Abstract
Hispidulin is a flavonoid compound which is an active ingredient in a number of traditional Chinese medicinal herbs. However, it’s therapeutic activity remains poorly understood. The present study investigated the pro-apoptotic effects and mechanism by which Hispidulin induces apoptosis in human hepatoblastoma cancer (HepG2) cells. The results showed that Hispidulin induced cell death in a dose- and time-dependent manner in HepG2 cells whereas no toxic reaction was observed in normal human liver cells at indicated concentration. This study also demonstrated that Hispidulin induces apoptosis through mitochondrial dysfunction, which is characterized by decreased Bcl-2/Bax ratio, disrupted mitochondrial membrane potential and increased release of cytochrome C and activated capase-3. Our results also showed that mitochondrial dysfunction was triggered by Hispidulin-induced excessive ROS generation. Hispidulin also significantly inhibited Akt activation. ROS inhibitor NAC abrogated the inhibitory effect of Hispidulin on P13k/Akt signalling pathway and the proapoptotic effect in HepG2 cells. Our results demonstrate for the first time that Hispidulin induces apoptosis in HepG2 cells and suggested that the pro-apoptotic effect of Hispidulin was mediated through mitochondrial dysfunction and inhibition of P13k/Akt signalling pathway. Since no toxic effect was observed when normal liver cells were treated with Hispidulin, Hispidulin may have the potential to be used as therapeutic for liver cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is currently the most common primary malignancy in liver and the third leading cause of cancer related death worldwide, accounting for more than 600,000 deaths annually [1, 2]. Current standard therapy for HCC consists of surgery and chemotherapy. However, the majority of patients with HCC die within one year of diagnosis despite significant efforts in improving treatment strategies [3]. Nowadays, the most widely used agent against HCC is doxorubicin, either as a single agent or in combination with other chemotherapeutics like cisplatin, which has shown only a minimal survival advantage due to the hepatotoxicity of doxorubicin [4]. Therefore, novel therapeutic agents with high antitumour potency and low hepatotoxicity are subjects of continued investigation.
Hispidulin (4′,5,7-trihydroxy-6-methoxyflavone) is one of the active ingredients in a number of traditional Chinese medicinal herbs [5, 6]. Previous studies have reported it’s antifungal, anti-inflammatory, antioxidant, anti-thrombosis and anti-epileptic activities [7–11]. A few researches have also demonstrated that Hispidulin could exert potent antitumour effect on a variety cancer cells. In vitro studies by Chen’s group showed that Hispidulin could potentiate apoptosis of human ovarian cancer cells and human glioblastoma multiforme cells by activating AMP-activated protein kinase (AMPK), leading to mammalian target of rapamycin (mTOR) inhibition [12, 13]. Besides inhibiting cell growth and inducing apoptosis in tumour cells, further study showed that Hispidulin could suppress angiogenesis of human pancreatic cancer and prevent pancreatic cancer growth in xenograft mice model by targeting vascular endothelial growth factor 2-mediated P13K/Akt/mTOR signalling pathway [14].
Apoptosis is a programmed cell death and induction of apoptosis has been evaluated as a major mechanism to eliminate cancer cells. Apoptosis can be driven by cell death receptor-mediated extrinsic pathway and mitochondria-mediated intrinsic pathways, which could be triggered by various stimuli including cellular reactive oxygen species (ROS), reactive nitrogen species (RNS), hormones, cell–cell interaction, growth factor withdrawal, antigens and chemotherapeutics [15, 16]. Many chemotherapeutic agents induce cell apoptosis by increasing oxidant stress in cancer cells via production of ROS and/or the depletion of glutathione (GSH). The imbalance redox status due to excessive oxidative stress can further trigger downstream cellular events such as mitochondrial dysfunction and other tumour necrosis factor receptor (TNF-R) signalling pathways that lead to apoptosis. Keeping in mind the ability of Hispidulin to inhibit enzymes of the mitochondrial respiratory chain and reduce fluidity of mitochondrial membrane [17, 18], we hypothesized that Hispidulin can disrupt the intracellular redox status and induce mitochondrial dysfunction which may eventually lead to apoptosis of cancer cells. To evaluate this hypothesis, we investigated the effect of Hispidulin on ROS generation as well as downstream signalling pathways in HepG2. Our study showed that Hispidulin induce apoptosis in HepG2 cells via ROS generation and activation of mitochondrial intrinsic apoptotic cascade.
Materials and Methods
Cell Culture and Treatment
Human HepG2 and HL7702 cells were obtained from Shanghai Cell Bank, China, and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (Invitrogen, USA). Cells were grown at 37 °C in a 5 % CO2 (v/v) in humidified atmosphere.
Determination of Cell Viability
Cell viability was determined by MTT assay as previously described [19]. Briefly, HepG2 cells were treated with different concentrations of Hispidulin for 24, 48 and 72 h. Following treatment, cells were further incubated with MTT reagents (500 g/ml) at 37 °C for 4 h before DMSO was added, to dissolve farmazan crystals, and absorbance was measured at 570 nm in a microplate reader (Bio-Rad Laboratories Ltd., Shanghai, China).
Determination of Cell Cycle Distribution and Apoptotic Cells
To determine cell cycle distribution analysis, HepG2 cells (1 × 105 cells/ml) were plated in 30-mm dishes and treated with vehicle control and Hispidulin (50, 100 and 200 μM) for 24 h. Cells were resuspended in 1 ml of PBS buffer and 4 ml of 70 % ice-cold ethanol. Cells were incubated overnight at 4 °C before washing with PBS buffer. The cells were then resuspended in 250 μl of propidium iodide solution (50 μg/ml final concentration) containing 1 μl of 20 μg/ml RNase A. The cells were incubated in the dark setting for 15 min at room temperature and analysed by flow cytometer (Elite ESP, Beckman Coulter, Brea, CA) [20]. A minimum of 10,000 cells were collected and the cell distribution in each phase (G0/G1, S and G2/M phases) of the cell cycle was determined using windows multiple document interface (WinMDI) software, including sub-G1 phase of apoptotic cells.
Measurement of ROS
The intracellular changes in ROS generation were detected by staining the cells with 2,7-dichlorodihydrofluorescein-diacetate (DCFH-DA) as described previously [21]. Briefly, HepG2 cells were treated with different concentrations of Hispidulin (50, 100 and 200 μM) for 24 h. After treatment, cells were further incubated with 20 μM DCFH-DA at 37 °C for 30 min. Subsequently cells were harvested, rinsed and resuspended in PBS before the fluorescence was analysed by flow cytometry.
Measurement of Mitochondrial Membrane Potential (MMP)
Fluorochrome dye JC-1 was used to evaluate the changes in MMP as described previously [18]. Briefly, HepG2 cells were incubated with 50, 100 or 200 μM Hispidulin for 24 h. Following incubation, cells were collected and stained with JC-1. Cells were then harvested and the cell pellet was gently rinsed with PBS once and then resuspended in 500 l PBS. Fluorescent intensity of cells was quantitatively analysed by flow cytometry.
Western Blotting
Proteins were isolated from control and Hispidulin-treated cells as previously described [22]. 40 g proteins were electrophoresed on 12 % SDS-PAGE before transferring to PVDF membrane. The membrane was then blocked with 5 % (w/v) non-fat milk and washed with Tris-buffered saline-Tween solution (TBST) and the membranes were incubated overnight at 4 °C with primary antibodies according to manufacturer’s instructions. After another washing with TBST, secondary antibody was added and incubated for 2 h. The blots were washed with TBST before signals were detected using chemiluminescent substrate (KPL, Guildford, UK). BandScan software (Glyko, Novato, CA) was used to quantify the blot density.
Tumour Xenograft Study
Tumours were established by injecting 5 × 106 HepG2 cells into the right flank of 5-week-old BALB/cA nude mice (National Rodent Laboratory Animal Resource, Shanghai, China). After tumour sizes reached approximately 50 mm3, mice were divided into two groups and treated with DMSO or Hispidulin at a dosage of 10, 20 or 40 mg/kg/day for 35 days. Hispidulin was dissolved in minimum DMSO and subcutaneously injected around the solid tumours. The body weight of tumour-bearing mice was recorded every week and tumour volume was calculated according to the formula A × B 2 × 0.5, where A is the length and B is width of the tumour.
Statistical Analysis
All experimental results are expressed as mean ± SD. Statistic analysis was performed using Student’s test and one-way ANOVA test (SPSS, Chicago, IL). P value of <0.05 was considered statistically significant.
Results
Hispidulin Inhibits Growth of HepG2 Cells In Vitro
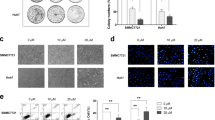
Hispidulin exhibited dose- and time-dependent anti-proliferative effects as evaluated by MTT assay. As shown in Fig. 1a, treatment with Hispidulin (50, 100 and 200 μM) for 24 h resulted in reduction in cell viability by 24.3 ± 3.2, 45.5 ± 5.2 and 53.6 ± 6.7 %, respectively. After 48-h incubation with Hispidulin, cell viability decreased by 36.3 ± 6.2, 51.2 ± 7.3 and 68.3 ± 9.6 %, respectively. When incubation was prolonged to 72 h, further decrease in cell viability was observed as shown in Fig. 1. In order to assess the safety of Hispidulin, the toxicity was examined in human liver cells (HL7702). No significant effect was found on HL7702 cell growth as shown in Fig. 1b.
Hispidulin reduces the viability of HepG2 cells. Cells were incubated with Hispidulin at indicated concentration for 24, 48 or 72 h and then proceeded for MTT assay. a Analysis of the viability of HepG2 cells after Hispidulin treatment. b Analysis of the cytotoxicity of Hispidulin in HL-7720 cells. The results are shown as mean ± SD of nine experiments. *P < 0.05 versus control, **P < 0.01 versus control
Hispidulin Induces Cell Cycle Arrest and Apoptosis
To further elucidate the mechanism of cell growth inhibition, the cell cycle distribution was examined following Hispidulin treatment. Previous study by He’s group in glioblastoma cells showed that Hispidulin treatment caused G0/G1 phase arrest [14]. Consistent with their results, our study also showed that Hispidulin treatment resulted in accumulation of G0/G1 phase and considerable drop in G2/M phase and S phase as shown in Fig. 2a, b. Apoptotic cell death was determined by examining the flow cytometry. After 24-h treatment with Hispidulin, apoptotic cell death increased in a dose-dependent manner compared to control. Apoptotic markers including cleaved caspase-3 and PARP cleavage were also observed in Hispidulin-treated cells, which further suggested that Hispidulin-induced cell death is mainly due to apoptosis (Fig. 2c).
Hispidulin-induced apoptosis in HepG2 cells. Change in cell cycle distribution and apoptosis was examined by flow cytometry after HepG2 cells were incubated with Hispidulin at indicated concentration for 24 h. a Representative flow cytometry diagram of cell cycle distribution analysis. b Analysis of cell cycle distribution. The results were expressed as the mean ± SD of nine separate experiments. c Caspase 3 and PARP cleavage were examined by Western blot with β-actin as an internal control
Hispidulin Activates Mitochondria-Mediated Apoptosis Pathway
To further confirm the role of mitochondria in Hispidulin-induced apoptosis, the change in MMP and release of cytochrome C from mitochondria to cytosol were examined. Experimental data demonstrated that Hispidulin treatment caused an obvious loss in MMP relative to corresponding control as shown in Fig. 3a, b. Western blot showed increase in cytosol cytochrome c and decrease in mitochondrial cytochrome c, which also supported the role of mitochondria in Hispidulin-induced apoptosis (Fig. 3c). Along with MMP collapse and release of cytochrome c, downregulation of Bcl-2 and upregulation of Bax were observed.
Hispidulin-induced mitochondrial dysfunction in HepG2 cells. Cells were incubated with Hispidulin at indicated concentration for 24 h before tests were performed. a Representative flow cytometric diagram of MMP analysis. b Fluorescence intensity analysis of JC-1 staining cells. The results were expressed as the mean ± SD of nine separate experiments. c Representative image of immunoblots for Bcl-2, Bax and Cytochrome c (in cytosol) with actin as an internal control. *P < 0.05 versus control
Inhibition of the P13k/Akt Pathway by Hispidulin
P13k/Akt pathway plays an important role in apoptosis protection. To confirm the role of inhibition of Akt activation in Hispidulin-induced apoptosis, total and phosphorylated Akt were examined by Western blotting. After treated by Hispidulin, the levels of p-Akt in cells were reduced whereas total Akt level was not markedly affected (Fig. 4a).
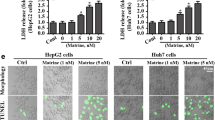
Effect of Hispidulin on P13k/Akt signalling pathway and ROS generation and Effect of ROS inhibitor on Hispidulin-induced apoptosis and P13k/Akt signalling pathway. Cells were incubated with Hispidulin at indicated concentration for 24 h before tests were performed. a Representative image of immunoblots for Akt and p-Akt. b Representative flow cytometric diagram for ROS analysis. c Analysis of ROS generation. The results are expressed as the mean ± SD of nine separated experiments. d Effect of NAC on the expression level of p-Akt, Akt, Cytochrome c and caspase-3 cleavage. Cells were treated with NAC for 30 min before incubating with Hispidulin. Diagram showed representative immunoblot of Western blot analysis with β-actin as internal standard
Hispidulin Induces Apoptosis and Inhibited P13k/Akt in ROS-Dependent Manner
To investigate the role of ROS generation in Hispidulin-induced apoptosis and P13k/Akt signalling pathway inhibition, HepG2 cells were treated with Hispidulin in the presence or absence of antioxidant NAC. As shown in Fig. 4d, NAC almost blocked Hispidulin-induced release of cytochrome c and Caspase-3 cleavage. These observations suggest that Hispidulin-induced ROS generation is an early event that triggers mitochondrial apoptotic pathways in HepG2 cells. Interestingly, pretreatment with NAC abolished the inhibitory effect of Hispidulin on Akt phosphorylation, indicating that Hispidulin-induced ROS generation was relevant to its effect on P13k/Akt signalling pathway. Taken together, these results indicate that Hispidulin could induce mitochondria-mediated apoptosis by inhibiting the P13k/Akt pathway.
Hispidulin Inhibits Tumour Growth in Xenografted Nude Mice Model
To further evaluate whether Hispidulin had an effect to inhibit tumour growth in vivo, we measured the tumour volume in a xenograft tumour model in which HepG2 cells were injected subcutaneously into nude mice. 10, 20 and 40 mg/kg of Hispidulin was selected for in vivo experiments [14]. When transplant tumours reached a mean group size of approximately 50 mm3, mice were treated every day for 35 days with varying dose of Hispidulin (i.p., 10, 20 and 40 mg/kg). Results clearly show that compared with the control group, Hispidulin showed significant inhibitory effect on tumour size (Fig. 5).
Hispidulin inhibits tumour growth in xenograft mice model. Measurements of tumour volume at indicated time points depicted the in vivo therapeutic efficacy of Hispidulin at indicated dosage. N = 15 per group. *P < 0.05 versus animals treated with vehicle. **P < 0.01 versus animals treated with vehicle
Discussion
HCC accounts for 90 % of all primary liver cancers and the median survival duration for patients with HCC is only 7–8 months from the time of diagnosis [23]. Owing to the high hepatotoxicity, currently available chemotherapeutics remain ineffective and half of the patients now have no adequate treatment. Therefore, novel therapeutic agents that kill tumour cells without harming normal hepatocytes are highly desirable. As a natural small molecule of flavonoid, Hispidulin has been reported to possess anti-inflammatory, anti-epileptic and antifungal activities. The antitumour activity of Hispidulin has been examined in human ovarian cells, human glioblastoma multiform cells and human pancreatic cancer cells [12–14]. However, the effects and mechanism of Hispidulin on human HCC cells have not been elucidated. In our study, HepG2 cells were used as model cell line to investigate the antitumour activity. Results showed that Hispidulin treatment not only results in dose- and time-dependent inhibition of viability, but also trigger apoptosis in HepG2 cells. Experimental data showed that Hispidulin provoked the generation of ROS in HepG2 cells. In addition, Hispidulin-induced inhibition on cell growth and apoptosis were completely abolished when the cells were treated with NAC, suggesting that disruption in intracellular redox balance was responsible for apoptotic cell death. Furthermore, we found that Hispidulin treatment also blocked activation of P13K/Akt signalling pathways in a ROS-dependent manner.
Decreased apoptosis and enhanced proliferation of cancer cells are early events in the progress of cancers, which makes the induction of apoptosis an important strategy to treat cancers. A series of studies showed that flavonoid compounds can lead to apoptotic cell death via mitochondria-mediated pathway. In line with those findings, our study found that Hispidulin triggered the mitochondria-mediated apoptosis pathway as evidenced by an increased ratio of Bax/Bcl-2, subsequent release of cytochrome c and sequential activation of caspase-3. Activation of intrinsic apoptosis pathway is also characterized by loss of integrity of mitochondrial membrane, which is regulated by Bcl-2 family. Therefore, we measured the change in MMP. Our data showed that Hispidulin treatment caused a significant decrease in MMP in HepG2 cells. Taken together, our results indicated that Hispidulin induced apoptosis by activating mitochondria-dependent apoptosis pathway.
Accumulating evidence showed that excessive ROS generation and/or antioxidant depletion such as GSH could disrupt intracellular balance, which then triggers mitochondria-dependent apoptosis pathway in tumour cells [22, 24, 25]. Early study showed that Hispidulin exerted inhibitory effect on mitochondrial respiratory chain, promoting alteration of the electron flux in the respiratory chain and increasing the ROS generation [17, 26]. Therefore, we were interested whether Hispidulin induced mitochondrial dysfunction through generation of excessive oxidative stress. Our data showed that the intracellular ROS levels were significantly increased in Hispidulin-treated HepG2 cells. Treatment with antioxidant (NAC) effectively protected against Hispidulin-induced cell death. All these results suggest that the ROS acted as upstream signalling molecule to initiate mitochondria-mediated cell apoptosis.
Recent studies highlighted that ROS was involved in the phosphorylation of Akt as secondary signalling molecules [27, 28]. In this study, we found that ROS inhibitor (NAC) strongly abrogated the inhibitory effect of Hispidulin on P13k/Akt activation. Furthermore, NAC caused significantly reduced cytochrome c release in the cytosol and caspase-3 cleavage, suggesting that Hispidulin mediated mitochondria dysfunction via inhibition P13k/Akt pathway through generating excessive ROS. P13k/Akt signalling pathway also plays a crucial role in cell survival by directly regulating apoptotic proteins, such as Bcl-2 and Bcl-xL [29, 30]. P13k/Akt signalling pathway could regulate other crucial targets which play crucial role in cell metabolism, growth, proliferation and survival, such as NF-κB and mTOR [31, 32]. Therefore, by inhibiting P13k/Akt signalling pathway, Hispidulin may act as a multi-target therapeutic.
To examine whether the enhanced cell growth inhibition and apoptosis induction could be validated in vivo, an animal model using xenograft was employed. Our results showed that Hispidulin treatment led to a significant inhibition of tumour growth. Pang’s group [14] reported that Hispidulin could suppress the angiogenesis of human pancreatic cancer, Hispidulin treatment might also be able to inhibit cell invasion in HCC, in addition to suppression of tumour growth.
In conclusion, our results demonstrate for the first time that Hispidulin induces HCC cell apoptosis through mitochondrial dysfunction and inhibiting the P13k/Akt pathway in a ROS-dependent manner. Hispidulin also suppressed tumour growth in vivo. Therefore, we consider Hispidulin as a potential drug for HCC treatment. However, further studies, including in vivo toxicity of Hispidulin, are needed to fully evaluate Hispidulin as a novel therapeutic in cancer prevention and treatment.
References
Ferlay, J., Shin, H. R., Bray, F., Forman, D., Mathers, C., & Parkin, D. M. (2008). Estimates of worldwide burden of cancer in 2008: GLOBOCAN. International Journal of Cancer, 127(2010), 2893–2917.
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., & Forman, D. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61, 69–90.
Andreana, L., Isgro, G., Marelli, L., Davies, N., Yu, D., Navalkissoor, S., et al. (2012). Treatment of hepatocellular carcinoma (HCC) by intra-arterial infusion of radio-emitter compounds: Trans-arterial radio-embolisation of HCC. Cancer Treatment Reviews, 38, 641–649.
Thomas, M. B., O’Beirne, J. P., Furuse, J., Chan, A. T., Abou-Alfa, G., & Johnson, P. (2008). Systemic therapy for hepatocellular carcinoma: Cytotoxic chemotherapy, targeted therapy and immunotherapy. Annals of Surgical Oncology, 15, 1008–1014.
Way, T. D., Lee, J. C., Kuo, D. H., Fan, L. L., Huang, C. H., Lin, H. Y., et al. (2010). Inhibition of epidermal growth factor receptor signaling by Saussurea involucrata, a rare traditional Chinese medicinal herb, in human hormone-resistant prostate cancer PC-3 cells. Journal of Agriculture and Food Chemistry, 58, 3356–3365.
Yin, Y., Gong, F. Y., Wu, X. X., Sun, Y., Li, Y. H., Chen, T., et al. (2008). Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. Journal of Ethnopharmacology, 120, 1–6.
Kavvadias, D., Sand, P., Youdim, K. A., Qaiser, M. Z., Rice-Evans, C., Baur, R., et al. (2004). The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects. British Journal of Pharmacology, 142, 811–820.
Tan, R. X., Lu, H., Wolfender, J. L., Yu, T. T., Zheng, W. F., Yang, L., et al. (1999). Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Medica, 65, 64–67.
Nagao, T., Abe, F., Kinjo, J., & Okabe, H. (2002). Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biological and Pharmaceutical Bulletin, 25, 875–879.
Chen, Y. T., Zheng, R. L., Jia, Z. J., & Ju, Y. (1990). Flavonoids as superoxide scavengers and antioxidants. Free Radical Biology and Medicine, 9, 19–21.
Bourdillat, B., Delautier, D., Labat, C., Benveniste, J., Potier, P., & Brink, C. (1988). Mechanism of action of hispidulin, a natural flavone, on human platelets. Progress in Clinical and Biological Research, 280, 211–214.
Yang, J. M., Hung, C. M., Fu, C. N., Lee, J. C., Huang, C. H., Yang, M. H., et al. (2010). Hispidulin sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by AMPK activation leading to Mcl-1 block in translation. Journal of Agriculture and Food Chemistry, 58, 10020–10026.
Lin, Y. C., Hung, C. M., Tsai, J. C., Lee, J. C., Chen, Y. L., Wei, C. W., et al. (2010). Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK). Journal of Agriculture and Food Chemistry, 58, 9511–9517.
He, L., Wu, Y., Lin, L., Wang, J., Chen, Y., Yi, Z., et al. (2011). Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3 K/Akt/mTOR signaling pathway. Cancer Science, 102, 219–225.
Ou, H. C., Lee, W. J., Lee, S. D., Huang, C. Y., Chiu, T. H., Tsai, K. L., et al. (2010). Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3 K/Akt/eNOS pathway. Toxicology and Applied Pharmacology, 248, 134–143.
Chen, X., Zhong, Z., Xu, Z., Chen, L., & Wang, Y. (2011). No protective effect of curcumin on hydrogen peroxide-induced cytotoxicity in HepG2 cells. Pharmacological Reports, 63, 724–732.
Dabaghi-Barbosa, P., Mariante Rocha, A., Franco da Cruz Lima, A., Heleno de Oliveira, B., Benigna Martinelli de Oliveira, M., Gunilla Skare Carnieri, E., et al. (2005). Hispidulin: Antioxidant properties and effect on mitochondrial energy metabolism. Free Radical Research, 39, 1305–1315.
Herrerias, T., Oliveira, A. A., Belem, M. L., Oliveira, B. H., Carnieri, E. G. S., Cadena, S. M. S. C., et al. (2010). Effects of natural flavones on membrane properties and citotoxicity of HeLa cells. Revista Brasileira de Farmacognosia, 20, 403–408.
Khan, M., Zheng, B., Yi, F., Rasul, A., Gu, Z., Li, T., et al. (2012). Pseudolaric Acid B induces caspase-dependent and caspase-independent apoptosis in u87 glioblastoma cells. Evidence-Based Complementary and Alternative Medicine, 2012, 957568.
Chou, T. H., & Liang, C. H. (2009). The molecular effects of aloe-emodin (AE)/liposome-AE on human nonmelanoma skin cancer cells and skin permeation. Chemical Research in Toxicology, 22, 2017–2028.
Chen, G., Wang, K., Yang, B. Y., Tang, B., Chen, J. X., & Hua, Z. C. (2012). Synergistic antitumor activity of oridonin and arsenic trioxide on hepatocellular carcinoma cells. International Journal of Oncology, 40, 139–147.
Yuan, L., Wang, J., Xiao, H., Xiao, C., Wang, Y., & Liu, X. (2012). Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3 K/Akt signaling pathway in HepG2 cancer cells. Toxicology and Applied Pharmacology, 265, 83–92.
Wilson, J. F. (2005). Liver cancer on the rise. Annals of Internal Medicine, 142, 1029–1032.
Li, S., Dong, P., Wang, J., Zhang, J., Gu, J., Wu, X., et al. (2010). Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Letters, 298, 222–230.
Raza, H., John, A., & Benedict, S. (2011). Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. European Journal of Pharmacology, 668, 15–24.
Valdameri, G., Herrerias, T., Carnieri, E. G., Cadena, S. M., Martinez, G. R., & Rocha, M. E. (2010). Importance of the core structure of flavones in promoting inhibition of the mitochondrial respiratory chain. Chemico-Biological Interactions, 188, 52–58.
Le Belle, J. E., Orozco, N. M., Paucar, A. A., Saxe, J. P., Mottahedeh, J., Pyle, A. D., et al. (2011). Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell, 8, 59–71.
Woo, J. H., Kim, Y. H., Choi, Y. J., Kim, D. G., Lee, K. S., Bae, J. H., et al. (2003). Molecular mechanisms of curcumin-induced cytotoxicity: Induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome C and inhibition of Akt. Carcinogenesis, 24, 1199–1208.
Datta, S. R., Dudek, H., Tao, X., Masters, S., Fu, H., Gotoh, Y., et al. (1997). Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell, 91, 231–241.
del Peso, L., Gonzalez-Garcia, M., Page, C., Herrera, R., & Nunez, G. (1997). Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science, 278, 687–689.
Cho, D. H., Choi, Y. J., Jo, S. A., Ryou, J., Kim, J. Y., Chung, J., et al. (2006). Troglitazone acutely inhibits protein synthesis in endothelial cells via a novel mechanism involving protein phosphatase 2A-dependent p70 S6 kinase inhibition. American Journal of Physiology Cell Physiology, 291, C317–C326.
Deeb, D., Jiang, H., Gao, X., Al-Holou, S., Danyluk, A. L., Dulchavsky, S. A., et al. (2007). Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. Journal of Pharmacology and Experimental Therapeutics, 321, 616–625.
Acknowledgments
This work was supported by the funds of Chongqing Normal University (No. 12XLB025).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hui Gao and Hui Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, H., Wang, H. & Peng, J. Hispidulin Induces Apoptosis Through Mitochondrial Dysfunction and Inhibition of P13k/Akt Signalling Pathway in HepG2 Cancer Cells. Cell Biochem Biophys 69, 27–34 (2014). https://doi.org/10.1007/s12013-013-9762-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9762-x