Abstract
The purpose of this study is to compare the efficacy and safety of Gefitinib versus VMP in combination with three-dimensional conformal radiotherapy (3D-CRT) for multiple brain metastases from non-small cell lung cancer (NSCLC). A total of 73 NSCLC patients with brain metastases from January 2010 to August 2013 were randomly divided into Gefitinib group (37 patients) and VMP chemotherapy group (36 patients). Patients in VMP group recieved VM-26 100 mg/day by intravenous injection, from day 1 to day 3, cisplatin 25 mg/m2 by intravenous injection, from day 1 to day 3. One cycle was defined as a 21-day therapy duration, with a total of 3 cycles; 2 cycles were used for consolidation. Patients in Gefitinib group received Gefitinib orally. Both groups received 3D-CRT, DT50 Gy/25f/35d from first day and target areas were treated with whole brain radiotherapy. The results of the study are listed below: There was no significant difference in the short-term effects of the two groups (P > 0.05). Median survival time (MST) of Gefitinib was 13.3 months whereas median survival time of VMP group is 12.7 months (P < 0.05). In Gefitinib group, we did not observe any difference of the median survival time between the patients with and without mutation EGFR. Toxicity of Gefitinib groups were characterized by rash, whereas chemotherapy resulted in hematologic toxicities, which included 6 cases of III/IV leucopenia (17.6 %), 3 cases of anemia (8.8 %), and 5 cases of thrombocytopenia (14.7 %), and non-hematological toxicity which was less serious symptoms for gastrointestinal disorders, hair loss, etc. These adverse reactions can be released after symptomatic treatment. No treatment-related deaths occurred. Two patients in VMP group quit due to IV leucopenia. Both oral Gefitinib and systemic VMP chemotherapy in combination with three-dimensional conformal radiotherapy (3D-CRT) could be used to treat brain metastases from non-small cell lung cancer. There were no difference in the short-term effects of the two groups, but long-term effect of Gefitinib group was slightly better than VMP group. Moreover, Gefitinib group showed low toxicity. All together, our finding implicated that Gefitinib is an effective method for patients with brain metastases from NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the highest incidences of malignant tumors, of which non-small cell lung cancer accounts for about 80 %. In recent years, considerable progress in treatment has been made for non-small cell lung cancer (NSCLC) and patient survival times were prolonged. However, incidence of brain metastases from NSCLC is also increased up to 25–54 % [1, 2]. Brain metastases from NSCLC cause not only shorter median survival time and poor prognosis, but seriously affect the quality of life. Brain radiotherapy remains a standard treatment of multiple brain metastases; however, it only prolonged median survival time in most of the patients with 4–6 months [3]. The commonly used chemotherapy drugs have the limited role of brain metastases owing to difficulties in entering or through the blood–brain barrier. VM-26 is a semi-synthetic derivative of podophyllotoxin, lipophilic, which can penetrate the blood–brain barrier [4]. Small-molecule tyrosine kinase inhibitors (TKIs), Gefitinib, targeting the epidermal growth factor receptor (EGFR), is able to competitively combine ATP and inhibit self-phosphorylation of EGFR in the intracellular tyrosine kinase domain and blocks downstream signaling, so as to inhibit cancer cell proliferation, invasion, and metastasis [5, 6]. For lung cancer patients with brain metastases, fewer studies are available for targeting drugs in combination with radiotherapy. Here, in order to clarify the efficacy and side effects of Gefitinib, we randomly divided NSCLC patients with multiple brain metastases into Gefitinib group and VMP regimes group in combination with radiotherapy and compared their short-term effect, overall survival and toxicity.
Patients and Method
Patients
A total of 73 patients were enrolled for the study from January 2010 to January 2013 (Table 1). Eligibility criteria: All patients had to have histologically or cytologically diagnosed NSCLC, with measurable brain metastases assessed with contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the brain; Patients should have no other serious medical problems and history of brain radiation therapy. Moreover, blood test, and liver and kidney functions should be normal prior to treatment; Karnofsky score ≥70 and age ≤75 years old. Gefitinib group: 25 males, 12 females, median age of 61 years old; VMP chemotherapy group: 23 males and 13 females, median age 62 years. Gefitinib group: 19 cases of adenocarcinoma and 18 cases of non-adenocarcinoma; VMP group: 17 cases of adenocarcinoma and 19 cases of non-adenocarcinoma. In Gefitinib Group, 9 patients were detected with EGFR mutations.
Treatment
(1) Gefitinib group: daily oral administration of 250 mg Gefitinib started at first day of radiation therapy until disease progression or patients drop because of unacceptable side effects, deaths, and economic reasons; VMP regimen group: intravenous infusion of 100 mg/d VM-26 from day 1 to day 3; intravenous infusion of cisplatin 25 mg/m2 from day 1 to day 3; one cycle was defined as a 21-day therapy duration, with a total of 2 cycles; radiotherapy starting from the first day of chemotherapy. Under allowable physical conditions, concurrent chemotherapy and radiotherapy for another 2–4 cycles. (2) Radiotherapy: Siemens linear accelerators equipped with 6-MV energy X-ray, three-dimensional treatment planning systems TPS STAR2000, multi-leaf collimator technology, and dose-volume histogram (DVH) optimization for treatment planning was used for the therapy. Patients were treated in supine position with hands along sides. Mobile laser beacons were used to mark relative position of patients and patients were immobilized with vacuum mattress. Positions of patients, vacuum mattress, and bed were kept unmovable during the therapy. Once finished CT scan, CT reconstruction figures were input into three-dimensional treatment plans system. Border clinical target district volume (CTV) was defined with whole cranial bone window and planning target district volume (PTV) was determined by clinical target district volume (CTV) plus 0.5–1 cm. Target district volumes were finalized by imaging technician and radiologist. Organ-threatening dose limits of three-dimensional conformal radiotherapy were as follows: brainstem maximum ≤50 Gy dose; prescription dose 50 Gy.
Evaluation Criteria
Response evaluation was performed 2 months after the end of radiotherapy in accordance with response evaluation in solid tumors (RECIST) criteria. Responses of the tumors were classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Toxicity was evaluated by CTCAE 3.0 criteria. All cases were followed up after the end of treatment every 3 months in first year, thereafter every 6 months. The follow-up consisted of CT or MRI of the brain, and quality of life (ADL scoring criteria: all patients were measured after 2 months after treatment. Score ≥10 indicated improvement. Score ≥10 indicated deterioration. In between was stable). OS definition: duration from first day of Gefitinib or VMP to patient death or end of follow-up time.
Statistics
SPSS16.0 was used for statistical purpose and Kaplan–Meier method was used for survival analysis.
Results
Therapy and Follow-up
Except 2 patients in VMP Group who quit due to IV leucopenia, the remaining finished the treatment. As of June 5, 2014, the rate of follow-up was 94.5 % at a median follow-up of 13.6 months. 4 cases failed with follow-up.
Short-term Effects
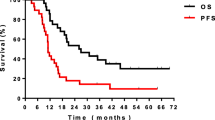
The efficacy was evaluated 2 month after the end of radiotherapy. CR, PR, SD, and PD rates of Gefitinib group were 7.4 % (2/37), 48.6 % (18/37), 40.54 % (15/37), and 5.4 % (2/37), respectively. CR, PR, SD, and PD rates of VMP group were 2.9 % (1/34), 44.1 % (15/34), and 47 % (16/34), 5.8 % (2/34) respectively. Total response rate (RR) was 54 % of Gefitinib group and 47 % of VMP group. Difference between the two groups was not statistically significant (x 2 = 0.0076, P = 0.9306) (Fig. 1).
Long-term Effects
Median survival time (MST) of Gefitinib group was 13.3 months whereas median survival time of VMP group was 12.7 months (P < 0.05), 1-year overall survival rate of Gefitinib group was 51.35 % (19/37) and 50 % (17/34) for VMP group. There was no statistically significant difference for that (x 2 = 0.379, P = 0.538) (Fig. 1).
Toxicity
There were no treatment-related deaths, no allergic reactions, no fluid retention, no peripheral nerve toxicity, and no hepatic and renal dysfunction found during treatment. Principal adverse reactions of Gefitinib were characterized by rashes 70.3 % (26/37). One of the VMP group was mainly for hematologic toxicities, which results in III/IV Leucopenia, anemia, and thrombocytopenia and there were 6 cases (17.6 %), 3 (8.8 %), and 5 (14.7 %), respectively. Other toxicities are mainly gastrointestinal symptoms 79.4 % (27/34). These adverse reactions could be released after symptomatic treatment. 2 patients in VMP group quit due to IV leucopenia.
Discussion
The most common brain metastases are from lung cancer. NSCLC accounts for 70–80% of lung cancer. There were 19.9 % brain metastases from newly diagnosed NSCLC patients according to reports. Lung cancer patient with brain metastases had shorter median survival time, poor prognosis, and poor quality of life. Only a few patients with brain metastases can be treated by surgery or stereotactic radiosurgery, but for most of them, whole brain radiotherapy remains a standard treatment though it has less effect on the survival time of the patients. As known, most of the chemotherapy drugs are limited for intracranial lesions because of the existence of the blood–brain barrier [7]. Therefore, finding a drug that can target lesions and work both inside and outside brain becomes particularly important.
VM-26 is a highly lipid-soluble drug, small molecular weight, able to cross the blood–brain barrier, which mainly targets cancer cells in G2 and M phase [4]. DDP can sensitize patients to radiotherapy; meanwhile, radiation therapy can promote the chemotherapy drugs across the blood–brain barrier. Small brain lesions, which are undetected by current imaging method, are susceptible to chemotherapy drugs. Furthermore, chemotherapy can kill primary and other parts of subclinical lesions. Chemotherapy in combination with whole brain radiation therapy can further stimulate permeability of blood–brain barrier, in that it will facilitates chemotherapy drugs into the brain and enhances radiation therapy effects. In this study, our data also showed that VMP in combination with whole brain radiation therapy was an effective treatment and had beneficial outcomes for patients’ short-term and long-term observation. Our data supported its effectiveness in clinical application.
Gefitinib is a highly selective epidermal growth factor receptor tyrosine kinase inhibitor and hence, it has been recommended for two or three lines treatment of advanced non-small cell lung cancer [8–10]. Pre-clinical studies [11] showed that HC radiolabeled Gefitinib could be found in the CNS of healthy mice after oral dose of Gefitinib reached peak plasma concentrations, which suggested that Gefitinib could penetrate the blood–brain barrier. When brain or meningeal metastasis occurred, immature angiogenesis and edema caused by tumor might lead to the destruction of the blood–brain barrier to help TKIs uptake and increase TKIs concentration in cerebrospinal fluid [12]. Wang et al. [13] reported Gefitinib in the cerebrospinal fluid of patients with brain metastases had higher permeability than patients without metastases. In 2003, Villano, [14] first reported treatment of Gefitinib in 1 case of brain metastases from NSCLC was effective. Since then, more related studies on Gefitinib in treatment of brain metastases from lung cancer were conducted by scholars at home and abroad [15, 16]. Here, we found that median survival time, OS, and 1-year survival rate of Gefitnib group were beyond most of the previously reported, which might be due to the increased radiotherapy dose to 50 Gy and brain lesions were better controlled by radiotherapy, at the same time radiotherapy improved permeability of blood–brain barrier and increased eligibility of Gefitinib to intracranial lesions. Improved long-term effects by Gefitinib might owe to less side effects and improvement of patients’ general situation.
With the advancement of modern medicine, strategy of cancer patient treatment has being changed. It required that clinicians should consider not only how to prolong their lifetime, more importantly, but how to improve the patient’s quality of life. In this study, we compared clinical efficacy and toxicity of Gefitinib verus VMP in combination with whole brain radiotherapy for advanced non-small cell lung cancer patients with brain metastases and found that both were efficient, both group of short-term curative effects had no difference but median survival time of Gefitinib group was better than VMP group, which might be due to Gefitinib showing less side effects. Toxicity of Gefitinib versus VMP: rashes and diarrhea were more common in Gefitinib group; hematological toxicity and gastrointestinal toxicity were more common in VMP group with rare cases of rashes and diarrhea. Compared to the unspecific cell toxicity of chemotherapy, targeted drugs have less side effects, especially third-generation all human derived small-molecule targeting drugs have even lower immune-related toxicity. Since qualities of life between the two groups were significantly different, we believe that the application of Gefitinib could improve the quality of life of lung cancer patients with brain metastasis more, though most of patients couldn’t get genetic testing in time.
In conclusion, lung cancer patients with brain metastases receiving Gefitinib and VMP regimen in combination with whole brain radiation therapy showed well responses: progression-free survival and overall survival were satisfactory, and toxicity could be tolerated. Both could be used as treatment options for lung cancer patients with brain metastases. However, Gefitinib in combination with radiotherapy would be a better choice for the patients who could not tolerate toxicity of chemotherapy. We should note that there are still difficulties for Gefitinib clinical applications. For example, it is hard to obtain brain metastases sample and detect EGFR mutations, which would hinder its application and get tumor molecular characteristics [17] Because of heterogeneity of EGFR mutations between primary tumors and metastases, there are still problems in forecasting metastases EGFR mutations according to primary tumor characteristics. So far we can’t decide whether it is worthwhile to apply targeted drugs in combination with radiotherapy in patients with good physical conditions and financial conditions. This requires more work and needs a large prospective study. However, we believe that we will be closer to bright future in the treatment of lung cancer with brain metastasis as time goes on and the research deepens.
References
Schouten, L., Rutten, J., Huveneers, H., & Twijnstra, A. (2002). Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer, 94, 2698–2705.
Siker, M. L., & Mehta, M. P. (2007). Resection versus radiosurgery for patients with brain metastases. Future Oncology, 3, 95–102.
Soffietti, R., Ruda, R., & Mutani, R. (2002). Management of brain metastases[J]. Journal of Neurology, 249(1O), 1357–1369.
Postmus, P. E., Haaxma-Reiche, H., Smit, E. F., et al. (2000). Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with wholebrain radiotherapy-a phase III study of the European organization for the research and treatment of lung cancer cooperative group [J]. Journal of Clinical Ontology, 18(19), 3400–3409.
Maemondo, M., Inoue, A., Kobayashi, K., et al. (2010). Gefitinib or chemot herapy for non-small-cell lung cancer with mutated EGFR [J]. New England Journal of Medicine, 362(25), 2380–2388.
Zou, Y. M., Xiong, H., & Yu, S. Y. (2008). The effect of Gefitinib on the quality of life in patients with advanced non-small cell lung cancer[J]. China Oncology, 18(12), 925–928.
Furuse, K., Kamimofi, T., kawahara, M., et al. (1997). A pilot study of concurrent w hole-brain radiotherapy and chemotherapy combined with cisplatin, vindesine and mitomycin in non-small-cell lung cancer with brain metastases[J]. British Journal of Cancer, 75(4), 614–618.
Mckillop, D., Hutchison, M., Partridge, E. A., et al. (2004). Metabolic disposition of Gefitinib, an epidermal growth factor receptor tyrosine kinase inhibit or in rat, dog and m an[J]. Xenobiotica, 34(10), 917–934.
Yan, D. F., Yan, S. X., Yang, J. S., et al. (2010). Hemrrhage of brain metastases from non- small cell lung cancer post Gefitinib therapy: two case reports and review of the literature[J]. BMC Cancer, 10, 49.
Katz, A., & Zalewski, P. (2003). Quality-of-life benefits an devidence of antitumour activity for patients with brain metastases treated with Gefitinib[J]. British Journal of Cancer, 89(Suppl 2), S15–S18.
Mckillop, D., Hutchison, M., Partridge, E. A., et al. (2004). Metabolic disposition of Gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor in rat, dog and man [J]. Xenobiotica, 34(10), 917–934.
Fukuhara, T., & Sakakibara, T. (2008). Successful treatment of carcinomatous mengingitis with Gefitinib in a patient with lung adenocarcinoma harboring a mutated EGF receptor gene[J]. Tohoku Journal of Experimental Medicine, 214(4), 359–363.
Wang, M.,Jing, Z., Min jiang, C.(2011). Cerebral penetration of Gefitinib inpatients with lung adenecarcinoma [J]. Journal of Clinical Oncology 29 ,(Supp1):a7608 .
Villano, J. L., Maucer, A. M., & Vokes, E. E. (2003). A case study documenting the anticancer activity of ZD1839(Iressa) in the brain[J]. Annals of Oncology, 14(4), 656–658.
Ceresoli, G. L., Cappuzzo, F., Gregorc, V., et al. (2004). Gefitinib in patients with brain metastases from non small cell lung cancer: a prospective trial [J]. Annals of Oncology, 15(7), 1042–1047.
Wu, C., Li, Y. L., Wang, Z. M., et al. (2007). Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain [J]. Lung Cancer, 57(3), 359–364.
Moran, C. (2011). Importance of molecular features of non-small cell lung cancer for choice of treatment[J]. American Journal of Pathology, 178(5), 1940–1948.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Ning, F., Liu, C. et al. Comparison of Gefitinib Versus VMP in the Combination with Radiotherapy for Multiple Brain Metastases from Non-small Cell Lung Cancer. Cell Biochem Biophys 71, 1261–1265 (2015). https://doi.org/10.1007/s12013-014-0286-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0286-9