Abstract
Gelatinase A (MMP-2) and gelatinase B (MMP-9) are proteolytic enzymes involved in process of tumor invasion, and they are considered as possible tumor markers in breast cancer patients. In this study, we measured activity of latent and active form of MMP-2 and MMP-9 in tumor and adjacent tissue of 60 breast cancer patients by SDS-PAGE zymography. The activity of both form of gelatinases significantly increased with each advancing clinical stage of disease. ProMMP-9 and aMMP-9 activity in tumor tissue shows a positive association with tumor size. Patients with lymph node involvement have higher proMMP-2, aMMP-2 and aMMP-9 activity than node negative patients. Steroid receptor-negative tumors had enhanced aMMP-2 and aMMP-9 activity. Patients with basal-like cancers had higher proMMP-2 tumor activity and aMMP-2 adjacent tissue activity compared to patients with luminal A tumors. Patients with negative hormone receptors are associated with increased activity of both form of gelatinases in adjacent tissue. Reported increased activity of MMP-2 in tumor and adjacent tissue of basal-like tumors implicates that MMP-2 might have a role in aggressive biology of basal-like cancers. Additional investigations regarding molecular pathways in adjacent tissue could give better insight into aggressive nature of basal-like carcinomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases causally involved in tumor progression [1]. While some of these MMPs appear to be involved in stimulating tumor cell growth, others are causally involved in invasion and metastasis [2]. Two of the MMPs implicated in the spread of cancer are MMP-2 and -9, also known as gelatinase A and B. These MMPs are thought to mediate invasion and metastasis by catalyzing degradation of type IV collagen, the main component of basement membranes and inducing angiogenesis. MMP-2 and -9, like all other known mammalian MMPs, are initially synthesized as inactive precursors [3]. The mechanism of activation in vivo is largely unknown but is likely to involve proteolytic processes mediated by other MMPs and/or serine proteases [1]. In tumor tissue samples, MMPs can occur in different forms such as inactive pro-enzymes and active enzymes.
In breast cancer, both gelatinases seem to be expressed in cancer tissue, although the results have not been consistent [4–6]. To date, only a few studies have investigated the activity of MMP-2 and MMP-9 in tumor tissue and their relation to clinicopathological parameters in breast cancer patients [5, 7]. It has been shown that tumor cell MMP-9 expression is significantly associated with tumor histological type and hormone receptor status. Jinga et al. explained this fact through activation of the proMMP-9 by estradiol via estrogen receptors [8]. However, Sullu et al. [9] have been shown that MMP-2 and MMP-9 expression was increased in hormone-negative breast cancer. HER2 is established molecular prognostic markers in breast cancer and is often targeted with therapeutic intention in both localized and metastatic breast cancer. Aberrant expressions of hormone receptors and HER2 oncogene are related to disease progression and increased invasive capacity in breast cancer [10], which is due in part to increased MMP-2 and MMP-9 activity [11]. Gelatinase activity in relation to hormone and HER2 receptor expression was not investigated.
However, despite the massive efforts invested in the identification of immunohistochemical biomarkers in breast cancer, the majority have not proven to be of value in multivariate analyses and only estrogen receptor, progesterone receptor, and HER2 expression have remained essential components of pathological examination [12]. These three markers were initially employed for prognostication but their role in treatment also rendered them of predictive value. Newer molecular methods have shown that even morphologically similar subtypes of breast cancer can show molecular heterogeneity; moreover, breast carcinoma can be separated into at least 4 molecular subtypes designated luminal (ER+, PR+, and HER2−), HER2 overexpressing (ER−, PR−, and HER2+), basal-like (ER−, PR−, HER2−, and CK5/6+/EGFR+), and normal breast-like (ER−, PR−, and HER2−), each with different clinical outcome [13]. Although basal-like cancers show a high response to neoadjuvant antracycline plus taxane neoadjuvant chemotherapy, survival with basal-like tumors is still poor, and there is a need for additional systemic treatments that are effective against these tumors [14]. Thus, evaluation of MMP activity profiles may contribute to the development of these needed therapies.
The aim of this study was to examine the activity of gelatinases in the tumor and adjacent tissue of breast cancer patients and to correlate them with TNM clinical stage of the disease, together with investigation of the activity of MMP-2 and MMP-9 in relation to tumor size, lymph node involvement and steroid and HER2 receptor status.
Materials and Methods
Patients
In this study, we examine the activity of MMP-2 and MMP-9 in the tumor and adjacent tissue samples of 60 breast cancer patients (clinical stage I, II and III) by gelatin zymography. The study was carried out after fulfilling all required ethical standards, and tumor tissues were investigated according to the ethical standards, with informed consent of patients at the Institute of Oncology and Radiology of Serbia.
Tissue Samples
Malignant and adjacent breast tissues (~100 mg) were quickly weighed and homogenized on ice. Homogenized tissue samples were treated with 200 μL of lysing buffer containing 20 mM Tris–HCl buffer, pH 7.5, 150 mM NaCL, 5 mM EDTA, 1 % TRITON X-100, 0.5 % for 1 h on 4 °C. After centrifugation at 10,000 rpm for 10 min at 4 °C, the obtained supernatant fluid presents the total cell lysate. The total protein concentrations were measured by Bradford assay [14] that has been adapted to microplates. Tissue lysates equivalent to 50 μg protein were mixed with equal volumes of sample buffer (4 % SDS, 20 % Glycerol, 0.004 % Bromophenol, 0.125 M Tris HCL) and kept at room temperature for 30 min.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Tumor and adjacent tissue extracts was diluted in 200 g/L sucrose to prepare the samples. The samples were analyzed by SDS-PAGE to determine the molecular massSDS-PAGE was performed with 75 g/L polyacrylamide gel [15, 16] under no reducing conditions using a solution mixture of protein markers containing ovalbumin (45 kDa), bovine serum albumin (BSA, 67 kDa), β-galactosidase (116 kDa) and myosin (200 kDa).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis Zymography
Samples were analyzed by SDS-PAGE zymography according to the method of Kleiner and Stetler-Stevenson [17]. For each sample, 28 mg of total tissue protein was loaded. Samples were incubated for 40 min at 37 °C and electrophoresis was performed without reduction on 75 g/L polyacrylamide gels copolymerized with 0.01 g/L gelatin at 4 °C at a constant current of 15 mA. When the tracking dye at the front reached the bottom of the gel, the gel was removed and shaken gently for 45 min in 0.25 g/L Triton X-100 to remove SDS. Then, the gel slabs were transferred to a bath (without Triton X-100) and washed for 20 min to remove Triton X-100. Then, the gels were incubated and shaken for 60 h in 0.1 mol/L glycine, 50 mmol/L Tris–HCl, 5 mmol/L CaCl2, 1 μmol/L ZnCl2, 0.5 mol/L NaCl, and pH 8.3, at 37 °C. Regions of proteolytic activity were visualized as clear zones against a blue background after 3-h staining with Coomassie brilliant blue G-250 dye [18]. The activity was measured using a gel image system (Kodak Image 1D 3.6.). Following gelatin zymography, gels containing samples run in triplicate were subjected to densitometric analysis to quantify the relative activity of the two gelatinases. Densitometric data in OD were then normalized for 1 mg of total tumor tissue proteins and plotted in graph using MS Excel software.
Quantification of MMP-2 and MMP-9 Activity
Following zymography, the degree of gelatin digestion was quantified using a scanner equipped with a transparency option interfaced to an IBM PC. Gels were scanned using gel image system (Kodak Image 1D 3.6.), in a gray scale mode at 169 mm pixel size and 1250–1650 (X–Y) pixel count, using the autodensity feature on a scale ranging from 0 (clear) to 255 (opaque). The image was digitally inverted, so that the integration of bands was reported as positive value. The pixel density was determined after background subtraction and used to calculate the integrated density of a selected band. Values of integrated density were reported in volume units of pixel intensity per mm2. The integrated density of each band is reported as the mean of three different measurements of the same gel for each sample examined in triplicate.
MMP Inhibition Test
In order to verify that the clear zones on blue background represent the activity of MMPs, 5 mmol/L EDTA was added into samples before incubation to inhibit MMP activities on gelatin zymography.
Pathological Assessment of Primary Tumors
This study included 60 breast cancer patients who underwent surgery as primary treatment. The age of the patients ranged from 38 to 82 years (median 59 years).
According to the TNM classification of the UICC, tumor size (T) was classified by the pathologist after surgery as T1, T2 or T3. The presence of regional lymph node involvement (N) was assessed histologically as No (lymph node negative) or N+ (lymph node positive). The presence of distant metastases (M) was excluded by clinical, X-ray and ultrasound examination in all cases (Mo). Typing of primary tumors was performed according to the WHO classification, while for grading the Ellis and Elston system was used. Immunostaining was performed on formalin-fixed paraffin-embedded 4 μm tissue sections using the primary mouse monoclonal antibodies for estrogen receptor (ER), progesterone receptor (PR), HER receptor, Citokeratin 5/6 (CK5/6), and epidermal growth factor receptor (EGFR, HER1), respectively. Staining was visualized using the Envision method (Dakocytomation, Copenhagen, Denmark) and DAB. For assessment of ER, PR, HER2, CK5/6 and EGFR staining the Allred score was used [19]. Samples were considered hormone receptor negative when staining of both steroid receptors was negative and hormone receptor positive when positive staining for both receptors was observed. The DAKO-HercepTest scoring system was used to evaluate the HER2 staining. Samples with a score of 0 or 1 were defined negative and samples with a score of 2+ or 3+ were defined as positive or strongly positive, respectively [20]. Samples were considered according to the new molecular classification as luminal A (ER+, PR+, and HER2−), luminal B (ER+, PR+, and HER2+), HER2 subtype (ER−, PR−, and HER2+), basal-like (ER−, PR−, HER2−, and CK5/6+/EGFR+), and normal breast-like (ER−, PR−, and HER2−) [19].
Statistical Analysis
We measured activity of latent (proMMP) and active (aMMP) form of MMP-2 and MMP-9 in tumor and adjacent tissue and data obtained were analyzed by the non-parametric Mann–Whitney U test. We correlated gelatinase activity and clinicopathological features using Fisher’s exact test with Bonferroni corrections. Assumption of normality was verified using the normal probability plot, Shapiro–Wilk’s W test and the Levene’s test for homogeneity of variances. Statistical analyses were performed with the software package Statistica version 6, the level of significance being set at p < 0.05.
Results
Detection of Latent and Active form of MMP-2 and MMP-9 Activities
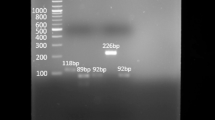
In Fig. 1., SDS-PAGE zymography shows activity of both latent and active form of MMP-2 and MMP-9 in breast cancer tissue in patients without (a) and with (b) specific inhibitor of metalloproteases. It is obvious that in the presence of EDTA, as specific inhibitor of metalloproteases, there is no clear zone on a dark background in bands at 72 kDa (proMMP-2), 64 kDa (aMMP-2), 92 kDa (proMMP-9), and 83 kDa (aMMP-9) in tumor tissue of breast cancer patients by biochemical analyses. Figure 1 also shows the latent and active form of MMP-2 and MMP-9 in adjacent tissue (c) of breast cancer patients and electrophoretic traces of standard protein markers (d) used in this study.
Electrophoretic traces of SDS-PAGE zymography. The activity of latent and active form of MMP-2 and pMMP-9 in tumor tissue of breast cancer patients (a). Inhibited MMP-2 and MMP-9 activity in tumor tissue of breast cancer patients with EDTA (there is no presence of clear zones at corresponding positions compared to the shown identical patient tumor specimen) (b). The activity of latent and active form of MMP-2 and pMMP-9 in adjacent tissue of breast cancer patient (c). Standard proteins marker (d)
Representative zymograms of active and latent MMP-2 and MMP-9 activity in breast cancer patients show that MMPs activity (at positions in gels which correspond to proMMP-2 at 72 kDa, aMMP-2 at 64 kDa, proMMP-9 at 92 kDa, and aMMP-9 at 83 kDa) had more intense gelatinolytic bands for both latent and active enzyme forms of MMP-2 (72 kDa, 64 kDa) and MMP-9 (92 kDa, 83 kDa) in tumor tissue, when compared to adjacent normal tissue (Fig. 1).
More detailed analyses of the mean values with standard deviation (mean ± SD) of MMP-2 activity show significant increase of proMMP-2 and aMMP-2 in tumor tissue compared to adjacent tissue (p = 0.001, p < 0.001, respectively, Mann–Whitney, U test) (Fig. 2a, b). The activity of proMMP-9 in tumor tissue of breast cancer patients significantly increases compared to adjacent patient tissue (179 ± 8.79 vs. 154.4 ± 8.42, p = 0.007, Mann–Whitney, U test) (Fig. 2c). Moreover, tumor tissue had a significantly higher aMMP-9 activity compared to adjacent tissue (139.4 ± 4.52 vs. 112.5 ± 3.58, p < 0.001, Mann–Whitney, U test) (Fig. 2d).
Activities of MMP-2 and MMP-9 Correlate with Clinical Stage of Breast Cancer Patients
The activity of pro and active MMP-2 increased significantly with each advanced clinical stage of disease (proMMP-2: p = 0.003 to p = 0.008, aMMP-2: p = 0.009 to p = 0.0168, Mann–Whitney, U test) (Fig. 3a). Analyses of the mean values with standard deviation (mean ± SD) of proMMP-9 activity show significant increase in tumor tissue of breast cancer patients in clinical stage III compared to patients in clinical stage II and I (229.2 ± 58.54 vs. 157.1 ± 38.83, p < 0.0001, 229.2 ± 58.54 vs. 99.31 ± 55.34, p < 0.0001, 164.4 ± 31.61 vs. 137.4 ± 25.85, p = 0.006, respectively, Mann–Whitney, U test), as well as significant increase of activity of aMMP-9 in patients in clinical stage III compared to patients in clinical stage II and I (229.2 ± 58.54 vs. 99.31 ± 55.34, p < 0.0001, p < 0.001, respectively Mann–Whitney, U test) (Fig. 3a).
The activity of pro and active form of MMP-9 and MMP-2 showing stage-dependent increase in activity of proMMP-2 (p = 0.003 to p = 0.008), aMMP-2 (p = 0.009 to p = 0.0168), proMMP-9 (p = 0.006 to p < 0.001) and aMMP-9 (p < 0.001, Mann–Whitney, U test) in evaluated breast cancer tissue of patients in clinical stage I, II and III (a). Association of activity of proMMP-2 (T2 vs. T1 p = 0.039; T3 vs. T1 p = 0.033; T3 vs. T2 p = 0.18), aMMP-2 (T2 vs. T1 p = 0.01; T3 vs. T1 p = 0.07; T3 vs. T2 p = 0.36), proMMP-9 (T2 vs. T1 p = 0.05; T3 vs. T1 p = 0.006; T3 vs. T2 p = 0.043) and aMMP-9 (T2 vs. T1 p = 0.002; T3 vs. T1 p = 0.031; T3 vs. T2 p = 0.05, Mann–Whitney U tests) in the tumor tissue of breast cancer patients with respect to tumor size (b). Activity of proMMP-2 (p = 0.005), aMMP-2 (p = 0.007) and aMMP-9 (p = 0.013) significantly increasing with respect to lymph node involvement, while there is no significant difference between proMMP-9 activity and lymph node involvement (p = 0.1, Mann–Whitney, U test) (c). * indicates a significant difference, p ≤ 0.05; ns indicates no significant difference, p > 0.05
MMP-2 and MMP-9 Activity with Respect to Patient’s Characteristics
Patients' clinical and pathological characteristics was estimated and presented in Table 1. The patients were classified according to the TNM and UICC classification by: tumor size (T) as T1 (n = 21), T2 (n = 32), T3 (n = 7); the presence of regional lymph node involvement (N) as node negative (N0)—31 patients, lymph node positive (N+)—29 patients. The patients were classified according to the Bloom-Richardson classification by the histological grade of the tumors as: grade I (G1)—12, grade II (G2)—35, and grade III (G3)—13 patients.
Based on histological type of tumors, there are 31 invasive ductal carcinoma (IDC), 26 invasive lobular carcinoma (ILC), and 3 of mixed, rare or unknown histology. All patients with breast cancer were also estimated with respect to hormone receptor status as hormone receptor negative—8 and hormone receptor positive—52. From total number of 60 patients analyzed for HER2 receptor status, 53 were negative (0, 1+) and 7 were positive (2+, 3+) (Table 1). Based on receptors status of tumors there are 7 patients basal-like tumors (ER negative, PR negative, HER2 negative, CK5/6 positive or EGFR positive), 46 patients luminal A (ER positive PR positive HER2 negative), 4 patients luminal B (ER positive, PR positive, HER2 positive), and 3 patients have HER2 subtype (ER negative, PR negative, HER2-positive receptors).
In Fig. 4, we show photomicrographs of tumor sample of basal–like breast cancer displaying the typical morphological features of triple-negative/basal-like cancer such as a high-grade ductal carcinoma (grade 3) associated with prominent lymphoid aggregates; cytologically, the tumor cells with marked nuclear pleomorphism and conspicuous mitotic activity and prominent membranous expression of CK5/6 or EGFR.
The typical morphological features of triple-negative/basal-like breast cancer are shown: marked nuclear pleomorphism, conspicuous mitotic activity, high-grade ductal carcinoma associated with prominent lymphoid aggregates (a). Immunohistochemistry for ER (b), PR (c), and HER2 (d) shows negative reaction. The tumor cells also show prominent membranous expression of CK5/6 (e)
In addition, we analyzed MMP-2 and MMP-9 activity with respect to tumor size and lymph node involvement and present individual data for each patient, since there is scarce data regarding the association of these factors. It is obvious from Fig. 3b that higher proMMP-2 activity had T2 tumors compared to T1 patients (T2 vs. T1 p = 0.039; T3 vs. T1 p = 0.033; T3 vs. T2 p = 0.18, Mann–Whitney U tests). Similar results were obtained with respect to tumor size and aMMP-2 activity (T2 vs. T1 p = 0.01; T3 vs. T1 p = 0.07; T3 vs. T2 p = 0.36) (Fig. 3b). However, patients in group T3 have higher values of proMMP-9 activity compared to T2 and T1 patient groups (T2 vs. T1 p = 0.05; T3 vs. T1 p = 0.006; T3 vs. T2 p = 0.043, Mann–Whitney U tests) (Fig. 3b). Also, higher aMMP-9 activity have tumors in group T3 compared to T2 and T1 patients group (T2 vs. T1 p = 0.002; T3 vs. T1 p = 0.031; T3 vs. T2 p = 0.05, Mann–Whitney U tests) (Fig. 3b).
Moreover, latent and active form of MMP-2 (Fig. 3c) in patients with axillary lymph node involvement N+ (positive) with respect to N0 (negative) show significantly increased activity (p = 0.005, p = 0.007, respectively, Mann–Whitney, U test) in the group of patients with lymph node involvement. We found no significant difference between axillary lymph node involvement and proMMP-9 activity (p = 0.1, Mann–Whitney, U test), while there is significant difference between axillary lymph node involvement and aMMP-9 activity in tumor tissue (p = 0.013, Mann–Whitney, U test) (Fig. 3c).
MMP-2 and MMP-9 Activity in Tumor Tissue with Respect to Receptor Status
Since estrogen and progesterone receptor status and HER2 expression have an important role in breast cancer, we analyzed MMP-2 and MMP-9 activity with respect to their expression in tumor tissue samples. We found no significant difference between hormone receptor expression and proMMP-2 (p = 0.074) and proMMP-9 (p = 0.49) activity (Fig. 5a). In this study, we show that there is a significant difference between aMMP-2 and aMMP-9 activity and hormone receptor expression (p = 0.007, p = 0.013, respectively) (Fig. 5a).
The steroid hormone receptor expression in relation to proMMP-2 (p = 0.074), aMMP-2 (p = 0.007), proMMP-9 (p = 0.49), and aMMP-9 (p = 0.013, Mann–Whitney, U test) activity in tumor tissue of breast cancer patient (a). Lack of association of HER2 receptor expression with respect to proMMP-2 (p = 0.88), aMMP-2 (p = 0.06), proMMP-9 (p = 0.6), and aMMP-9 (p = 0.09, Mann–Whitney, U test) activity in the tumor tissue of breast cancer patients (b). Values of integrated density were reported in volume units of pixel intensity per mm2
No significant difference was found between HER2 expression and proMMP-2 (p = 0.88) and proMMP-9 (p = 0.6) activity, as well as between HER2 expression and aMMP-2 (p = 0.06) and aMMP-9 activity (p = 0.09) (Fig. 5b).
In this study, we show that patients with basal-like cancers had significantly higher proMMP-2 activity (p = 0.015) compared to patients with luminal A tumors (Fig. 6a). However, no significant difference was found between activity of aMMP-2 (p = 0.07), ProMMP-9 (p = 0.21) and aMMP-9 (p = 0.11) in basal-like cancers and patients with luminal A (Fig. 6b, c, d).
MMP-2 and MMP-9 Activity in Adjacent Tissue with Respect to Receptor Status
The latent and active form of MMP-2 and MMP-9 (Table 2) in patients with negative hormone receptor show significantly increased activity (p = 0.05, p = 0.02, p = 0.001, p = 0.003, respectively, Mann–Whitney, U test) in adjacent tissue compared to the group of patients with positive hormone receptor. Moreover, we found a significant increase of aMMP-2 activity in adjacent tissue of patients with basal-like tumors compared to patients with luminal A tumors (p = 0.04, Mann–Whitney, U test) (Table 2). However, there is no significant difference in proMMP-2 and MMP-9 (latent, active) activity in adjacent tissue between basal-like and luminal A tumors (p = 0.12, p = 0.16, p = 0.07, Mann–Whitney, U test) (Table 2).
Discussion
MMP-2 and MMP-9 activity in the tumor tissue of breast cancer patients have been shown to be possible molecular markers of tumor invasiveness, as well as therapeutic targets [21, 22]. This study shows significantly increased activity of latent and active forms of MMP-2 and MMP-9 are detected in tumor tissues of breast cancer patients, as opposed to paired adjacent breast tissue, an analysis that has been shown in very few previous studies [4, 8]. In this study, we also show significant stage-dependent increase of proMMP-2, aMMP-2, proMMP-9, and aMMP-9 activity in tumor tissue of breast cancer patients. Increasing tumor MMP-2 and MMP-9 activity with clinical stage suggests the usefulness of these parameters as staging markers for breast cancer patients.
We show that patients with larger tumor size (T3 or T2) had significantly higher activity of proMMP-2 and aMMP-2 compared to patients with smaller tumors (T1). It is of interest that proMMP-9 and aMMP-9 activity in tumor tissue shows a more significant positive association with tumor size. Li et al. [6] have reported that the positive immunostaining of MMP-2 significantly correlated with tumor size. Furthermore, patients with lymph node positive cancer have significantly higher proMMP-2, aMMP-2, and aMMP-9 activity than node negative. This finding is in concordance with the only previous study published for breast cancer patients showing that MMP-9 activity in gelatin zymography correlates inversely with number of axillary nodal involvement [23, 24]. All these results implicate that MMP-2 and MMP-9 may play an important role in breast cancer progression.
Importantly, although markers such as ER and PR may have limited value as pure prognostic indicators, they may in combination with other parameters, have predictive value for response to therapy [25]. However, we did find that estrogen or progesterone receptor-negative tumors had increased activity of aMMP-2 and aMMP-9 in tumor tissue. In this sense, as a well-known risk factor in breast carcinoma is hormone receptor negativity, Talvensaari-Mattila et al. showed that MMP-2 negativity could serve as a marker for favorable prognosis in breast carcinoma patients with a hormone receptor-negative tumor [26, 27]. These results are in agreement with our results, indicating that MMP-2 and MMP-9 activity may play a role in the invasive and migratory phenotype of hormone-negative tumors.
Overexpression of the HER2 is found in 10–20 % of breast cancers and is associated with a worse prognosis [28]. The effect of HER2 overexpression on the invasion capacity of tumor cells is related, at least in part, to the up-regulation of MMP-2 and MMP-9 expression and their proteolytic activity [11]. We did not find significant difference between aMMP-2 activity and HER2 receptor expression in tumor tissue, although HER2-positive tumors had increased activity of aMMP-2.
Luminal A tumors usually have well/moderate differentiation, low proliferating index, and better prognosis [12]. The pattern of metastatic spread of tumors with a basal-like phenotype is different from that of luminal A cancers: as they are reported to less frequently disseminate to axillary nodes and bones and to favor a hematogenous spread [19, 29]. It has been shown that proMMP-2 directly regulates angiogenesis and that cleavage of collagen type IV by MMP-2 exposes a cryptic αvβ3 integrin binding site within collagen on the surface of angiogenic blood vessels [30]. In concordance with these findings, we did find a higher activity of proMMP-2 in tumor tissue of patients with basal-like, as compared to luminal A subtype. Thus, higher activity of proMMP-2 in basal-like groups suggests the MMP-2 may play an important role in hematogenic spread of basal-like carcinoma.
It has been shown that MMP-2 and MMP-9 are predominantly made by stromal cells [31], and we show significant MMP-2 and -9 activity in adjacent tissue, although at lower level compared to cancer tissue. Cancer cells might stimulate stromal cells in adjacent tissue to synthesize MMPs in a paracrine manner through secretion of interleukins, interferons, emmprin, and growth factors [32]. Moreover, we also show positive association between gelatinase activity of adjacent tissue and tumor steroid receptor negativity. Furthermore, we show increased adjacent tissue activity of aMMP-2 in basal-like tumors. These results suggests that increased invasive and angiogenic capacities of basal-like cancers are in part due to increased aMMP-2 activity. This implies that MMP-2 up-regulation appears to be independent of steroid hormones. In this sense, additional biochemical investigations regarding molecular pathways in adjacent tissue could give better insight into aggressive nature of basal-like carcinomas.
Taken together, our results show that gelatinases could be considered as valuable markers for diagnosis and staging of breast cancer. The reported increased activity of aMMP-2 and aMMP-9 in tumor tissue of patients with hormone receptor negativity implicates that MMP-2 and MMP-9 activity may be poor clinicopathological markers of hormone-negative breast cancer. Reported increased activity of MMP-2 in tumor and adjacent tissue of basal-like tumors implicates that MMP-2 might have a role in aggressive biology of basal-like cancers. The measurement of MMPs in primary cancers may be of benefit in designing MMP inhibitors for therapy.
Abbreviations
- MMPs:
-
Matrix metalloproteinases
- DAB:
-
3,3diaminobenzidine
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- CK5/6:
-
Citokeratin 5 or 6
References
Woessner, J. F., Jr. (1991). Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB Journal, 1991(5), 2145–2154.
Hofmann, U. B., Houben, R., Brocker, E. B., & Becker, J. C. (2005). Role of matrix metalloproteinases in melanoma cell invasion. Biochimie, 87, 307–314.
Liu, S. C., Yang, S. F., Yeh, K. T., Yeh, C. M., Chiou, H. L., Lee, C. Y., et al. (2006). Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clinica Chimica Acta, 371(1–2), 92–96.
Shah, F. D., Shukla, S. N., Shah, P. M., Shukla, H. K., & Patel, P. S. (2009). Clinical significance of matrix metalloproteinase 2 and 9 in breast cancer. Indian Journal of Cancer, 46, 194–202.
Remacle, A. G., Noel, A., Duggan, C., McDermott, E., O’Higgins, N., Foidart, J. M., et al. (1998). Assay of matrix metalloproteinases types 1,2,3 and 9 in breast cancer. British Journal of Cancer, 77(6), 926–931.
Li, H. C., Cao, D. C., Liu, Y., Hou, Y. F., Wu, J., Lu, J. S., et al. (2004). Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Research and Treatment, 88(1), 75–85.
Hanemaaijer, R., Verheijen, J. H., Maguire, T. M., Visser, H., Toet, K., McDermott, E., et al. (2000). Increased gelatinase-A and gelatinase-B activities in malignant vs. benign breast tumors. International Journal of Cancer, 86(2), 204–207.
Jinga, D. C., Blidaru, A., Condrea, I., Ardeleanu, C., Dragomir, C., Szegli, G., et al. (2006). MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: Correlations with prognostic factors. Journal of Cellular and Molecular Medicine, 10(2), 499–510.
Sullu, Y., Demirag, G. G., Yildirim, A., Karagoz, F., & Kandemir, B. (2011). Matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in invasive ductal carcinoma of the breast. Pathology, Research and Practice, 207(12), 747–753.
Pellikainen, J. M., Ropponen, K. M., Kataja, V. V., Kellokoski, J. K., Eskelinen, M. J., & Kosma, V. M. (2004). Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clinical Cancer Research, 10, 7621–7628.
Konecny, G., Untch, M., Arboleda, J., Wilson, C., Kahlert, S., Boettcher, B., et al. (2001). HER-2/neu and urokinase-type plasminogen activator and its inhibitor in breast cancer. Clinical Cancer Research, 7(8), 2448–2457.
Weigelt, B., & Reis-Filho, J. S. (2009). Histological and molecular types of breast cancer: Is there a unifying taxonomy? Nature Reviews Clinical Oncology, 6(12), 718–730.
Abd El-Rehim, D. M., Pinder, S. E., Paish, C. E., Bell, J., Blamey, R. W., Robertson, J. F., et al. (2004). Expression of luminal and basal cytokeratins in human breast carcinoma. Journal of Pathology, 203(2), 661–671.
Reis-Filho, J. S., & Tutt, A. N. (2008). Triple negative tumours: A critical review. Histopathology, 52(1), 108–118.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 1976(72), 248–254.
Stetler-Stevenson, W. G. (1990). Type IV collagenases in tumor invasion and metastasis. Cancer and Metastasis Reviews, 9(4), 289–303.
Kleiner, D. E., & Stetler-Stevenson, W. G. (1994). Quantative zymography detection of picogram quantities of gelatinases. Analytical Biochemistry, 218(2), 325–329.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1970(227), 680–685.
Lavasani, M. A., & Moinfar, F. (2012). Molecular classification of breast carcinomas with particular emphasis on “basal-like” carcinoma: a critical review. Journal of Biophotonics, 5(4), 345–366.
Stankovic, S., Konjevic, G., Gopcevic, K., Jovic, V., Inic, M., & Jurisic, V. (2010). Activity of MMP-2 and MMP-9 in sera of breast cancer patients. Pathology, Research and Practice, 206(4), 241–247.
Zucker, S., Hymowitz, M., Conner, C., Zarrabi, H. M., Hurewitz, A. N., Matrisian, L., et al. (1999). Measurement of matrix metalloproteinases and tissue inhibitors of metalloproteinases in blood and tissues. Clinical and experimental applications. Annals of the New York Academy of Sciences, 878, 212–227.
Jezierska, A., & Motyl, T. (2009). Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Medical Science Monitor, 15(2), 32–40.
Talvensari-Mattila, A., Pakko, P., Blanco-Sequeiros, G., & Turpeenniemi-Hujanen, T. (2001). Matrix metalloproteinases-2 is associated with the risk for a relapse in postmenopausal patients with node positive breast carcinoma treated with antiestrogen adjuvant therapy. Breast Cancer Research and Treatment, 65(1), 55–61.
Hirvonen, R., Talvensari-Mattila, A., Pakko, P., & Turpeenniemi-Hujanen, T. (2003). Matrix metalloproteinases-2 (MMP-2) in T (1–2) N0 breast carcinoma. Breast Cancer Research and Treatment, 77(1), 85–91.
Ali, H. R., Dawson, S. J., Blows, F. M., Provenzano, E., Pharoah, P. D., & Caldas, C. (2011). Cancer stem cell markers in breast cancer: Pathological, clinical and prognostic significance. Breast Cancer Research, 13(6), R118.
Talvensaari-Mattila, A., Paakko, P., & Turpeenniemi-Hujanen, T. (2003). Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. British Journal of Cancer, 89, 1270–1275.
Rahko, E., Jukkola, A., Melkko, J., Paavo, P., Bloigu, R., Talvensaari-Mattila, A., et al. (2004). Matrix metalloproteinase-9 (MMP-9) immunoreactive protein has modest prognostic value in locally advanced breast carcinoma patients treated with an adjuvant antiestrogen therapy. Anticancer Research, 24(6), 4247–4253.
Banerjee, S., & Smith, I. E. (2010). Management of small HER2-positive breast cancers. Lancet Oncology, 11(12), 1193–1199.
Fulford, L. G., Reis-Filho, J. S., Ryder, K., Jones, C., Gillett, C. E., Hanby, A., et al. (2007). Basal-like grade III invasive ductal carcinoma of the breast: Patterns of metastasis and long-term survival. Breast Cancer Research, 9(1), R4.
Hornebeck, W., Emonard, H., Monboisse, J. C., & Bellon, G. (2002). Matrix-directed regulation of pericellular proteolysis and tumor progression. Seminars in Cancer Biology, 12(3), 231–241.
Guo, W., & Giancotti, F. G. (2004). Integrin signalling during tumour progression. Nature Reviews Molecular Cell Biology, 5, 816–826.
Bourboulia, D., & Stetler-Stevenson, W. G. (2010). Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Seminars in Cancer Biology, 20(3), 161–168.
Acknowledgments
This study was supported by the Grant of the Ministry of Science of Serbia No. 175056. The authors also thank Marija Jankovic for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radenkovic, S., Konjevic, G., Jurisic, V. et al. Values of MMP-2 and MMP-9 in Tumor Tissue of Basal-Like Breast Cancer Patients. Cell Biochem Biophys 68, 143–152 (2014). https://doi.org/10.1007/s12013-013-9701-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9701-x