Abstract
Ovarian cancer is one human malignancy which has response portly to doxorubicin. The anti-cancer activity of gambogic acid has been tested in in vitro and in vivo studies. In this study, we showed that gambogic acid, a natural compound, could potentiate the anticancer activity of doxorubicin in ovarian cancer through ROS-mediated apoptosis. Platinum-resistant human ovarian cancer cell line (SKOV-3) was treated with gambogic acid, doxorubicin, or the combination of both to investigate cell proliferation and apoptosis. We found that the combination of gambogic acid and doxorubicin causes synergistic loss of cell viability in SKOV-3 cells and this synergistic effect correlated with increased cellular ROS accumulation. Moreover, in vivo results showed that gambogic acid and doxorubicin combination resulted in a synergistic suppressing effect on tumor growth in ovarian cancer mice model. Taken together, the results suggested that doxorubicin in combination with gambogic acid might provide a promising therapeutic strategy to enhance chemosensitivity of ovarian cancer to doxorubicin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the third most common gynecologic malignancies and the leading cause of female reproductive system cancers [1]. In 2010, approximately 21,880 ovarian cases were diagnosed, and about 13,850 deaths occur due to ovarian cancer worldwide [2]. Because of the lack of effective screening strategies, most patients with ovarian cancer are diagnosed in advanced-stage of the disease and the average 5-year survival rate is <25 %. Currently, primary treatment for patients with ovarian cancer is cytoreductive surgery followed by platinum-based chemotherapy. Although 70 % patients achieved complete clinical remission after primary treatment, the majority of patients (more than 85 %) will ultimately relapse [3]. Most patients develop resistance to platinum-based drugs, which makes the management of recurrent malignancies challenging [4, 5].
Doxorubicin (Dox), an anthracyclin anticancer-drug, is classified as topoisomerase II inhibitor and can also interfere with DNA replication and transcription [6]. Although Dox has been employed to treat recurrent platinum-resistant ovarian cancer, its usage is limited because of severe dose-dependent side effects including acute nausea and vomiting, stomatitis, neurologic disturbances, myocardial toxicity, alopecia, and bone marrow aplasia [6]. Alternatively, Pegylated liposomal doxorubicin (PLD), a pegylated (polyethylene glycol coated) liposome-encapsulated form of Dox with relatively lower toxicity has been designed and approved by US FDA in the treatment of recurrent ovarian cancer [7]. However, only 10–15 % patients with recurrent platinum-resistant ovarian cancer responded to PLD.
In recent years, combined treatment of conventional anticancer agents with natural compounds has been a focus of study because natural compounds are multi-targeted compared with designed mono-target agents and hence can overcome intrinsic cancer cell resistance to apoptosis [8, 9].
Gambogic acid is the principle active component derived from gamboge, a dry resin of various Garcinia species. The therapeutic effects of gambogic acid including anti-inflammatory, anti-oxidant, anti-viral, and anti-infectious have been recorded in Traditional Chinese Medicine documents [10]. In addition, it is reported that gambogic acid could induce apoptosis in human breast cancer cells and hepatoma cells [11, 12]. Studies also reveal that gambogic acid can significantly suppress the growth of various cancer cells in vitro and in vivo [11–18]. Besides its role as a single-agent anticancer drug, gambogic acid exhibited synergistic effect with conventional chemotherapeutics including 5-fluoracil, oxaliplatin, and docetaxel against human gastric intestinal cancer cells and with proteasome inhibitor murine hepatoma cells [19–21]. However, whether gambogic acid could enhance cytotoxicity of Dox has yet to be investigated.
In this present study, we reported that gambogic acid combined with doxorubicin could produce significant synergistic anti-tumor effect on platinum-resistant ovarian cancer cell line in vitro and in vivo. Furthermore, our results revealed that the synergistic interaction between doxorubicin and gambogic acid is associated with increased ROS accumulation and ensuing ROS-dependent apoptosis.
Methods and Materials
Cell Cultures
Cisplatin-resistant SKOV-3 cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and maintained in RPMI 1640 culture medium supplemented with 10 % fetal bovine serum (FBS). The cells were cultured in a humidified incubator containing 5 % CO2 in the air at 37°.
Cell Growth Inhibition Assay
Cell growth inhibition was assessed according to the methods previously described [22]. Briefly, cells were seeded at a density of 5 × 103 cells/well in 96-well culture plate. Cells were treated with gambogic acid, doxorubicin or in combination of both for 24 h. After treatment, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL in phosphate-buffered saline (PBS)) was added to each well, and the plates were incubated for 4 h at 37 °C. The supernatant was aspirated and the formazan crystals formed by viable cells were dissolved in DMSO. The microplates were agitated for 30 s at a medium rate prior to the spectrophotometric measurement at a wavelength of 570 nm on an ELISA reader (BIO-Tek Elx800, Winooski, VT, USA).
Flowcytometric Analysis of Cell Apoptosis
Apoptosis was determined using an FITC Annexin V apoptosis kit (BD Pharmingen, Franklin Lakes, NJ) according to manufacturer’s instructions. In brief, cells were washed with ice-cold phosphate buffered saline and resuspended in binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, and 2.5 mM CaCl2) at a concentration of 1 × 106 cells/ml. Cells were stained with annexin V-FITC and propidium (PI) for 15 min in dark before being analyzed with flow cytometer (Beckman Coulter Inc., Miami, Florida, USA).
Western Blotting Analysis
Proteins were extracted from washed cells following treatment, subjected to SDS polyacrylamide gel electrophoresis (PAGE) as previously described [23]. The separated proteins were transferred to polyvinylidene difluoride blots (Bio-Rad Laboratories, Hercules, CA), which were probed with specific rabbit polyclonal antibodies (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). The membrane was then extensively washed with PBS containing 0.05 % Tween 20 before incubation for 1 h at room temperature with horseradish peroxidase-conjugated goat antibodies to rabbit immunoglobulin G (Sigma, St. Louis, MI). Immune complexes were finally detected with Westzol enhanced chemiluminescence kit (Intron, Sungnam, Korea).
Caspase-3 Activity Assay
Caspase-3 activity was measured in SKOV3 cell lysate by a colorimetric assay according to the manufacturer’s instruction (Keygen Biotech, Nanjing, China). Following treatment, 1 × 106 cells were extensively washed with PBS before resuspension in 50 μl lysis buffer. The resuspended solution was incubated in ice-bath for 60 min followed by centrifuging at 10,000 g for 1 min. The supernatant was collected and incubated with enzyme-specific colorimetric substrate at 37 °C for 4 h. The colorimetric product was measured using ELISA reader at a wavelength of 405 nm (BIO-Tek Elx800, Winooski, VT, USA).
Reactive Oxygen Species (ROS) Determination
Reactive oxygen species was measured using ROS assay kit according to manufacturer’s instructions. Briefly, 1 × 105 cells were harvested and resuspended in serum-free medium containing 100 μM dihydrodichlorofluorescein diacetate (H2DCFDA). Intracellular H2DCFDA was deesterified and oxidized by ROS to form the fluorescent compound dichlorofluorescein. After a 30-minute incubation at 37 °C, the fluorescence intensity was measured using the fluorescence plate reader (BD Bioscience, San Jose, CA) at Ex./Em. = 488/525 nm.
Experimental Animal and Xenograft Experimental
The animal experiments were performed in accordance with the CAPN (China Animal Protection Law) and the protocols were approved by Institutional Animal Care and Use Committee at Jiangyin Hospital Affiliated to Nanjing University of Traditional Chinese Medicine. Female BALB/c (nu/nu) mice, 6 weeks old, were purchased from Animal Center of China (Beijing, China) and were housed in a light/dark cycle of 12/12 h and allowed free access to rodent chow and water. SKOV-3 cells (1 × 107 cells in 200 μl PBS per mouse) were injected subcutaneously on the right hind flank of mice [24]. Following establishment of tumor (average tumor volume over 150 mm3), mice were randomly allocated into four groups: (A) vehicle control (0.9 % saline); (B) gambogic acid alone (1 mg/kg, Alexis Laboratories, San Diego, CA) [25]; (C) Dox alone (5 mg/kg, Zhejiang Hisun Pharmaceutical, Taizhou, China) [26]; (D) gambogic acid and Dox in combination thrice a week for 2 weeks. Tumor length, width, and body weight of murine model were measured every 5 days. Tumor volume was calculated according to the formula: volume = (length × width2)/2. At endpoint of the study, all mice were killed and pathological examination was performed on major organs including heart, liver, and kidney to evaluate damage.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 11.5 software (SPSS Inc., Chicago, IL). Comparison between groups was performed with Student’s t test. A P value of <0.05 was considered statistically significant.
Results
Gambogic Acid Sensitizes Ovarian Cancer Cells to Doxorubicin
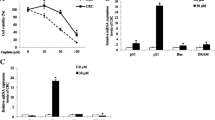
To determine whether gambogic acid could increase chemosensitivity of ovarian cancer cells to doxorubicin, MTT assay was performed to assess cell viability. As shown in Fig. 1a, cell growth was effectively inhibited by Dox in a dose-dependent manner. Next, we tested the cytotoxicity of gambogic acid and found gambogic acid at tested concentration (0.2 and 0.4 μM) was not able to cause significant loss of cell viability (survival rate >80 %, Fig. 1a). However, combinational treatment with Dox and gambogic acid showed a significant sensitization with cell growth inhibition as shown in Fig. 1a. The interaction between Dox and gambogic acid was evaluated by combination index (CI) value calculated according to Chou’s methods [27]. As demonstrated by CI values listed in Fig. 1b, gambogic acid and Dox exerted synergistic effects on inhibition of SKOV-3 cell viability (CI < 1).
Combinational treatment of ovarian cancer cell lines with gambogic acid and doxorubicin reduces cell viability. SKOV-3 cells were treated with gambogic alone, doxorubicin alone, or in combination of these two agents at indicated doses for 24 h, respectively, followed by MTT assay. Cell growth inhibition is demonstrated (a) and CI of doxorubicin plus gambogic acid is presented (CI < 1 indicates synergistic effect) (b). Data presented mean ± SD from four experiments
Gambogic Acid Enhanced Doxorubicin-Induced Apoptosis
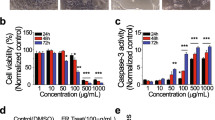
Next flow cytometry analysis was performed to examine whether enhanced cytotoxicity by gambogic acid was mediated by apoptosis. The untreated cells had a background apoptotic rate of 4.8 ± 0.8 %. Single treatment with gambogic (0.4 μM) resulted in a similar apoptotic cell population as shown in Fig. 2a. In contrast, the treatment with Dox at 2 μM showed an increased apoptotic cell population of 15.5 ± 2.8 %, which was further augmented by concurrent gambogic acid (33.7 ± 4.7 %).
Combinational treatment of ovarian cancer cell lines with gambogic acid and doxorubicin potentiates apoptosis. SKOV-3 cells were treated with gambogic alone, doxorubicin alone, or in combination of these two agents at indicated doses for 24 h, respectively, followed by flow cytometry analysis, western blot, and caspase-3 activity analysis. The representative results of flow cytometry are presented and summarized in bar graph (a), caspase-3 and PARP levels are determined using western blot (blots shown are representative of four observations) (b) and caspase-3 activity was measured by colorimetric assay (c). Data presented mean ± SD from four experiments. *P < 0.05, **P < 0.01 (Color figure online)
To confirm the results of cell apoptosis assay, we examined the activity of caspase-3 in cell lysates as well as level of caspase-3 and PARP. As shown in Fig. 2b, combination of gambogic acid and Dox caused additional decrease in caspase-3 level and increase in PARP level. In addition, relative to treatment with single agent, combination of gambogic acid and Dox elicited significant increase in caspase-3 activity (Fig. 2c). Taken together, these results suggested that gambogic acid sensitized SKOV-3 cells to Dox though inducing cell apoptosis.
Gambogic Acid Potentiates Doxorubicin-Induced Apoptosis by Increasing ROS Generation
Generation of excess amount of ROS leads to cancer cell death, and accumulation of ROS is reported to contribute to gambogic acid-induced mitochondrial apoptosis [28, 29]. Therefore, we measured ROS level to examine whether ROS is involved in the synergistic mechanism between gambogic acid and doxorubicin. As shown in Fig. 3a, treatment with gambogic acid lead to a slight increase in ROS generation as demonstrated by increased fluorescence intensity. When cells were exposed to Dox at 1 μM, a significant increase in ROS generation was observed, which is consistent with previous studies [28, 30, 31]. Results also showed that combinational treatment with Dox and gambogic acid generated more ROS relative to Dox alone (Fig. 3a). To further confirm the role of ROS generation in enhanced cytotoxicity by gambogic acid, we tested cell viability in SKOV-3 cells in the presence or absence of antioxidant N-acetylcysteine (NAC, Sigma, St Louis, MI). SKOV-3 cells were preincubated with the antioxidant (1 mM NAC) for 1 h before treatment started and incubation then continued for 24 h. As shown in Fig. 3b, NAC treatment alone did not affect the viability of SKOV-3 cells and the growth inhibitory effect of Dox was only slightly reduced. However, the cytotoxic effect of gambogic acid/Dox combination was eliminated by NAC (P < 0.05), indicating that enhanced cytotoxicity by gambogic acid was mainly mediated by increased generation of ROS.
Potentiation of cytotoxicity is mediated by ROS-dependent JNK and p38 activation. SKOV-3 cells were treated with gambogic alone, doxorubicin alone, or in combination of these two agents at indicated doses for 24 h, respectively, in the presence or absence of NAC as indicated. ROS level is elevated as demonstrated by fluorescence intensity (a), cell viability in the presence of NAC is presented (b), JNK and p38 activation are shown by representative blots (c) and effect of NAC on apoptosis is shown (d). Data presented mean ± SD from four experiments. *P < 0.05, **P < 0.01 (Color figure online)
Gambogic Acid and Doxorubicin ROS-dependently Potentiates JNK and P38 Activation
JNK and p38 activation can lead to cytotoxic effect in cancer cells and ROS has been reported to be able to activate these two pathways [32, 33]. Therefore, we examined activation of JNK and p38 by western blot analysis. As shown in Fig. 3c, combinational treatment with gambogic acid and DOX resulted in marked activation of JNK and p38, which was associated with increased apoptosis (Fig. 3d). To further explore the role of ROS in JNK and p38 activation, cells were treated with gambogic acid and Dox in the presence of NAC. As shown in Fig. 3c and d, NAC significantly inhibited JNK and p38 activation and suppressed apoptosis. In summary, these in intro results showed that combinational treatment with gambogic acid and Dox potentiated growth inhibitory effect and proapoptotic JNK and p38 signaling pathway by generating additional ROS.
In Vivo Anti-tumor Activity of Co-treatment of Gambogic Acid and Doxorubicin
To study the effect of Dox and gambogic acid alone or in combination on tumor growth in vivo, mouse tumor xenografts were used. As shown in Fig. 4, treatment with Dox and gambogic acid suppressed SKOV-3 tumor growth to a markedly greater extent than did treatment with either single agent. In addition, no decrease in average body weight was found at the endpoint of in vivo study, suggesting that little pronounced tissue toxicity or damage was caused by the combined treatment of gambogic acid and doxorubicin.
Tumor growth in xenograft mice model is significantly suppressed by combinational treatment of ovarian cancer cell lines with gambogic acid and doxorubicin. Treatment started after average tumor size reached 150 mm2. Mice were treated with gambogic acid (1 mg/kg) and doxorubicin (10 mg/kg) alone or in combination, thrice a week for 2 weeks. Tumor size and body weight were measured every 5 days. Body weight is expressed relative to the corresponding value on the day treatment started. All data are mean ± SD from 15 mice per group. *P < 0.05 vs. Control, *P < 0.05 vs. Dox or GA
Discussion
Despite the established therapeutic protocol for ovarian cancer, the prognosis of patients with ovarian cancer is extremely poor due to late presentation and high recurrence rate [3]. Currently, a treatment for patients with recurrent ovarian cancer after first-line platinum-based chemotherapy is highly desirable. Treatment with single anticancer agent cannot provide satisfactory therapeutic effect on these patients due to drug resistance and dose-limiting side effect [34]. On the other side, combinational use of chemotherapeutic agents always caused profound tissue toxicity. Since natural compounds are generally safe, combined therapy with conventional cytotoxic agents with natural compound might provide a promising therapeutic strategy with less toxicity. Moreover, due to the ability to bind multiple targets, combinational use of natural compounds might also help overcome chemo-resistance. One natural product that has been explored for anti-tumor activity is gambogic acid, which has been approved by Chinese Food and Drug Administration in clinical trials of various cancers [35].
The anti-tumor effect of gambogic acid has been reported in human cell lines and animal models. Many studies have been conducted to understand the underlying mechanisms by which gambogic acid inhibits cancer cell growth and induces cancer cell apoptosis. The proposed mechanisms include regulating apoptosis relevant proteins Bcl-2, Bax [36, 37], p53, pro-caspase-3 [14, 38, 39], and survivin [40]; activation of c-Jun-N-terminal protein kinase (JNK), p38 [41], and glycogen synthase kinase 3β (GSK3β) signaling pathways [42]; inhibiting NF-κB and its down-dream gene expression [16]. Furthermore, a few mechanisms have also been proposed to elucidate the synergistic effect of gambogic acid with other anti-cancer agents. Wang et al. [19] showed that gambogic acid potentiated the cytotoxic effect of 5-fluorouracil by modulating the level of thymidine synthetase which is the metabolic enzyme of 5-flurouracil. Recently, it has been suggested that enhancement of anticancer efficacy of oxaliplatin, 5-flurouracil, and docetaxel could be attributed to gambogic acid-induced downregulation of chemotherapeutic agent-associated gene including BRCA1, tau, thymidine synthetase, excision repair cross-complementing (ERCC1), and β-tubulin III [21]. Here, our study showed that synergistic effect of combinational use of gambogic acid and Dox was associated with increased ROS accumulation. These results suggest that like other natural compounds, gambogic acid is also a multi-targeted anti-cancer agent.
ROS are byproducts of normal cellular metabolism, which play a crucial role in cell regulation and signal transduction [43]. The level of cellular ROS is tightly balanced by ROS production and elimination by a cell’s antioxidant capacity. Excessive cellular ROS may cause cellular components damage as well as detrimental effects on cellular function and viability. More and more studies showed that cancer cells are associated with elevated ROS levels relative to their normal counterparts, which make cancer cells more susceptible to oxidative damage due to further ROS increase [44]. Therefore generating excess amount of ROS has been proposed to be a mechanism for targeting cancer cells. Although Dox is classified as a topoisomerase II inhibitor, a few studies have demonstrated that the formation of ROS and radicals serves as one of the underlying mechanisms of the cytotoxic activity of Dox [28, 30, 31, 45]. A few studies also showed that chemosensitivity to Dox could be enhanced by increase in cellular ROS [46–48]. In consistency with those previous studies, here we showed that combination of gambogic acid and Dox exhibited potentiated cytotoxicity by augmenting ROS accumulation and consequently activating pro-apoptosis pathways. Moreover, we also found that antioxidant treatment significantly abrogated the enhanced cytotoxicity, indicating the crucial role of ROS accumulation in synergistic mechanism between gambogic acid and doxorubicin.
Both JNK and p38 belong to MAPKs serine/threonine kinases. JNK and p38 activations are reported to be associated with stress-induced apoptosis by a wide range of cytotoxic stimuli including H2O2, UV light, X-rays, γ-irradiation, tumor necrosis factor-α, growth factor, heat shock, and chemotherapeutic drugs [33, 49]. Our findings here demonstrated that potentiated JNK and p38 activation by combined treatment of gambogic acid and Dox was concomitant with increased apoptosis, suggesting that potentiation of JNK and p38 activation might be the predominant mechanism underlying gambogic acid and Dox combination-induced apoptosis.
In addition to these in vitro data, in vivo results from our study also demonstrated that gambogic acid given at 1 mg/kg significantly enhanced the anti-tumor effect of Dox in a xenograft murine ovarian model. Toxicologic studies of gambogic acid have established 4 mg/kg as innocuous dose [50]. Moreover, it has been reported that gambogic acid had selective anticancer activity due to its higher distribution and longer retention time in cancer cells than normal cells [11]. All these suggested that gambogic acid used in our study (1 mg/kg) has little toxicity to normal tissues and combinational therapy of gambogic acid and Dox will not cause additional side effect compared with treatment with Dox as single agent.
In conclusion, our findings, described in this study, showed that gambogic acid potentiates the cytotoxicity activity of Dox in vitro and in vivo. This synergistic cytotoxicity activity of concomitant gambogic acid and Dox correlated with increased cellular ROS accumulation. However, further studies are warranted to fully evaluate the efficacy and safety of gambogic acid in combination with Dox in cancer treatment.
References
Posadas, E. M., Davidson, B., & Kohn, E. C. (2004). Proteomics and ovarian cancer: implications for diagnosis and treatment: a critical review of the recent literature. Current Opinion in Oncology, 16, 478–484.
Jemal, A., Siegel, R., Xu, J., & Ward, E. (2010). Cancer statistics, 2010. CA: A Cancer Journal for Clinicians, 60, 277–300.
Ozols, R. F. (2005). Treatment goals in ovarian cancer. International Journal of Gynecological Cancer, 15(Suppl 1), 3–11.
Ozols, R. F., Bookman, M. A., Connolly, D. C., et al. (2004). Focus on epithelial ovarian cancer. Cancer Cell, 5, 19–24.
Landen, C. N, Jr, Birrer, M. J., & Sood, A. K. (2008). Early events in the pathogenesis of epithelial ovarian cancer. Journal of Clinical Oncology, 26, 995–1005.
Carvalho, C., Santos, R. X., Cardoso, S., et al. (2009). Doxorubicin: the good, the bad and the ugly effect. Current Medicinal Chemistry, 16, 3267–3285.
Campos, S. M., Penson, R. T., Mays, A. R., et al. (2001). The clinical utility of liposomal doxorubicin in recurrent ovarian cancer. Gynecologic Oncology, 81, 206–212.
Prasad, S., Pandey, M. K., Yadav, V. R., & Aggarwal, B. B. (2011). Gambogic acid inhibits STAT3 phosphorylation through activation of protein tyrosine phosphatase SHP-1: potential role in proliferation and apoptosis. Cancer prevention research (Philadelphia, Pa.), 4, 1084–1094.
Bharti, A. C., & Aggarwal, B. B. (2002). Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Annals of the New York Academy of Sciences, 973, 392–395.
Panthong, A., Norkaew, P., Kanjanapothi, D., et al. (2007). Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. Journal of Ethnopharmacology, 111, 335–340.
Yang, Y., Yang, L., You, Q. D., et al. (2007). Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Letters, 256, 259–266.
Zhang, H. Z., Kasibhatla, S., Wang, Y., et al. (2004). Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorganic & Medicinal Chemistry, 12, 309–317.
Liu, W., Guo, Q. L., You, Q. D., et al. (2005). Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World Journal of Gastroenterology, 11, 3655–3659.
Gu, H., Wang, X., Rao, S., et al. (2008). Gambogic acid mediates apoptosis as a p53 inducer through down-regulation of mdm2 in wild-type p53-expressing cancer cells. Molecular Cancer Therapeutics, 7, 3298–3305.
Guo, Q. L., You, Q. D., Wu, Z. Q., et al. (2004). General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacologica Sinica, 25, 769–774.
Pandey, M. K., Sung, B., Ahn, K. S., et al. (2007). Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood, 110, 3517–3525.
Yi, T., Yi, Z., Cho, S. G., et al. (2008). Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Research, 68, 1843–1850.
Yu, J., Guo, Q. L., You, Q. D., et al. (2007). Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis, 28, 632–638.
Wang, J., Liu, W., Zhao, Q., et al. (2009). Synergistic effect of 5-fluorouracil with gambogic acid on BGC-823 human gastric carcinoma. Toxicology, 256, 135–140.
Zou, Z. Y., Wei, J., Li, X. L., et al. (2012). Enhancement of anticancer efficacy of chemotherapeutics by gambogic acid against gastric cancer cells. Cancer Biotherapy and Radiopharmaceuticals, 27, 299–306.
Huang, H., Chen, D., Li, S., et al. (2011). Gambogic acid enhances proteasome inhibitor-induced anticancer activity. Cancer Letters, 301, 221–228.
Gerlier, D., & Thomasset, N. (1986). Use of MTT colorimetric assay to measure cell activation. Journal of Immunological Methods, 94, 57–63.
Iwasa, T., Okamoto, I., Takezawa, K., et al. (2010). Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. British Journal of Cancer, 103, 36–42.
Lee, M. H., Choi, B. Y., Kundu, J. K., et al. (2009). Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: eukaryotic elongation factor 1A2 as a potential target. Cancer Research, 69, 7449–7458.
Ding, L., Huang, D., Wang, J., & Li, S. (2007). Determination of gambogic acid in human plasma by liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 846, 112–118.
Colombo, P. E., Boustta, M., Poujol, S., et al. (2011). Intraperitoneal administration of novel doxorubicin loaded polymeric delivery systems against peritoneal carcinomatosis: experimental study in a murine model of ovarian cancer. Gynecologic Oncology, 122, 632–640.
Chou, T. C., & Talalay, P. (1984). Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation, 22, 27–55.
Kim, K. K., Kawar, N. M., Singh, R. K., et al. (2011). Tetrathiomolybdate induces doxorubicin sensitivity in resistant tumor cell lines. Gynecologic Oncology, 122, 183–189.
Nie, F., Zhang, X., Qi, Q., et al. (2009). Reactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cells. Toxicology, 260, 60–67.
Swift, L. P., Rephaeli, A., Nudelman, A., et al. (2006). Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Research, 66, 4863–4871.
Gewirtz, D. A. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology, 57, 727–741.
Osone, S., Hosoi, H., Kuwahara, Y., et al. (2004). Fenretinide induces sustained-activation of JNK/p38 MAPK and apoptosis in a reactive oxygen species-dependent manner in neuroblastoma cells. International Journal of Cancer, 112, 219–224.
Mansouri, A., Ridgway, L. D., Korapati, A. L., et al. (2003). Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. Journal of Biological Chemistry, 278, 19245–19256.
Naumann, R. W., & Coleman, R. L. (2011). Management strategies for recurrent platinum-resistant ovarian cancer. Drugs, 71, 1397–1412.
Wang, J., & Zhou, Z. (2007). Phase I human tolerability trial of gambogic acid. Chinese Journal of New Drugs, 16, 79–82.
Gu, H., Rao, S., Zhao, J., et al. (2009). Gambogic acid reduced bcl-2 expression via p53 in human breast MCF-7 cancer cells. Journal of Cancer Research and Clinical Oncology, 135, 1777–1782.
Zhao, L., Guo, Q. L., You, Q. D., et al. (2004). Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biological & Pharmaceutical Bulletin, 27, 998–1003.
Xu, X., Liu, Y., Wang, L., et al. (2009). Gambogic acid induces apoptosis by regulating the expression of Bax and Bcl-2 and enhancing caspase-3 activity in human malignant melanoma A375 cells. International Journal of Dermatology, 48, 186–192.
Rong, J. J., Hu, R., Song, X. M., et al. (2010). Gambogic acid triggers DNA damage signaling that induces p53/p21(Waf1/CIP1) activation through the ATR-Chk1 pathway. Cancer Letters, 296, 55–64.
Wang, T., Wei, J., Qian, X., et al. (2008). Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Letters, 262, 214–222.
Chen, J., Gu, H. Y., Lu, N., et al. (2008). Microtubule depolymerization and phosphorylation of c-Jun N-terminal kinase-1 and p38 were involved in gambogic acid induced cell cycle arrest and apoptosis in human breast carcinoma MCF-7 cells. Life Sciences, 83, 103–109.
Li, R., Chen, Y., Zeng, L. L., et al. (2009). Gambogic acid induces G0/G1 arrest and apoptosis involving inhibition of SRC-3 and inactivation of Akt pathway in K562 leukemia cells. Toxicology, 262, 98–105.
Thannickal, V. J., & Fanburg, B. L. (2000). Reactive oxygen species in cell signaling. American Journal of Physiology. Lung Cellular and Molecular Physiology, 279, L1005–L1028.
Trachootham, D., Alexandre, J., & Huang, P. (2009). Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews Drug Discovery, 8, 579–591.
Sinha, B. K., Mimnaugh, E. G., Rajagopalan, S., & Myers, C. E. (1989). Adriamycin activation and oxygen free radical formation in human breast tumor cells: protective role of glutathione peroxidase in adriamycin resistance. Cancer Research, 49, 3844–3848.
Staedler, D., Idrizi, E., Kenzaoui, B. H., & Juillerat-Jeanneret, L. (2011). Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemotherapy and Pharmacology, 68, 1161–1172.
Uguz, A. C., Cig, B., Espino, J., et al. (2012). Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. Journal of Pineal Research, 53, 91–98.
Das, A., Durrant, D., Mitchell, C., et al. (2010). Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proceedings of the National Academy of Sciences USA, 107, 18202–18207.
Saitoh, M., Nishitoh, H., Fujii, M., et al. (1998). Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO Journal, 17, 2596–2606.
Guo, Q., Qi, Q., You, Q., et al. (2006). Toxicological studies of gambogic acid and its potential targets in experimental animals. Basic & Clinical Pharmacology & Toxicology, 99, 178–184.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Yuan, Z. Gambogic Acid Sensitizes Ovarian Cancer Cells to Doxorubicin Through ROS-Mediated Apoptosis. Cell Biochem Biophys 67, 199–206 (2013). https://doi.org/10.1007/s12013-013-9534-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9534-7