Abstract
The objective of this study was to evaluate the clinical efficacy and safety of nitroprusside injection for preventing the slow-flow/no-reflow phenomenon after percutaneous coronary intervention (PCI). We searched the Cochrane Central Register of Controlled Trials (Issue 2, 2011), PubMed, EMbase, and Google Scholar for data. Two reviewers independently evaluated the quality of the included studies and extracted the data. A meta-analysis was performed using RevMan 5.0 software. Four randomized controlled trials (RCTs) involving 319 patients were included. The results of the meta-analyses showed that intracoronary nitroprusside is beneficial in preventing no-reflow/slow-flow, in reducing corrected TIMI frame count, and in improving left ventricular ejection fraction. It also likely reduces adverse reactions in patients after PCI and rehospitalization due to cardiovascular events. However, we must caution that in this review, there is a moderate possibility of bias with regard to patient selection, performance, and publication because of the small number of included studies. A larger sample size and high-quality RCTs are needed for a more reassuring analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous coronary intervention (PCI) is the most pivotal means of reperfusion for acute myocardial infarction (AMI) patients. The application of PCI has significantly improved the short- and long-term prognosis of AMI patients [1]. However, numerous studies have indicated that despite the opening of the epicardial coronary vessels, in some AMI patients, ischemic myocardia cannot be effectively reperfused. It expresses as limited microcirculation blood flow, stagnation of contrast agent flow, persistent elevated ST segment in ECG, and persistent chest pain, a phenomenon known as no-reflow [2]. Some reports suggested that in AMI patients undergoing PCI, the incidence of no-flow is about 25 % in the saphenous vein grafts (SVGs) and this no-flow is independently associated with sudden cardiac death and cardiac events [3, 4]. Nitroprusside is a strong vasodilator and its activity relies on the direct formation of nitric oxide which plays a powerful role in relaxation of vascular smooth muscle [5]. Therefore, the application of sodium nitroprusside in preventing no-flow is a very important and effective method. Although some clinical trials studied the effect of nitroprusside in preventing no-reflow after PCI, the efficacy and clinical applications of nitroprusside are still controversial. There are no standardized clinical guidelines for the application of nitroprusside, leading to a lack of standardized treatment of no-flow after PCI in AMI patients. In order to provide a scientific basis for clinical use of nitroprusside, it is necessary to evaluate the drug efficacy and safety in preventing no-flow using the method of the Cochrane systematic review.
Materials and Methods
Criteria of Ruling In and Ruling Out
Study Type
Randomized controlled trials of the effect of nitroprusside in preventing no-flow after PCI, regardless of blinding or not, were chosen.
Rule in Criteria
Patients with AMI, in line with the WHO diagnostic criteria for the diagnosis of AMI, who underwent PCI were chosen. Patients with AMI combined with old myocardial infarction, ischemic cardiomyopathy, severe heart failure, moderate to severe liver and kidney dysfunction, left coronary disease, and with contraindication of anticoagulant were excluded.
Interventions
We chose nitroprusside versus placebo (including the blank control, saline, and nitroglycerin), requiring drugs to be administered by intracoronary injection.
Indicator Measurement
Primary indicators: corrected TIMI frame count (CTFC), thrombolysis in myocardial infarction trial (TIMI) <grade 3; secondary indicators: adverse cardiac events (MACE, including heart failure, target vessel revascularization, myocardial reinfarction, postoperative death) rate, readmission rates due to cardiac causes, left ventricular ejection fraction, ST segment regression, the final infarct size.
Search Strategy
The Cochrane Library of Controlled Trials databases (2011 No. 2), PubMed (1966 ~ 2011.4), EMbase (1900 ~ 2011.4), and Google Scholar were searched. Searching key words included slow-flow/no-reflow, PCI, nitroprusside, etc.
Data Extraction and Quality Assessment
Using a pre-designed data extraction form, two independent researchers extracted the following information: (a) the basic information of experiments and patients; (b) Interventions, outcome indicators, and loss of follow-up; (c) Methodology quality. If the information in clinical trials was incomplete, the original author was contacted. In case of different opinions held by the two researchers, third-party arbitration was applied. The methodological quality was evaluated using a cochrane collaboration recommended method; (d) Prevention of selection bias: if random was complete or if the allocation concealment was complete; (e) Prevention of the implementation of bias: if patients and investigators were blinded; (f) Prevention of the attrition bias: if the patient withdrawals and loss of follow-up were completely described and if intention to treat analysis (ITT) was applied; and (g) Prevention of the measurement bias: if the blinded measurement was applied.
Statistical Analysis
The Cochrane Collaboration’s RevMan 5.0 software was used in the meta-analysis. In the enumeration data, risk ratio (RR) was used for the efficacy analysis of statistics; in the measurement data, mean difference (MD) was used. The effects of volume are expressed with 95 % CI. The heterogeneity of the results was measured using χ 2 test. When the studies were statistically homogeneous (P > 0.1, I 2 < 50 %), the fixed effects model was applied; otherwise, a random effects model was applied.
Results
Search Results
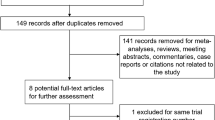
In the primary search, 178 publications were selected, in which 21 were from PubMed, 56 from EMbase, 7 from Cochrane Library, and 94 from Google Scholar. After reading the titles and abstracts, we excluded 174 studies: 87 publications were reviews and animal experiments, 36 reports did not meet the criteria, and 51 publications were repetitions of earlier literature. Ultimately, only 4 studies were included in our study [5–8] including 319 cases of patients. The basic information of these 4 studies is given in Table 1.
Quality Assessment of the Methodology
In all 4 studies, random methods were employed. However, only 2 RCTs [6, 8] reported the specific random methods. In one study [6], a random number table was used. In another study [8], a block random was applied. In none of the 4 studies were the allocation concealments described in detail. In two studies [7, 8], blinded methods were used. Of these two studies, a single-blind study was applied [7] in one and a double-blind study [8] was used in the other. Detailed methods are given in Table 2.
Statistical Analysis
TIMI Flow
Three studies [6–8] reported the incidence of no-reflow/slow-flow by coronary artery injection of sodium nitroprusside after coronary stent placement. There is no significant heterogeneity among these studies. So, the fixed effects model was applied for the Meta-analysis. The results showed that the differences were statistically significant [RR = 0.49, 95 % CI (0.25, 0.95)] (Fig. 1).
Corrected TIMI Frame Count (CTFC)
Four studies [6–9] reported the CTFC of no-flow by injection of sodium nitroprusside. There are no statistical heterogeneities among these studies, so the fixed effects model was applied for the Meta-analysis. The results showed that the differences were statistically significant [MD = −4.24, 95 % CI (−6.40, −2.08)] (Fig. 2).
Final Infarct Size
One study [9] reported the infarct myocardial size after 3 months of coronary artery injection of sodium nitroprusside. The results showed that in comparison with the control group, there was no significant difference between the infarct sizes in the patient groups (16.7 ± 21.5 vs. 20.2 ± 19.3 %, P = 0.403).
Left Ventricular Ejection Fraction
One study [7] reported the LVEF 1 month after the coronary artery injection of sodium nitroprusside preventing no-reflow and the results showed that compared with the control group, the difference of the left ventricular ejection fractions was statistically significant (P < 0.05).
ST Segment Regression
One study [8] reported the ST segment regression after the coronary artery injection of sodium nitroprusside preventing no-reflow and the results showed that compared with the control group, both in 1 and 24 h, there were no significant differences between the two groups (P > 0.05).
The Incidence of Adverse Cardiac Events
Two studies [6, 8] reported the incidence of adverse cardiac events after the injection of sodium nitroprusside. There was no statistical heterogeneity between the studies, so the fixed effects model was applied and Meta-analysis was performed. The results showed that the differences were statistically significant [RR = 0.27, 95 % CI (0.11, 0.63)] (Fig. 3).
Readmission Rates Due to Cardiac Causes
Two studies [6, 8] reported the rehospitalization rates due to the adverse cardiac events. There was no statistical heterogeneity between studies, so the fixed effects model was applied and Meta-analysis was performed. Results showed that the differences were statistically significant [RR = 0.44, 95 % CI (0.21, 0.91)] (Fig. 4).
Discussion
Sodium nitroprusside (SNP) is an inorganic nitrite, which can dilate arteries and veins, relax vascular smooth muscle, reduce the pre- and after-loads of the heart, and decrease ventricular ejection resistance. SNP also increases cardiac output under the condition of low-oxygen consumption and therefore improves the heart function. As a direct nitric oxide donor, SNP plays a strong role in vasodilation by activating guanylate cyclase in vascular smooth muscle cells leading to the increase of cytoplastic cG cyclophosphamide [10, 11]. Meanwhile, SNP can primitively dilate the coronary microcirculation, improve the dysfunction of coronary small blood vessels and microvessels, and therefore effectively induce coronary hyperemia [12–14].
In this study, using Meta-analysis, we quantitatively analyzed the efficacy and safety of injection of SNP in the prevention of coronary no-reflow after PCI. The results show that the injection of SNP can significantly improve TIMI blood flow and CTFC and reduce the incidence of the no-reflow phenomenon. For adverse heart events, SNP can significantly reduce the incidence of adverse events and reduce the readmission rate caused by cardiac events.
Among the 4 RCTs included in this study, 2 RCTs [6, 8] illustrate the methods of randomization. All 4 do not describe the process of allocation concealment; therefore, the possibility cannot be ruled out that selection bias exists in some studies, although due to the specialty of PCI, it is impossible to blind doctors to avoid the effect of implementation bias. However, for the most subjective measurements, such as TIMI flow, CTFC, etc., it is necessary to measure personnel using a blinded method to avoid the effect of bias on the research results. However, only one study of the four RCTs included [8] a double-blind method for patients and researchers. One study [7] used a single-blind method for researchers. The others did not mention whether or not the blind method was used. Therefore, in terms of the measurements of those subjective indicators, the possibility of measurement bias cannot be ruled out. In addition, due to the variety of the patient ages, there is some certain degree of heterogeneity among the studies. All of these will affect the research results.
Currently, many complementary medicines are considered to improve the treatment of AMI and the clinical outcomes of myocardial function [15]. Although the no-reflow phenomenon occurs only in a small proportion of patients, these patients are at a high risk of fatal complications and poor clinical prognosis. Therefore, prevention of microvascular dysfunction can reduce the number of such type of patients, but the significant effect cannot be reflected in the general population. Atrial natriuretic peptide (ANP) is a very good drug. It limits infarct size after myocardial infarction, improves left ventricular ejection fraction, and reduces the incidences of sudden cardiac death and heart failure. However, ANP does not improve the no-reflow phenomenon [16]. Although adenosine can reduce the incidence of no-reflow after initial PCI [17], it cannot improve the clinical outcome after myocardial infarction [18]. Compared with these drugs, SNP can both prevent the occurrence of no-reflow and improve cardiac function and clinical outcomes. Therefore, SNP would be a more promising adjuvant drug treatment.
In summary, intracoronary injection of SNP can improve TIMI flow and CTFC, serving to prevent no-reflow/slow-flow. In addition, SNP is also effective in increasing the left ventricular ejection fraction and reducing major adverse cardiovascular event occurrence. However, due to the methodological limitations in the ruled-in studies, we think that a larger number of samples and high-quality RCTs need to be performed to further demonstrate SNP’s efficacy and safety.
References
Keeley, E. C., Boura, J. A., & Grines, C. L. (2003). Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet, 361, 13–20.
Kloner, R. A., Ganote, C. E., & Jennings, R. B. (1974). The no-reflow phenomenon after temporary coronary occlusion in the dog. Journal of Clinical Investigation, 54, 1496–1508.
Kelly, R. V., Cohen, M. G., & Stouffer, G. A. (2005). Incidence and management of “no-reflow” following percutaneous coronary interventions. American Journal of the Medical Sciences, 329, 78–85.
Akdemir, R., Karakurt, O., Kilic, H., et al. (2010). Effect of reperfusion therapy on index of myocardial performance in acute myocardial infarction: Thrombolytics versus primary angioplasty. Heart and Vessels, 25, 87–91.
Bates, J. N., Baker, M. T., Guerra, R, Jr, & Harrison, D. G. (1991). Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochemical Pharmacology, 42(Suppl), S157–S165.
Pan, W., Wang, L. F., Yu, J. H., et al. (2009). Intracoronary nitroprusside in the prevention of the no-reflow phenomenon in acute myocardial infarction. Chinese Medical Journal, 122, 2718–2723.
Hendler, A., Aronovich, A., Kaluski, E., et al. (2006). Optimization of myocardial perfusion after primary coronary angioplasty following an acute myocardial infarction. Beyond TIMI 3 flow. Journal of Invasive Cardiology, 18, 32–36.
Amit, G., Cafri, C., Yaroslavtsev, S., et al. (2006). Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial. American Heart Journal, 152, 887.e9–887.e14.
Sakamoto, H., Shimamoto, S., Kawase, Y., Ueda, H., Murakami, T., & Tamura, T. (2010). Effect of nitroprusside on reducing infarct size after primary percutaneous coronary intervention for acute myocardial infarction. Journal of the American College of Cardiology, 55, A104.E968.
Valero, S. J., Moreno, R., Reyes, R. M., et al. (2008). Pharmacological approach of no-reflow phenomenon related with percutaneous coronary interventions. Cardiovascular and Hematological Agents in Medicinal Chemistry, 6, 125–129.
Gibson, C. M., & Schomig, A. (2004). Coronary and myocardial angiography: Angiographic assessment of both epicardial and myocardial perfusion. Circulation, 109, 3096–3105.
Tesic, M. B., Stankovic, G., Vukcevic, V., & Ostojic, M. C. (2010). The use of intracoronary sodium nitroprusside to treat no-reflow after primary percutaneous coronary intervention in acute myocardial infarction. Herz, 35, 114–118.
Airoldi, F., Briguori, C., Cianflone, D., et al. (2007). Frequency of slow coronary flow following successful stent implantation and effect of nitroprusside. American Journal of Cardiology, 99, 916–920.
Pasceri, V., Pristipino, C., Pelliccia, F., et al. (2005). Effects of the nitric oxide donor nitroprusside on no-reflow phenomenon during coronary interventions for acute myocardial infarction. American Journal of Cardiology, 95, 1358–1361.
Kiernan, T. J., Ruggiero, N. J, I. I., Bernal, J. M., et al. (2009). The no-reflow phenomenon in the coronary circulation. Cardiovascular and Hematological Agents in Medicinal Chemistry, 7, 181–192.
Kitakaze, M., Asakura, M., Kim, J., et al. (2007). Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): Two randomised trials. Lancet, 370, 1483–1493.
Marzilli, M., Orsini, E., Marraccini, P., & Testa, R. (2000). Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation, 101, 2154–2159.
Ross, A. M., Gibbons, R. J., Stone, G. W., Kloner, R. A., & Alexander, R. W. (2005). A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). Journal of the American College of Cardiology, 45, 1775–1780.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, Q., Li, L., Naing, K.A. et al. Safety and Effectiveness of Nitroprusside in Preventing No-Reflow During Percutaneous Coronary Intervention: A Systematic Review. Cell Biochem Biophys 68, 201–206 (2014). https://doi.org/10.1007/s12013-013-9690-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9690-9