to the DCF. The incubation was carried out under room temperature for 30 min away from light. With the addition of 6 ml PBS into a centrifuge tube (15 ml in volume), the activated DCF was added and the tube was then placed over ice for reserve. After treatment, the cells were washed with PBS and DCF probe (1:20) was diluted with 2 % serum/PBS. Then, the cells were put in the wells for 1 h of culture. After the probe was loaded, the cells were collected and washed twice with PBS. Flow cytometry was applied to detect the reactive oxygen system (ROS) content in the cells.
Measurement of Ape1 Protein Expression by Western Blot
After IR, the protein was extracted from the mice right thoracic tissues. Then, the proteins were incubated with rabbit anti-human APE1/Ref1 polyclonal antibody (1:200, Novus Company) or rabbit anti-human β2 actin monoclonal antibody (1:2000, Pierce Inc.) for 11/2 h and incubated with HRP-labeled goat anti-rabbit secondary antibody (1:2,000, Pierce) for 1 h. The chemiluminescence kit (Pierce) was used for chromogenic assay.
Determination of NF-κB Expression by EMSA Method
The 2.0 × 105/well of A549 cells were inoculated on a 24-well plate for the following groups: the control group, pure genistein group, pure Ad5/F35 APE1 siRNA recombinant adenovirus group, genistein + Ad5/F35 APE1 siRNA recombinant adenovirus group, pure IR 5 Gy group, genistein + IR 5 Gy group, Ad5/F35 APE1 siRNA recombinant adenovirus + IR 5 Gy group, and genistein + Ad5/F35 APE1 siRNA + IR 5 Gy group. Genistein with 30 μmol/l of the final concentration was added 24 h before IR. The Ad5/F35 APE1 siRNA recombinant adenovirus (with infective dose of 10 MOI) was added 12 h before IR. IR with 5 Gy was done with 8 MV X-ray for all groups, with absorption dose rate of 0.5 Gy/min. After the IR, the culture was continued for 12 h. The cells were collected and nuclear protein (Pierce Company) was extracted for reserve according to the kit specification. The nuclear protein (Pierce Company) was extracted from the right thoracic tissues of each group after 3 days of IR in accordance with kit specification for reserve.
Determination of Inflammatory Factors in Serum and BALF by Using Double Antibody Sandwich ELISA Assay
After the mice were irradiated for 2 weeks, 1 % amyl barbital sodium (50 mg/kg) was intramuscularly injected for full anesthesia. Then, the eyeball was removed and the orbital venous blood was collected in a clean EP tube. After deposition for 30 min, the orbital venous blood was centrifuged for 3 min at 1,000 rpm, followed by collection of the supernatant in a clean EP tube for preservation at −70°C in the refrigerator. The neck skin was cut and the indwelling needle was used for endotracheal intubation. We used 1 ml of normal saline to wash the whole thorax for collection of the BALF. The procedure was repeated three times and a total of 2 ml BALF was collected. Then, the BALF was centrifuged at 4°C and at 1,000 rpm for 10 min and the supernatant was collected and preserved in −70°C refrigerator. The ELISA method (NeoBioscience) was used to determine the levels of IL-1β, TNF-α, IL-6, and TGF-β1 in the serum and BALF. A standard curve was drawn to calculate the concentration of these inflammatory factors.
Statistical Analysis
The SPSS 13.0 was used for statistical analysis and the results were expressed as mean ± SD. The pairwise comparisons of the single-factor analysis and t test were performed for the mean value of multiple samples. The differences were considered statistically significant at P < 0.05.
Results
Morphological Observation of Mice Thoracic Tissues
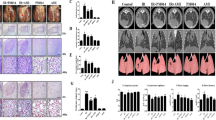
The thoracic tissue of mice in the control group had intact capillary wall, with no congestion of the pulmonary interstitial or inflammatory exudation of the alveolar and bronchiolar cavity (Fig. 1a). The pathological changes in the mice thorax in the pure IR group featured exudation at day 3 after IR, with congestion and edema in the pulmonary interstitial and capillary, many lymphocytes in the bronchoalveolar cavity and bronchioles, and edema fluid in some alveolus (Fig. 1b). The pathological changes at day 56 after IR, featured mainly chronic inflammation. The inflammatory cell infiltration of the lymphocytes could be seen around the bronchi and blood vessels, where the fibrinoid exudate could be found in the alveolar septa and part of the alveolar cavity (Fig. 1c). Acute inflammatory reaction was also found in the thoracic tissue of the genistein + IR group, but the congestion and edema of the alveolar cavity lymphocytes, thoracic interstitial and alveolar wall capillary and the alveolar inflammatory exudate were significantly alleviated in comparison to the pure IR group (Fig. 1d–f). Masson triple staining showed blue fibrous deposition in the pulmonary interstitial, while a little fibrous tissue could be found in the control group (Fig. 2a); the fibrous tissue started to proliferate in the pure IR group 14 days after IR (Fig. 2b); pulmonary fibrosis was formed at days 28 and 56 after IR, with disorder thoracic structure and a lot of green-dyed collagen fibers (Fig. 2c, d).
Determination of ROS Content
Compared with the control group, the content of intracellular ROS was significantly increased in the A549 cells after IR (P < 0.05). While the content of intracellular ROS in the genistein + IR 5 Gy group and genistein + IR 10 Gy group was significantly reduced, which showed insignificant difference in comparison with the control group but significant difference in comparison with IR 5 Gy group and IR 10 Gy group (P < 0.05) (Fig. 3), indicating that genistein can effectively suppress the production of ray-generated large number of reactive oxygen substances within the target cells.
Effect of Genistein on Ape1/Ref-1 Expression in Thoracic Tissues
Western blot results showed that APE1/Ref-1 expression of the thoracic tissue of the mice changed little at different time points in the control group but was first increased and then decreased in the pure IR group with the development of the RIP. At day 1 after IR, APE1/Ref-1 expression in the pure IR group began to increase and reached a peak on day 3, which was significantly higher (about 2.2 times) than that of the control group (P < 0.05). Then, with the development of RIP, APE1/Ref-1 expression in the pure IR group was gradually reduced to the level of the control group at day 28 and it was significantly lower than that of the control group at day 56, only about 1/5 of the control group (P < 0.05) (Fig. 4). Subcutaneous injection of 200 mg/kg of genistein was performed 24 h before IR. Western blot results after 3 days of IR showed that APE1/Ref-1 protein expression had reached the highest level in the pure IR group, followed by the control group and the genistein + IR group in descending order, suggesting that genistein could significantly antagonize the elevation of radiation-induced Ape1/Ref-1 expression (Fig. 5).
Detection of NF-κB Expression in Thoracic Tissues and A549 Cells With EMSA
After 3 days of IR, the nucleus protein was extracted from the right thorax of the mice from each group to detect NF-κB expression. The results showed that the NF-κB expression in the thoracic tissues of the control group was lower than the other groups, indicating that the NF-κB expression in the thoracic tissue was increased at various degrees after IR, with the most remarkable increase in the pure radiotherapy group. The NF-κB expression in the genistein 24 h + IR group showed no obvious increase, which indicates that rendering of genistein 24 h before IR could obviously reduce NF-κB expression in the thoracic tissues. While rendering of genistein 16 or 8 h before IR had no similar effect (Fig. 6). The NF-κB expression was clearly increased in the A549 cells after IR. While NF-κB expression in the A549 cells after pretreatment with genistein or Ad5/F35-APE1 siRNA was lowered to some extent. The NF-κB expression reached the lowest level after treatment with genistein + Ad5/F35-APE1 siRNA, as indicated that down-regulation of the Ape1/Ref-1 expression could restrain NF-κB activity (Fig. 7).
NF-κB expression in A549 cells by EMSA. 1. Control group; 2. pure genistein group; 3. pure Ad5/F35 APE1 siRNA recombinant adenovirus group; 4. genistein + Ad5/F35 APE1 siRNA recombinant adenovirus group; 5. pure IR 5 Gy group; 6. genistein + IR 5 Gy group; 7. Ad5/F35 APE1 siRNA recombinant adenovirus + IR 5 Gy group; and 8. genistein + Ad5/F35 APE1 siRNA + IR 5 Gy group
ELISA Analysis on Expressions of Inflammatory Cytokines in Serum and BALF of RIP in Mice
The ELISA method was used to measure the influence of genistein on protein expressions of IL-1β, TNF-α, IL-6, and TGF-β1 in serum and BALF of the mice.
IL-1β Protein Content
Compared with the control group (with the exception of the IL-1β protein content in BALF of the mice in genistein + IR group which was treated 24 h before IR), the IL-1β protein contents in the other groups had increased significantly (P < 0.05), indicating the existence of IL-1β high expression of IL-1β protein at radiation-induced thoracic injury. Compared with the pure radiation group, the expression of IL-1β proteins 24 h before IR was significantly decreased in the groups C and D (P < 0.05), while there was no significant changes of IL-1β protein expression in genistein + IR group at 8 and 16 h before IR (P > 0.05), suggesting that only subcutaneous injection of genistein 24 h before IR could achieve protections from thoracic injury and significantly lower the IL-1β protein content in the serum and BALF (Tables 1, 2).
TNF-α Protein Content
Compared to the control group, the serum TNF-α protein content of the mice in the genistein + IR group which was treated 24 h before IR showed no significant differences with that in the bronchoalveolar lavage of the genistein + IR group which was treated 16 and 24 h before IR (P > 0.05), but the TNF-α protein content was increased significantly in the other groups. Compared with pure IR group, the serum TNF-α protein contents of the mice were decreased significantly in the genistein + IR group before IR (P < 0.05), and the TNF-α content in BALF in the genistein + IR group which was treated 16 and 24 h before IR also had obvious difference (P < 0.05), which indicated that TNF-α was sensitive in predicting the influence of genistein on RIP (Tables 1, 2).
IL-6 Protein Content
Compared with the control group (except for the serum IL-6 of the mice in the genistein + IR group that was treated 16 h before IR), the IL-6 contents in the other groups was increased significantly (P < 0.05). Compared with pure IR group, the serum IL-6 content of the mice had increased significantly 8 h before IR in the genistein + IR group (P < 0.05) but decreased slightly in the other groups, with no significant change (P > 0.05). However, the IL-6 content in the BALF had decreased significantly in all groups (P < 0.05), indicating that the serum IL-6 content was not the proper forecasting index for measuring genistein’s influence on RIP in comparison with the bronchoalveolar lavage (Tables 1, 2).
TGF-β1 Protein Content
Compared with the control group, the TGF-β1 protein content showed high expression in the serum and the BALF of the mice with RIP in the other groups (P < 0.05), indicating that the TGF-β1 was a sensitive index for forecasting acute radioactive thoracic injury. Compared with the pure IR group, the serum TGF-β1 protein content of the mice 24 h before IR in the genistein + IR group (P < 0.05) and the TGF-β1 contents in the BALF in the genistein + IR group (P < 0.05) had decreased significantly, which indicated that TGF-β1 was sensitive in predicting the protective effect of genistein on the RIP (Tables 1, 2).
Discussion
Malignant thoracic tumors greatly threatens human life and radiotherapy is one of the most important treatment methods [15]. As the most common dose-limiting factor for thoracic tumor radiotherapy, the radiation-induced lung injury (RILI) including RIP and RIF has an incidence ratio as high as 5–15 %. RIP may lead to serious consequences and an ideal radioprotectant is urgently needed [16].
The animal models of RIP are essential to further investigate the mechanism and protection of RIP. Rodent models of RIP are currently the most widely used. Though small dosage with repeated IR corresponds with routine treatment, fractionated IR is commonly used in short-term research. William et al. [17] used 6 MV X-ray for single IR with mice with 30 Gy right thorax, and observed the changes for 2 months after IR. In this study, the 8-week-old female C57BL/6J mice were used and the mice RIP model was constructed using single IR of the whole thorax with 12 Gy. The inflammatory pathological change and collagenous fiber deposit of the thorax were tested via HE and Masson staining. The expressions in inflammatory factors included TNF-α, IL-1β, IL-6, and TGF-β1 and were tested by the ELISA method.
RILI is a complicated pathological process that is regulated by the various factors produced by the interaction of cells. Once the target cells are damaged by radiation, the cytokines (TNF-α, IL-1, IL-6, etc.) will be released to induce acute inflammatory response, which in turn induce cytokines to activate the macrophages releasing TGF-β1, boost fiber metrocyte transformation, fibroblast proliferation and matrix protein synthesis, and ultimately lead to pulmonary interstitial fibrosis [18, 19]. “Remote contingent effects” of the RIP further proves that the cascade effect of the inflammatory cytokines is the key in mediating and magnifying RIP. In this study, the ELISA method was used to test the protein expressions of TNF-α, IL-1β, IL-6, and TGF-β1 in the mice serum and BALF and found that ionizing radiation could cause a remarkable increase in the expression of the four inflammatory factors. The results of the study are in accordance with other domestic and foreign research results indicating that blood plasma TGF-β1 level could be a predictive factor for RIP [5, 20–22].
As the main component of the soy isoflavones, 5,7,4′/-genistein is a special type of polyphenolic compound. Our previous studies have demonstrated that genistein is a promising radio-protector [10]. NF-κB plays a key role in mediating release of the inflammatory cytokines [6, 7]. A number of recent studies have proved that genistein quenching radiation-induced radical and inhibiting oxidized stress-induced NF-κB DNA binding activity are the main molecular basis for its radiation protective effect [23–25]. Other studies have also demonstrated that genistein reduces the activity of NF-κB by decreasing Ape1/Ref-1 expression [9]. In this study, the Western blot method was used to observe Ape1/Ref-1 expression at different stages after RIP and the results showed that the Ape1/Ref-1 expression was increased on day 1 after IR, reached peak at day 3 (about 2.2 times of control group); later, with alleviation of RIP, Ape1/Ref-1 expression was lowered to the level of control group at day 28 and was obviously lower than the control group at day 56 (about 1/5 of the control group). This, on one hand, was possibly related with large amount of ROS produced by ionizing radiation, which exceed the capacity of antioxidant system in the thoracic cell. Ape1/Ref-1 protein was then activated to up-regulate of the DNA binding activity of the transcription factors in response to oxidative stress. On the other hand, with development of RIP, the imbalance between repair capacity of Ape1/Ref-1 and DNA damage is then formed during thoracic injury repair [26]. Genistein pretreatment was performed 24 h before IR. The Western blot method showed that Ape1/Ref1 protein expression in the pure IR group ranked the highest, with control group the second, pure genistein the third and genistein + IR the least, which indicates that pretreatment with genistein can reduce the radiotherapy-induced expression increase of Ape1/Ref1 protein. As the redox activator of NF-κB, Ape1/Ref-1 can restrain NF-κB activity under the down-regulated expression but activate NF-κB activity under the up-regulated expression [8, 9]. The in vitro and in vivo experiments in this study have displayed the similar results: NF-κB expression was obviously increased after IR, and obviously decreased after pretreatment with genistein or Ad5/F35-APE1 siRNA adenovirus and reached the lowest level after synergetic treatment with genistein + Ad5/F35-APE1 siRNA adenovirus, as further proves that Ape1/Ref-1 is the redox activator of NF-κB.
Because NF-κB plays a key role in mediating the release of the inflammatory cytokines [6, 7], it was presumed that down-regulating the Ape1/Ref-1 expression could influence inflammation and the release of the inflammatory cytokines. The results of this study indicated that subcutaneous injection of genistein at 24, 16, and 8 h before IR, showed a change in acute inflammation in all groups. However, compared with the pure IR group, the scope of inflammation decreased, the congestion in the thoracic mesenchyme and alveolar wall capillary was alleviated and the inflammatory exudation reduced. This demonstrated that the use of genistein before radiotherapy could obviously mitigate the acute radioactive pneumonia. The results also showed that radiation could significantly stimulate production of ROS, while genistein could effectively inhibit intracellular ROS production after IR. The ELISA method was used to test the influence of genistein on the expressions of inflammatory cytokines such as IL-1β, TNF-α, IL-6, and TGF-β1 in the serum and BALF and found that in order to reduce the expressions of the inflammatory cytokines, subcutaneous injection of genistein should be done within 24 h before IR. Compared with IL-1β, TNF-α was a more sensitive factor for predicting the protection role of genistein against RIP. While IL-6 level in BALF was a more reasonable parameter for predicting the protection role of genistein against RIP in comparison with IL-6 level in the serum. As for TGF-β1, it was a sensitive predictive parameter not only for acute RIP, but also for the protection effect of genistein against RIP.
In conclusion, genistein could protect against RIP, when one of the mechanisms is possibly to reduce synthesis of the inflammatory cytokine through decreasing Ape1/Ref-1 protein expression. With development of the molecular biology, more molecular mechanism of genistein protection will be disclosed, which will definitely provide a new and effective therapy for alleviation of RIP.
References
Hosseinimehr, S. J. (2007). Trends in the development of radioprotective agents. Drug Discovery Today, 12, 794–805.
Rubin, P., Finkelstein, J., & Shapiro, D. (1992). Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: Interrelationship between the alveolar macrophage and the septal fibroblast. International Journal of Radiation Oncology, Biology, Physics, 24, 93–101.
Beinert, T., Binder, D., Stuschke, M., Jorres, R. A., Oehm, C., Fleischhacker, M., et al. (1999). Oxidant-induced lung injury in anticancer therapy. European Journal of Medical Research, 4, 43–53.
Arpin, D., Perol, D., Blay, J. Y., Falchero, L., Claude, L., Vuillermoz-Blas, S., et al. (2005). Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. Journal of Clinical Oncology, 23, 8748–8756.
Chen, Y., Hyrien, O., Williams, J., Okunieff, P., Smudzin, T., & Rubin, P. (2005). Interleukin (IL)-1A and IL-6: Applications to the predictive diagnostic testing of radiation pneumonitis. International Journal of Radiation Oncology, Biology, Physics, 62, 260–266.
Haase, M. G., Klawitter, A., Geyer, P., Alheit, H., Baumann, M., Kriegel, T. M., et al. (2003). Sustained elevation of NF-kappaB DNA binding activity in radiation-induced lung damage in rats. International Journal of Radiation Biology, 79, 863–877.
Linard, C., Marquette, C., Mathieu, J., Pennequin, A., Clarencon, D., & Mathe, D. (2004). Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: Effect of an NF-kappaB inhibitor. International Journal of Radiation Oncology, Biology, Physics, 58, 427–434.
Ando, K., Hirao, S., Kabe, Y., Ogura, Y., Sato, I., Yamaguchi, Y., et al. (2008). A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Research, 36, 4327–4336.
Raffoul, J. J., Banerjee, S., Singh-Gupta, V., Knoll, Z. E., Fite, A., Zhang, H., et al. (2007). Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Research, 67, 2141–2149.
Zhou, Y., & Mi, M. T. (2005). Genistein stimulates hematopoiesis and increases survival in irradiated mice. Journal of Radiation Research, 46, 425–433.
Messina, M. J., & Loprinzi, C. L. (2001). Soy for breast cancer survivors: A critical review of the literature. Journal of Nutrition, 131, 3095S–3108S.
Day, R. M., Barshishat-Kupper, M., Mog, S. R., McCart, E. A., Prasanna, P. G., Davis, T. A., et al. (2008). Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. Journal of Radiation Research, 49, 361–372.
Calveley, V. L., Khan, M. A., Yeung, I. W., Vandyk, J., & Hill, R. P. (2005). Partial volume rat lung irradiation: Temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. International Journal of Radiation Biology, 81, 887–899.
Ghafoori, P., Marks, L. B., Vujaskovic, Z., & Kelsey, C. R. (2008). Radiation-induced lung injury. Assessment, management, and prevention. Oncology, 22, 37–47. discussion 52–53.
Milano, M. T., Constine, L. S., & Okunieff, P. (2007). Normal tissue tolerance dose metrics for radiation therapy of major organs. Seminars in Radiation Oncology, 17(2), 131–140.
Travis, E. L., & De Luca, A. M. (1985). Protection of mouse lung by WR-2721 after fractionated doses of irradiation. International Journal of Radiation Oncology, Biology, Physics, 11, 521–526.
Williams, J. P., Brown, S. L., Georges, G. E., Hauer-Jensen, M., Hill, R. P., Huser, A. K., et al. (2010). Animal models for medical countermeasures to radiation exposure. Radiation Research, 173, 557–578.
Wang, S., Liao, Z., Wei, X., Liu, H. H., Tucker, S. L., Hu, C. S., et al. (2006). Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). International Journal of Radiation Oncology, Biology, Physics, 66, 1399–1407.
Hart, J. P., Broadwater, G., Rabbani, Z., Moeller, B. J., Clough, R., Huang, D., et al. (2005). Cytokine profiling for prediction of symptomatic radiation-induced lung injury. International Journal of Radiation Oncology, Biology, Physics, 63, 1448–1454.
Johnston, C. J., Piedboeuf, B., Rubin, P., Williams, J. P., Baggs, R., & Finkelstein, J. N. (1996). Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiation Research, 145, 762–767.
Rube, C. E., Uthe, D., Schmid, K. W., Richter, K. D., Wessel, J., Schuck, A., et al. (2000). Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. International Journal of Radiation Oncology, Biology, Physics, 47, 1033–1042.
Sont, J. K., De Boer, W. I., van Schadewijk, W. A., Grunberg, K., van Krieken, J. H., Hiemstra, P. S., et al. (2003). Fully automated assessment of inflammatory cell counts and cytokine expression in bronchial tissue. American Journal of Respiratory and Critical Care Medicine, 167, 1496–1503.
Ullmann, K., Wiencierz, A. M., Muller, C., Thierbach, R., Steege, A., Toyokuni, S., et al. (2008). A high-throughput reporter gene assay to prove the ability of natural compounds to modulate glutathione peroxidase, superoxide dismutase and catalase gene promoters in V79 cells. Free Radical Research, 42, 746–753.
Zavodnik, L. B., Shkodich, A. P., Wielanek, M., Egorov, A. I., Zavodnik, I. B., Popov, Iu V, et al. (2006). Antioxidative effects of isoflavon genisteine-8-C-glycoside in vitro and in vivo. Eksperimental’naya i Klinicheskaya Farmakologiya, 69, 48–52.
Sarkar, F. H., & Li, Y. (2002). Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer and Metastasis Reviews, 21, 265–280.
Evans, A. R., Limp-Foster, M., & Kelley, M. R. (2000). Going APE over ref-1. Mutation Research, 461, 83–108.
Acknowledgments:
We thank Ms. Yu-Xin Yang, and Ms. Ling Liao for their kind and excellent technical assistance. This study was supported by grants from the National Natural Science Foundation of China (No. 30970865, 81272499, 81101783), National Natural Science Foundation of Chongqing (CSTC2010BB5030) and Initial Fund for Returnees of Third Military Medical University (2009XHG17) to Prof. Zhen-Zhou Yang and Military Twelfth Five Key Projects (BWS11J038) to Guo-Dong Liu.
Disclosures
There is no any conflict of interests to disclose.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guo-Dong Liu and Lei Xia contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, GD., Xia, L., Zhu, JW. et al. Genistein Alleviates Radiation-Induced Pneumonitis by Depressing Ape1/Ref-1 Expression to Down-regulate Inflammatory Cytokines. Cell Biochem Biophys 69, 725–733 (2014). https://doi.org/10.1007/s12013-014-9859-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9859-x