Abstract
This study aims to explore the changes in calcium regulation in the sarcoplasmic reticulum (SR) during doxorubicin (DOX) treatment. Sprague–Dawley rats were treated with intravenous DOX (1.5 mg/kg) twice weekly for 12 treatments. The hemodynamic changes, myocardial oxidative stress, levels of cardiac toxicity markers, and calcium handling of the myocardial SR were observed. When the accumulation of DOX reached 12 mg/kg, (1) heart weight, left ventricular mass, and lung congestion increased significantly, and ascites appeared; (2) SBP, DBP, MAP, +dP/dt, −dP/dt, and LVSP decreased significantly, and LVEDP increased (p < 0.01); (3) the iNOS activity and MDA and NO concentrations significantly increased, while the SOD decreased (p < 0.05 or 0.01); (4) the serum level of the AST, LDH CPK, cTnI, and BNP increased significantly (p < 0.01); (5) during DOX treatment, the rat SR Ca2+ absorption function and Ca2+-stimulated ATPase activity declined dramatically, as did the SERCA2 and phospholamban levels (p < 0.01). As expected, all these changes became evident with DOX accumulation in vivo (p < 0.05 or 0.01). In conclusion, DOX induces SR calcium regulation dysfunction via the decrease of SERCA2 and phospholamban expressions in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin (DOX) is a highly effective chemotherapeutic agent, but its clinical use is limited due to the development of dose-dependent cardiomyopathy [1]. Chemotherapy with DOX can cause severe cardiomyopathy, leading to fatal congestive heart failure [2]. The maximum dose is usually limited to 450–500 mg/m2 body surface area because the incidence of the cardiomyopathy is “low” below this dose. However, more than half of the patients could tolerate a higher dose without developing cardiomyopathy, whereas a small percentage of patients will, with these low doses [3, 4]. Endo-myocardial biopsy is used to monitor DOX cardiomyopathy. Billingham [5] described the morphological changes observed on patients’ biopsies receiving DOX. The earliest changes are distended sarcoplasmic reticulum (SR) and early myofibrillar loss. Later changes include diffuse cell damage with multiple cellular organelles. These morphologic SR abnormalities have been described in animal models as well [6]. DOX-induced cardiomyopathy is characterized by abnormal cytosolic concentration of Ca2+ [7–9]. In cardiomyocytes, the SR determines the cytosolic level of Ca2+ via the ATP-dependent Ca2+-pump mechanism [10]. The SR regulates the intracellular calcium stores on which adult mammalian cardiac muscle contracts [11]. Studies have focused on the in vitro effects of anthracyclines on the pump and channel function of this subcellular membrane system. DOX induces calcium release from isolated SR vesicles and in skinned cardiac fibers [12]. It binds to the calcium release channel in fractions, enriched in terminal cisternae, and increases the open probability of calcium release channels in reconstituted lipid bilayers [9]. Doxorubicinol, a metabolite of DOX, is a potent inhibitor of multiple intracellular pumps. However, higher anthracycline concentrations are required to mediate effects on the calcium-dependent ATPase rather than on the calcium release channel. Thus, there is compelling evidence that anthracyclines alter the calcium regulation function of the SR in vitro. However, the exact mechanism of SR calcium regulation dysfunction in vivo is unknown. The purpose of this study was to explore the effects and mechanism of DOX administration on SR calcium regulation function.
Materials and Methods
Ethics Statement
All protocols were approved by the Animal Care Committee of the Shanghai Jiaotong University in accordance with the standards of the China Council on Animal Care (SCXK (Hu) 2008-0016).
Experimental Model
Eighty healthy male Sprague–Dawley rats (180 ± 20 g) were used in this study. Animals were kept under controlled temperature conditions (20 ± 2 °C), relative humidity (55–65 %), and 12 h light/12 h dark cycle. The animals were fed standard chow, and tap water was supplied ad libitum.
Reagents
Doxorubicin (Pharmacia, North Peapack, NJ, USA) was dissolved in sterile saline and administered intravenously; each containing 1.5 mg/kg, twice a week, over a period of 6 weeks for a total cumulative dose of 18 mg/kg body weight.
Experimental Design
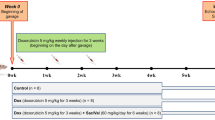
The rats were divided into the following groups. The sham control group comprised 20 animals that received a standard chow diet for 6 weeks. The DOX-treated groups comprised 20 animals each: The rats were treated with intravenous DOX (1.5 mg/kg) twice weekly for 2, 4, or 6 weeks.
Hemodynamics
Twenty-four hours after the last administration, the rats were anesthetized with urethane (1.2 g/kg, intraperitoneal) and then placed supine on an operating table. The right common carotid artery was isolated using the blunt dissection technique to measure systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR). A ventricular cannula was filled with 1 % heparin and inserted into the right carotid artery to connect to the biopac multichannel physiologic signal collection and processing system via a pressure transducer. Then, the cannula was slowly pushed and positioned in the left ventricular cavity to record the left ventricular end-diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP), maximum rate of contraction (+dP/dt max), and maximum rate of relaxation (−dP/dt max).
Oxidative Stress and Biochemical Examination
After 2, 4, or 6 weeks of DOX treatment, 4 ml blood from the abdominal aorta was collected from each sample. To half of the samples, we added 2 % EDTA·Na2, and to the other half we added nothing. After treatment, the samples were centrifuged for 15 min at 3,000 rpm. We isolated the plasma and serum in each sample and detected superoxide dismutase (SOD), malondialdehyde (MDA), nitric oxide (NO), inducible nitric oxide synthase (iNOS), lactate dehydrogenase (LDH), serum creatine phosphokinase (CPK), aspartate aminotransferase (AST), cardiac troponin I (cTnI), and brain natriuretic peptide (BNP) by Elisa according to the instructions.
Weight Measure
The rats were killed by KCl bolus injection, and the lungs and hearts were taken out immediately after thoracotomy. Fresh and dry lungs were weighed and the wet/dry weight ratio was calculated. The hearts were rinsed in cold saline and then dried by filter paper. The atriums and right ventricles were cut off along the coronary and interventricular groove, and were weighed.
Isolation of SR Membrane
The membrane fraction enriched in SR was isolated according to Ganguly et al. [13]. Briefly, viable left ventricular tissue from the hearts was homogenized in a Waring blender in medium containing 10 mM NaHCO3, 5 mM NaN3, and 15 mM Tris–HCl (pH 6.8) at a moderate speed for 45 s. The homogenate was then centrifuged at 10,000×g for 20 min. The resulting pellet was discarded, and the supernatant was centrifuged again at 40,000×g for 30 min. The pellets obtained during the second centrifugation were resuspended in 600 mM KCl and 20 mM Tris–HCl (pH 6.8) to solubilize contractile proteins, then re-centrifuged at 40,000×g for 5 min. The final pellets were washed and resuspended in 250 mM sucrose and 20 mM Tris–HCl. Biochemical measurements were carried out using freshly prepared SR membranes. This SR preparation was relatively free of mitochondria and contractile protein contamination. Membrane purity and protein concentration were determined as described elsewhere [14].
The SR Ca2+ Uptake Assay
The SR Ca2+ uptake activity was determined using the Millipore filtration technique [14]. Briefly, SR vesicles (0.03–0.08 mg/ml) were incubated in 100 mM KCl, 20 mM Tris–HCl (pH 6.8), 5 mM MgCl2, 5 mM K-oxalate, and 5 mM NaN3. The desired concentration of free 45Ca2+ was maintained by buffering the 45CaCl2 solution (Sigma) with EGTA [13]. The reaction was initiated by adding 5 mM ATP. At the desired time, a 200-µl aliquot of the reaction mixture was filtered through a Millipore filter (0.45 µM), after which the filter was immediately washed with 2 ml of ice-cold water. The filter was dried, and radioactivity was quantified using standard liquid scintillation counting. The ATP-independent Ca2+ uptake was determined in the absence of ATP, and this value was subtracted from the total Ca2+ uptake in the presence of ATP to obtain the ATP-dependent Ca2+ uptake activity.
Measurement of SR Ca2+-Stimulated ATPase Activity
Basal and total activities of Ca2+-stimulated ATPase were quantified in the incubation medium similar to the method used in the Ca2+ uptake assay [15]. Total ATPase activity was measured using non-radioactive CaCl2 (final concentration of free Ca2+: 10 µM), while basal ATPase activity was measured in the absence of Ca2+ and in the presence of 0.2 mM EGTA. After a 5 min pre-incubation of the reaction mixture with 0.05 mg/ml of membrane, the reaction was started by addition of 5 mM Tris–ATP and terminated with 12 % ice-cold trichloroacetic acid. Inorganic phosphate liberated during this reaction was estimated in a protein-free filtrate by a spectrophotometric method [15].
SDS–PAGE and Western Blot Analysis of SERCA2 and Phospholamban Expression in SR Membrane
Proteins were separated on 10 % SDS polyacrylamide gels according to the Laemmli’s method [16]. For this, samples (protein concentration of 2 mg/ml) were mixed with an equal volume of loading buffer containing 250 mM Tris–HCl (pH 6.8), 8 % (w/v) SDS, 4 % glycerol, 20 % β-mercaptoethanol, and 0.006 % bromophenol blue. Gels were run at 200 V for 40–45 min and electrotransferred onto PVDF membranes. The membranes were blocked overnight in Tris–saline buffer (TBS: 10 mM Tris, 150 mM NaCl) and 5 % fat-free powdered milk. The membranes were then incubated for 1 h at room temperature with mouse monoclonal anti-SR Ca2+ ATPase (1:3,000; Affinity Bioreagents, Golden, Colorado, USA) and phospholamban antibodies (1:3,000; Affinity Bioreagents) in TBS containing 1 % Tween-20 and 1 % fat-free powdered milk. The blots were subsequently incubated with biotinylated anti-mouse IgG antibody (1:3,000; Amersham, Shanghai, China) for 40 min and then with streptavidin-conjugated horseradish peroxidase (1:5,000; Amersham) for 30 min. After each step, the blots were rinsed 3 × 15 min in TBS buffer. Chemiluminescent detection was carried out using hyperfilm ECL (Amersham). Images were analyzed using a GS-670 imaging densitometer. Protein expression in the experimental groups was normalized to the respective expression in the sham control group.
Statistical Analysis
All results are presented as the mean ± SD. The results were analyzed using SPSS 13.0 software. Differences among the groups were analyzed by one-way analysis of variance (ANOVA). A p < 0.05 was considered statistically significant.
Results
General Parameters in DOX-Induced Cardiomyopathy Rats
Early during DOX treatment (2 weeks), the rat heart showed no significant pathological changes: the heart weight, left ventricular weight, and lung wet/dry weight ratio were similar to the control group (p > 0.05; Table 1). By the end of 4 weeks, DOX accumulation reached 12 mg/kg, and the rat heart weight, left ventricular mass, and lung congestion (as reflected by wet/dry lung weight ratio) were significantly increased compared to the control group (p < 0.01). Ascites became apparent in 4 weeks, and ascitic fluid accumulation was significantly more extensive in the DOX (6W) groups than the DOX (2W) group (p < 0.05).
Hemodynamic Parameters in DOX-Induced Cardiomyopathy Rats
We observed the following changes (Table 2): SBP, DBP, and MAP significantly decreased (p < 0.05 or p < 0.01 vs. control group); LVEDP significantly increased (p < 0.01 vs. sham control group), LVSP decreased (p < 0.01 vs. sham control group), and +dP/dt and −dP/dt decreased (p < 0.01 vs. sham control group). These changes were more pronounced as DOX accumulation increased [p < 0.05 or p < 0.01 vs. DOX (2W)].
Oxidative Stress Parameters in DOX-Induced Cardiomyopathy Rats
Compared to the control group, the activity of iNOS and the concentrations of MDA and NO were significantly increased while the activity of SOD significantly decreased in the DOX groups (p < 0.05 or p < 0.01 vs. control group). These changes became more pronounced as DOX accumulation increased [p < 0.05 or p < 0.01 vs. DOX (2W) group] (Table 3; Fig. 1).
Changes in MDA, NO, SOD, and iNOS in DOX-induced cardiomyopathy rats. Data are expressed as the mean ± SD. **p < 0.01 versus control group. # p < 0.05, ## p < 0.01 versus DOX (2W) group. DOX: doxorubicin; MDA: malondialdehyde; SOD: superoxide dismutase; NO: nitric oxide; iNOS: inducible nitric oxide synthase
Cardiac Enzymes and Proteins in DOX-Induced Cardiomyopathy Rats
Exposure to DOX led to a significant increase in serum levels and cardiac enzymes activity (AST, LDH, CPK, cTnI) and plasma level of BNP (p < 0.01 vs. sham control group; Table 4). These biochemical changes were more pronounced as the DOX accumulation increased (p < 0.05 or p < 0.01 vs. DOX (2 W) group) (Table 4; Figs. 2, 3).
Ca2+ Uptake by Left Ventricular SR of Cardiomyopathy Rats
In the DOX (2W) group, we observed a significant reduction of the SR Ca2+ uptake compared with the sham control group (p < 0.01). The SR Ca2+ uptake increased further as the DOX accumulation increased [p < 0.05 or p < 0.01 vs. DOX (2W) group] (Table 5; Fig. 4).
Activity of Left Ventricular SR Ca2+-Stimulated ATPase in Cardiomyopathy Rats
In the DOX (2W) group, we observed a significant decrease in Ca2+-stimulated ATPase activity compared with the sham control group (p < 0.01), and these changes increased as the DOX accumulation increased [p < 0.05 or p < 0.01 vs. DOX (2W) group] (Table 6; Fig. 5).
Expression of SERCA2 and Phospholamban
To examine whether the observed changes in SR Ca2+ transport activities were due to altered expression of the SERCA2 and phospholamban in the left ventricle, we quantified their levels in SR membranes by Western blot analysis. As expected, in the DOX (2W) rats, there were significant diminished levels of both SERCA2 and phospholamban compared with the sham control group (p < 0.01). Further, these changes were more obvious as DOX accumulation increased [p < 0.05 or p < 0.01 vs. DOX (2W) group] (Table 7; Fig. 6).
Discussion
In this study, we demonstrated that rats with DOX cardiomyopathy developed congestive heart failure. This type of congestive heart failure is dose dependent and irreversible. Under a cumulative dosage of DOX of 12 mg/kg, heart failure begins to occur in the experimental animals; with a cumulative dosage of 18 mg/kg, the resultant myocardial cells gradually apoptosis, and symptoms of the heart failure occur, with massive ascites. These changes are due to myocardial toxicity caused by DOX and are consistent with previous observations [4].
In addition, after 4 weeks of DOX treatment, we observed toxic myocardial oxidative stress injury. DOX significantly increased iNOS activity and MDA and NO concentrations, while it decreased the SOD activity. The activities of cardiac enzymes (AST, LDH, CPK, cTnI) and BNP level were significantly increased by DOX.
Although extensive investigations on DOX-induced cardiotoxicity have been conducted, the underlying mechanisms responsible have not been completely elucidated. DOX-induced cardiotoxicity has been reported to be associated with oxidative stress, free radicals, intracellular calcium overload, iron metabolism imbalance, mitochondrial lesions, and energy metabolism dysfunction. Intracellular calcium overload induced by SR dysfunction is considered an important factor in myocardial cell injury and apoptosis.
In this study, we found that the decreases in the SR Ca2+ uptake and Ca2+ pump ATPase activity during DOX treatment (2 weeks) were due to DOX myocardial toxicity, which was in line with earlier reports [9, 12, 17]. We provide further evidence that the expression of SERCA2 and phospholamban decreases DOX myocardial toxicity, and are consistent with reduced SR Ca2+ pump activities in rats with DOX-induced cardiomyopathy. Phospholamban, a protein expressed in the SR membrane, can regulate the SERCA2 activity. Phosphorylation of phospholamban enhances the SERCA2 activity, leading to augmentation of contractility and relaxation speed of the myocardium. Conversely, dephosphorylation of phospholamban decreases SERCA2 activity. As a result, decreased phospholamban expression in the failing heart, induced by DOX, could lead to decreased cardiac SR Ca2+ pump function. Besides, the decrease of SERCA2 expression aggravates SR regulation dysfunction and induces intracellular calcium overload. In conclusion, both the decreased expression of SERCA2 and phospholamban play important roles in SR calcium regulation dysfunction caused by DOX in rats.
References
Lipshultz, S. E., Colan, S. D., Gelber, R. D., Perez-Atayde, A. R., Sallan, S. E., & Sanders, S. P. (1991). Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. New England Journal of Medicine, 324, 808–815.
Nakamura, T., Ueda, Y., Juan, Y., Katsuda, S., Takahashi, H., & Koh, E. (2000). Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation, 102, 572–578.
Jakacki, R., Silber, J., & Larsen, R. (1991). Cardiac dysfunction following low-risk cardiotoxic treatment for childhood malignancy. Pediatric Research, 29, 143A.
Watts, R. G. (1991). Severe and fatal anthracycline cardiotoxicity at cumulative doses below 400 mg/mz: evidence for enhanced toxicity with multiagent chemotherapy. American Journal of Hematology, 36, 217–218.
Billingham, M. E. (1991). Role of endomyocardial biopsy in diagnosis and treatment of heart disease. In M. D. Silver (Ed.), Cardiovascular pathology (2nd ed., pp. 1465–1486). New York: Churchill Livingstone.
Fischer, V. W., Wang, G. M., & Hobart, N. H. (1991). Mitigation of an anthracycline-induced cardiomyopathy by pretreatment with razoxanes a quantitative morphological assessment. Virchows Archiv B Cell Pathology, 51, 353–361.
Kim, S. Y., Kim, S. J., Kim, B. J., Rah, S. Y., Chung, S. M., Im, M. J., et al. (2006). Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Experimental & Molecular Medicine, 38, 535–545.
Olson, R. D., Gambliel, H. A., Vestal, R. E., Shadle, S. E., Charlier, H. A., Jr, & Cusack, B. J. (2005). Doxorubicin cardiac dysfunction: Effects on calcium regulatory proteins, sarcoplasmic reticulum, and triiodothyronine. Cardiovascular Toxicology, 5, 269–283.
Ondrias, K., Borgatta, L., Kim, D. H., & Ehrlich, B. E. (1990). Biphasic effects of doxorubicin on the calcium release channel from sarcoplasmic reticulum of cardiac muscle. Circulation Research, 67, 1167–1174.
Dhalla, N. S., Shao, Q., & Panagia, V. (1998). Remodeling of cardiac membranes during the development of congestive heart failure. Heart Failure Reviews, 2, 261–272.
Fleischer, S., & Inui, M. (1989). Biochemistry and biophysics of excitation-contraction coupling. Annual Review of Biophysics and Biophysical Chemistry, 18, 333–364.
Kim, D. H., Landry, A. B., Lee, Y. S., & Katz, A. M. (1989). Doxorubicin induced calcium release from cardiac sarcoplasmic reticulum vesicles. Journal of Molecular and Cellular Cardiology, 21, 433–436.
Fabiato, A. (1988). Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods in Enzymology, 157, 378–417.
Afzal, N., & Dhalla, N. S. (1992). Differential changes in left and right ventricular SR calcium transport in congestive heart failure. American Journal of Physiology, 262, H868–H874.
Ganguly, P. K., Pierce, G. N., Dhalla, K. S., & Dhalla, N. S. (1983). Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. American Journal of Physiology, 244, E528–E535.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Chamberlain, B. K., & Fleischer, S. (1988). Isolation of canine cardiac sarcoplasmic reticulum. Methods in Enzymology, 157, 91–99.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81170125, 81270209), the Science and Technology Commission of Shanghai Municipality (No. 12JC1406400), and the Doctor Innovation Fund of Shanghai Jiao Tong University School of Medicine (BXJ201224).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yachen Zhang and Yu Chen contributed equally to this work and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, Y., Chen, Y., Zhang, M. et al. Doxorubicin Induces Sarcoplasmic Reticulum Calcium Regulation Dysfunction via the Decrease of SERCA2 and Phospholamban Expressions in Rats. Cell Biochem Biophys 70, 1791–1798 (2014). https://doi.org/10.1007/s12013-014-0130-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0130-2