Abstract
The present study investigated the roles of folic acid and DNA methyltransferases (DNMTs) in the differentiation of neural stem cells (NSCs). Neonatal rat NSCs were grown in suspended neurosphere cultures and identified by their expression of SOX2 protein and capacity for self-renewal. Then NSCs were assigned to five treatment groups for cell differentiation: control (folic acid-free differentiation medium), low folic acid (8 μg/mL), high folic acid (32 μg/mL), low folic acid and DNMT inhibitor zebularine (8 μg/mL folic acid and 150 nmol/mL zebularine), and high folic acid and zebularine (32 μg/mL folic acid and 150 nmol/mL zebularine). After 6 days of cell differentiation, immunocytochemistry and western blot analyses were performed to identify neurons by β-tubulin III protein expression and astrocytes by GFAP expression. We observed that folic acid increased DNMT activity which may be regulated by the cellular S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH), and the abundance of neurons but decreased the number of astrocytes. Zebularine blocked these effects of folic acid. In conclusion, folic acid acts through elevation of DNMT activity to increase neuronal differentiation and decrease astrocytic differentiation in NSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Folic acid supplementation has proven to be extremely effective in preventing neural tube defects and other congenital abnormalities in humans [1, 2]. There is also evidence of associations between either dietary or blood folic acid status and some adult neurodegenerative diseases [3–5]. Neural stem cells (NSCs) are undifferentiated brain cells that have the capacity to self-renew and to differentiate into neurons and astrocytes [6]. The idea that NSCs may be used therapeutically for treatment of neurodegenerative disorders is very appealing and has led to numerous studies of the mechanisms underlying NSC self-renewal and differentiation [7, 8]. In particular, folic acid supplementation has been shown to stimulate cell proliferation and decrease cell death in neurosphere cultures of rat NSCs [9, 10]. Additionally, folic acid dose-dependently induces NSC proliferation and stimulates hippocampal neurogenesis in a rat model of cerebral ischemia [11]. It remains to be determined if folic acid induces differentiation of NSCs into neurons.
Recent studies showed that epigenetic regulation may interact with transcription factors and environmental cues to modulate the differentiation of NSCs [12, 13]. DNA methylation, which is an example of epigenetic regulation, undergoes marked changes in the developing brain that suggest a role for DNMTs in brain cell differentiation [14]. Recent experiments have discovered that folic acid supplementation stimulates the regeneration of injured spinal neurons by a mechanism that depends on DNA methylation pathways [15]. Since folic acid is a methyl donor that stimulates DNA methylation and folate deficiency causes genomic hypomethylation [16, 17], we hypothesized that this nutrient affects NSC differentiation by altering DNMT activity.
An inhibitor of DNMTs was needed to test this hypothesis. Zebularine is a cytidine analog that binds DNMTs covalently and inhibits the enzymatic activity. Zebularine has advantages over previously studied DNMT inhibitors because it is stable in aqueous solution and less toxic [18]. Therefore, the present study’s hypothesis was tested by incubating NSCs with folic acid and zebularine.
Materials and Methods
Reagents
Folic acid-free Dulbecco’s modified Eagle’s medium (DMEM), N2, B27 supplement, fetal bovine serum (FBS), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF) were obtained from Gibco (Carlsbad, CA, USA). Folic acid and DAPI were purchased from Sigma (St Louis, CA, USA). Zebularine was purchased from Merck Chemicals (Darmstadt, Germany). DNA Methyltransferase Activity/Inhibition Assay kit was purchased from Active Motif (Carlsbad, CA, USA). Monoclonal anti-β tubulin III antibody, Polyclonal anti-GFAP antibody, and SOX2 were obtained from Abcam, Inc (Cambridge, MA, USA). Monoclonal anti-BrdU antibody and β-actin were obtained from Cell Signaling Technology, Inc (Boston, MA, USA). All fluorescent secondary antibodies were obtained from Zhongshan Goldbridge Biotechnology (Beijing, China). BCA protein assay kit was obtained from Boster Biological Technology (Wuhan, China).
Cell Culture
Brain tissue was isolated from neonatal rats and washed 3 times with DMEM. The tissue was cut into small pieces and then dissociated by incubation with 0.25 % parenzyme and 0.02 % EDTA. This step was followed by agitation, centrifugation, and resuspension of the cells in DMEM containing 2 % B27 supplement, 20 ng/mL EGF, 20 ng/mL bFGF, 2 μmol/mL l-glutamine, 100 U/mL penicillin and streptomycin. The resulting cell suspension was cultured at 37 °C in a humidified atmosphere containing 95 % air/5 % CO2. The culture medium was changed every 2 d, while the suspended cells formed neurospheres.
Some neurospheres were incubated with the proliferation marker BrdU (10 μg/mL) for 24 h before harvest on day 6. The remaining neurospheres were mechanically dissociated for subculture on day 6. The dissociated cells (NSCs) were plated at a density of 5 × 104 cell/mL and incubated in differentiation medium containing 5 % FBS and 2 % N2. These NSCs were assigned to 5 treatment groups: control (folic acid-free), low folic acid (8 μg/mL folic acid), high folic acid (32 μg/mL folic acid), low folic acid and DNMT inhibitor zebularine (8 μg/mL folic acid and 150 nmol/mL zebularine), and high folic acid and zebularine (32 μg/mL folic acid and 150 nmol/mL zebularine). The duration of the folic acid treatment was 6 d. In the cell cultures that received zebularine, the NSCs were exposed to zebularine for 48 h and then were incubated without the drug for 6 d, before being harvested for immunocytochemistry and western blot analysis.
Immunocytochemistry Analysis
The cells were fixed in 4 % paraformaldehyde for 20 min and incubated with blocking buffer for another 20 min. Subsequently, the cells were permeabilized with 0.1 % Triton X-100 in PBS for 15 min and then blocked for 1 h at room temperature with 1 % FBS. To detect NSCs and proliferating cells, respectively, the cells were incubated overnight at 4 °C with a mixture of 1:200 rabbit anti-rat monoclonal anti-SOX2 and mouse anti-rat monoclonal BrdU antibodies. The reason we identified SOX2 was because it is a persistent marker for multipotential NSCs [19, 20]. To detect neurons and astrocytes, cells were incubated overnight at 4 °C with a mixture of 1:100 rabbit anti-rat monoclonal anti-β-tubulin III and mouse anti-rat polyclonal GFAP antibodies. Subsequently, cells were incubated for 2 h at room temperature with TRITC (tetramethylrhodamine isothiocyanate)-conjugated goat anti-mouse IgG and FITC (fluorescein isothiocyanate)-conjugated goat anti-rabbit IgG. Cell nuclei were counterstained by incubating cells with 4′-6-diamidino-2-phenylindole (DAPI) for 5 min and then washing extensively with distilled water. Images were obtained using an IX71SIF-2 fluorescence microscope (Olympus, Tokyo) and displayed on a monitor, and at least six fields of view were selected randomly for cell counting at ×200 magnification. Results were expressed as the percentages of DAPI-positive cells that were also β-tubulin III-positive or GFAP-positive cells.
Western Blot Analysis
Cells were washed with ice-cold PBS and lysates were centrifuged at 12,000 rpm for 10 min at 4 °C, and then the pellets were discarded. Protein concentrations in the supernatants were determined by BCA protein assay kit, using bovine serum albumin as a standard. Equal amounts of protein were loaded in each well for sodium dodecyl sulfate 12 % polyacrylamide gel electrophoresis and then the separated proteins were transferred to nitrocellulose membranes. The membranes were blocked in Tris-buffered saline containing Blotto solution for 2 h at room temperature. The membranes were then incubated with the primary antibodies (rabbit anti-β-tubulin III antibodies, 1:300; mouse anti-GFAP antibodies, 1:1,000) and β-actin (1:1,000) overnight at 4 °C in Tris-buffered saline containing 0.1 % Tween 20 (TBST). Membranes were rinsed three times with TBST before being incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000 in TBST) for 2 h and detected by chemiluminescence. Quantitation of proteins was performed by densitometry with NIH Image software (version 1.61).
DNMTs Activity
DNA methylation is accomplished by three enzymes: DNMT1, DNMT3a, and DNMT3b. DNMT1 may be important, because experiments with conditional mutants that lack DNMT1 showed that DNMT1 deficiency in NSCs resulted in DNA hypomethylation in daughter cells and the latter died quickly [21]. DNMT 3a and 3b are de novo methyltransferases, capable of methylating previously unmethylated CpG sequences, and have been detected in NSCs and postmitotic young neurons in vivo [22]. DNMTs activity was measured using Active Motif’s DNA Methyltransferase Activity/Inhibition Assay kit, according to the manufacturer’s instructions, and expressed as OD/(h.mg) nuclear protein.
HPLC Analysis
Cell cultures were rinsed twice with PBS and frozen at −80 °C. After thawing, cells were scraped into 1 mL of deionised water and sonified for 15 s in ice. The macromolecules were precipitated using 1.5 mmol/mL PCA solution at 4 °C for 1 h adjusting the pH to 4–5 with KOH and then centrifuged for 15 min at 9,000×g. The supernatant was freeze-dried and then subjected to HPLC analysis using a Waters HPLC system (Framingham, MA, USA) equipped with a 600E pump and a 2487 UV detector set to 254 nm. Remote control of the HPLC system, data acquisition, and calculation of peak areas were performed via computer-based data system (Waters, USA). The samples were injected through an injection valve with a 20 μL sample loop. The mobile phase was pumped at a low-rate of 1.00 mL/min. The samples were hydrolyzed with perchloric acid and the hydrolysate was adjusted to centrifuged and filtrated with a 0.45 μm membrane, the supernatants were separated on the reverse-phase column (Agela, Venusil MP-C18 ODS, 4.6 × 250 mm, 5 μm).
The mobile phases were mixture of 50 μmol/mL NaH2PO4 (pH 4.38), C7H15NaO3S, and methanol. Standards of SAM and SAH, dissolved in 10−4 mmol/mL HCl, were run before the experimental samples.
Statistical Analysis
The data were expressed as mean ± SD values and analyzed by statistical software SPSS13.0. One-way analysis of variance and the Student-Newman-Keuls test for multiple comparisons were used to determine significant differences among the experimental groups. The criterion for statistical significance was P < 0.05.
Results
Identification of NSCs
Undifferentiated NSCs formed round neurospheres that remained suspended in the culture medium (Fig. 1a). Almost all cells in the neurospheres expressed SOX2 protein, which was detected as intense green staining of nuclei in the immunofluorescence assay (Fig. 1b). Many of the cells in neurospheres also incorporated BrdU, as indicated by the red staining of nuclei in Fig. 1c. Double immunofluorescence staining of cells showed colocalization of SOX2 and BrdU (Fig. 1d). Taken together, these results demonstrated that NSCs capable of self-renewal were isolated from neonatal rat brains and grown successfully in suspended neurospheres.
Folic Acid Induces Differentiation of NSCs into Neurons
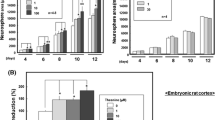
Next, we determined the effect of folic acid treatment for 6 d on the differentiation of NSCs that had been mechanically dissociated from neurospheres and subcultured. Neurons were identified by their expression of β-tubulin III protein and astrocytes by their expression of GFAP. Immunocytochemistry analysis showed that β-tubulin III and GFAP never co-localized (Fig. 2a–j), confirming that these detection of these proteins identified distinct cell phenotypes. Further, compared to control, folic acid increased the percentage of cells that were neurons (P < 0.05; Fig. 2k) and decreased the percentage that were astrocytes (P < 0.05; Fig. 2l). The effects of folic acid at 8 μg/mL were the same as those at 32 μg/mL (Fig. 2k, l). Immunocytochemistry analysis also showed that the DNMT inhibitor zebularine blocked the effects of both doses of folic acid (8 and 32 μg/mL) on NSC differentiation into neurons and astrocytes (P < 0.05; Fig. 2k, l).
Immunocytochemistry analysis showing that folic acid induces differentiation of NSCs into neurons instead of astrocytes. Differentiated cells were incubated with specific primary antibodies and fluorescent secondary antibodies to detect neuron marker β-tubulin III and astrocyte marker GFAP. a–j Fluorescence photomicrographs of cells expressing β-tubulin III (green, ×200) and GFAP (red, ×200) are shown. k The abundance of neurons (β-tubulin III-positive cells) expressed as a percentage of the total number of cells. The mean ± SD values from 3 experiments are shown. (l) The abundance of astrocytes (GFAP-positive cells) expressed as a percentage of the total number of cells. The mean ± SD values from 3 experiments are shown. * P < 0.05
The results of immunocytochemistry were confirmed by western blot analysis. Representative western blots of β-tubulin III and GFAP are shown in Fig. 3. Densitometry data from western blots showed that, compared to control, folic acid increased the expression of the neuron-specific protein β-tubulin III and decreased the expression of the astrocyte-specific protein GFAP (P < 0.05; Fig. 3a, b). The effects of the 2 doses of folic acid (8 and 32 μg/mL) did not differ (Fig. 3a, b). Zebularine blocked the effects of both doses of folic acid on the expression levels of β-tubulin III and GFAP (P < 0.05; Fig. 3a, b).
Western blot analysis showing that folic acid induces differentiation of NSCs into neurons. β-tubulin III and GFAP were detected by western blot analysis and normalized to β-actin expression. a Representative western blots and bar graph of β-tubulin III expression level (β-tubulin III: β-actin ratios) in differentiated NSCs. b Representative western blots and bar graphs of GFAP expression level (GFAP : β-actin ratios) in differentiated NSCs. Shown are mean ± SD values of 6 experiments. * P < 0.05
Taken together, these data show that folic acid acts on NSCs to stimulate differentiation into neurons and inhibit differentiation into astrocytes. These data also indicate an important role for zebularine-sensitive DNMT activity in the responses of NSC to folic acid.
Effect of Folic Acid on DNMT Activity
Folic acid supplementation increased DNMT activity compared to control (P < 0.05; Fig. 4). The DNMT activity after incubation with folic acid at 8 μg/mL was the same as that at 32 μg/mL. Zebularine decreased DNMT activity to lower levels in the cells that received the low dose of folic acid (8 μg/mL) than in those that received the high dose (32 μg/mL) (P < 0.05; Fig. 4). These results showed that the folic acid stimulation of NSC differentiation into neurons was accompanied by increased DNMT activity and that zebularine’s effect on neuron production was associated with inhibition of DNMTs.
Intracellular Level of SAM, SAH, and the Ratio of SAM/SAH
To examine the physiological consequences of increased methyl donor availability after folic acid treatment, and accordingly increase of DNMT activity, the cellular concentrations of SAM and SAH were measured by HPLC method. We verified that the SAM levels were significantly increased in two treatment groups compared with the control (P < 0.05; Fig. 5a); in contrast, the SAH levels were significantly decreased (P < 0.05; Fig. 5b). Furthermore, the SAM-to-SAH ratios were significantly decreased in two treatment groups (P < 0.05; Fig. 5c).
Discussion
One of the main findings of the present study is that folic acid supplementation induces neuronal differentiation of NSCs at the expense of astrocytic differentiation. Immunocytochemistry analysis of BrdU labeling and SOX2 expression showed that the neurospheres contained proliferating NSCs. When the neurospheres were dissociated and the NSCs were incubated in differentiation medium, folic acid increased the abundance of β-tubulin III-positive neurons and decreased the number of GFAP-positive astrocytes. These immunocytochemistry results were confirmed by western blot analysis. As we have known, astrocytes are the most abundant cells of the human brain. They perform many functions, including biochemical support of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, and a role in the repair and scarring process of the brain and spinal cord following traumatic injuries [23]. However, neurons are still considered as the most important cells responsible for brain function. A new study showed that newly generated or newborn neurons in the adult hippocampus are critical for memory retrieval [24]. Finding ways to make new brain cells are important steps in the search for treatments for brain-wasting diseases such as Alzheimer’s and Parkinson’s. In our report, folic acid facilitated the transformation of precursor cells into more neurons. Stimulatory effects of folic acid on NSC proliferation and neuronal differentiation (present experiments) may explain previous observations that folic acid enhances hippocampal neurogenesis and lessens the impairment of cognitive function that occurs after experimental stroke [11]. Stimulation of NSC proliferation and neuronal differentiation may also explain, at least in part, folic acid’s protective actions against neural tube defects and adult spinal nerve degeneration. Further, the effects of the nutrient on NSCs may account for the observed associations of folic acid status and adult neurodegenerative disease [25, 26]. Thus the present work provides insight into potential therapies for brain and spinal cord diseases.
Another main finding of the present study is that DNMTs mediates the stimulation by folic acid of neuronal differentiation of NSCs. The evidence is that, first, folic acid supplementation of NSC cultures stimulated DNMT activity. Second, partial inhibition of DNMT activity by zebularine treatment attenuated the effects of folic acid on NSC differentiation into neurons and astrocytes. These results are consistent with previous reports that DNMT activity is involved in initial specification of the neuronal cell phenotype as well as subsequent neuronal maturation and function [27, 28]. In contrast to the neuronal differentiation arising from increased DNMT activity in NSCs, hypermethylation of astrocytic gene promoters such as GFAP prevent NSCs from differentiating into astrocytes [29]. Thus folic acid-dependent DNMT activity may be a critical factor controlling NSC differentiation.
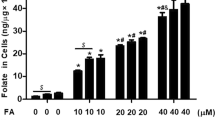
The DNMTs are a family of enzymes that catalyze the transfer of methyl groups from SAM to cytosine residue in DNA, thus producing 5-methylcytosine and SAH. SAH, as a product, can be recycled back to SAM as part of the methyl cycle. It has been reported that DNMT activity can be affected by SAM/SAH levels in the cell as SAH is a reversible DNMT inhibitor [30, 31]. A decrease in SAM or the accumulation of SAH could reduce DNMT activity [32]. In our study, to understand the mechanism underlying the change in DNMT activity caused by folic acid treatment, we measured the cellular concentration of SAM and SAH by HPLC method. The results showed that SAH and the ratio SAM/SAH were elevated in folic acid treated groups. Since folic acid, in the form of tetrahydrofolate coenzymes, is an essential carrier of methyl groups within cells, it is necessary in the conversion of homocysteine to methionine in the synthesis of SAM. Thus, there is no doubt that folic acid may alter SAM and SAH concentration by the methyl cycle. So we speculated that folic acid may indirectly increase DNMT activity by decreasing SAM and increasing SAH levels. Furthermore, DNA methylation is an important epigenetic modification that is catalyzed by specific DNMTs, including DNMT1, DNMT3a, and DNMT3b, and it has been proven to be an active component and an intrinsic program during differentiation of NSCs [33]. Intracellular SAM/SAH is considered as a more reliable biomarker for cellular methylation status [34]. Since folic acid can alter DNMT activity and SAM/SAH ratio, our results suggested that folic acid affects the differentiation of NSCs act, at least in part, by interfacing with DNA methylation.
Folic acid concentrations used in this study were set on the analysis of many other in vitro experiments, where concentrations up to 400 μg/mL [35–37]. In our previous study, NSCs were plated in complete growth media with no supplemental folic acid (0 μg/mL) or with addition of folic acid to final concentrations of 4, 8, 16, 32, 64 μg/mL. Cell proliferation was assessed 48 h later by MTT. The results showed that mild folic acid (8 μg/mL), which better promoted NSC survival (data not shown). In our present experiments, final concentration of 8 μg/mL was also the optimal concentration for NSC differentiation into more neurons (Fig. 2). In addition, some vivo studies have shown to be administered continuously at a lower dose of zebularine to maintain demethylation for a prolonged period [38, 39]. According to our present study, 0–200 nmol/mL zebularine treatment resulted in dose-dependent decreases of DNMT (Fig. 6; P < 0.05). A zebularine concentration of 150 nmol/mL which inhibited DMNT activity of 50 % was chosen to insure minimal cytotoxic in vitro, and showed a maximal effect on NSC differentiation into astrocytes (Fig. 2l, 3b).
Zebularine inhibited DNMT activity in a dose-dependent manner. Zebularine treatment was associated with a dose-dependent depletion of DNMT in all four doses. The NSCs were treated with various concentrations of Zebularine and DNMT inhibition was determined by Active Motif’s DNA Methyltransferase Activity/Inhibition Assay kit. A dependent decrease in DNMT was seen with the application of Zebularine (50, 100, 150, and 200 nmol/mL; P < 0.05). A Zebularine concentration of 150 nmol/mL accounted for the inhibition of DNMT inhibition of 50 %
In conclusion, the present study showed that folic acid supplementation alters the DNMT activity and cell differentiation of NSCs. Folic acid acts through elevation of DNMT activity to increase neuronal differentiation and decrease astrocytic differentiation in NSCs.
References
Blom, H. J., & Smulders, Y. (2011). Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. Journal of Inherited Metabolic Disease, 34, 75–81.
Daly, L. E., Kirke, P. N., Molloy, A., Weir, D. G., & Scott, J. M. (1995). Folate levels and neural tube defects. Implications for prevention. Journal of the American Medical Association, 274, 1698–1702.
Haan, M. N., Miller, J. W., Aiello, A. E., Whitmer, R. A., Jagust, W. J., Mungas, D. M., et al. (2007). Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. American Journal of Clinical Nutrition, 85, 511–517.
Morris, M. S. (2003). Homocysteine and Alzheimer’s disease. Lancet Neurology, 2, 425–428.
Van Dam, F., & Van Gool, W. A. (2009). Hyperhomocysteinemia and Alzheimer’s disease: A systematic review. Archives of Gerontology and Geriatrics, 48, 425–430.
Gage, F. H. (2000). Mammalian neural stem cells. Science, 287, 1433–1438.
Edlund, T., & Jessell, T. M. (1999). Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell, 96, 211–224.
Mu, Y., Lee, S. W., & Gage, F. H. (2010). Signaling in adult neurogenesis. Current Opinion in Neurobiology, 20, 416–423.
Zhang, X., Liu, H., Cong, G., Tian, Z., Ren, D., Wilson, J. X., et al. (2008). Effects of folate on notch signaling and cell proliferation in neural stem cells of neonatal rats in vitro. Journal of Nutritional Science Vitaminology, 54, 353–356.
Zhang, X. M., Huang, G. W., Tian, Z. H., Ren, D. L., & Wilson, J. X. (2009). Folate stimulates ERK1/2 phosphorylation and cell proliferation in fetal neural stem cells. Nutritional Neuroscience, 12, 226–232.
Zhang, X., Huang, G. W., Liu, H., Chang, H., & Wilson, J. X. (2012). Folic acid enhances Notch signaling, hippocampal neurogenesis, and cognitive function in a rat model of cerebral ischemia. Nutritional Neuroscience, 15, 55–61.
Juliandi, B., Abematsu, M., & Nakashima, K. (2010). Epigenetic regulation in neural stem cell differentiation. Development, Growth & Differentiation, 52, 493–504.
Mohamed Ariff, I., Mitra, A., & Basu, A. (2012). Epigenetic regulation of self-renewal and fate determination in neural stem cells. Journal of Neuroscience Research, 90, 529–539.
Tawa, R., Ono, T., Kurishita, A., Okada, S., & Hirose, S. (1990). Changes of DNA methylation level during pre- and postnatal periods in mice. Differentiation, 45, 44–48.
Iskandar, B. J., Rizk, E., Meier, B., Hariharan, N., Bottiglieri, T., Finnell, R. H., et al. (2010). Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. Journal of Clinical Investigation, 120, 1603–1616.
Jacob, R. A., Gretz, D. M., Taylor, P. C., James, S. J., Pogribny, I. P., Miller, B. J., et al. (1998). Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. Journal of Nutrition, 128, 1204–1212.
Pogribny, I. P., Miller, B. J., & James, S. J. (1997). Alterations in hepatic p53 gene methylation patterns during tumor progression with folate/methyl deficiency in the rat. Cancer Letters, 115, 31–38.
Champion, C., Guianvarc’h, D., Senamaud-Beaufort, C., Jurkowska, R. Z., Jeltsch, A., Ponger, L., et al. (2010). Mechanistic insights on the inhibition of c5 DNA methyltransferases by zebularine. PLoS ONE, 5, e12388.
Ellis, P., Fagan, B. M., Magness, S. T., Hutton, S., Taranova, O., Hayashi, S., et al. (2004). SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Developmental Neuroscience, 26, 148–165.
Favaro, R., Valotta, M., Ferri, A. L., Latorre, E., Mariani, J., Giachino, C., et al. (2009). Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nature Neuroscience, 12, 1248–1256.
Fatemi, M., Hermann, A., Gowher, H., & Jeltsch, A. (2002). Dnmt3a and Dnmt1 functionally cooperate during de novo methylation of DNA. European Journal of Biochemistry, 269(20), 4981–4984.
Feng, J., Chang, H., Li, E., & Fan, G. (2005). Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. Journal of Neuroscience Research, 79, 734–746.
Ricci, G., Volpi, L., Pasquali, L., Petrozzi, L., & Siciliano, G. (2009). Astrocyte-neuron interactions in neurological disorders. Journal of Biological Physics, 35, 317–336.
Gu, Y., Arruda-Carvalho, M., Wang, J., Janoschka, S. R., Josselyn, S. A., Frankland, P. W., et al. (2012). Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nature Neuroscience, 15, 1700–1706.
Mattson, M. P., & Shea, T. B. (2003). Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neuroscience, 26, 137–146.
Steinfeld, R., Grapp, M., Kraetzner, R., Dreha-Kulaczewski, S., Helms, G., Dechent, P., et al. (2009). Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. American Journal of Human Genetics, 85, 354–363.
Feng, J., Zhou, Y., Campbell, S. L., Le, T., Li, E., Sweatt, J. D., et al. (2010). Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nature Neuroscience, 13, 423–430.
Levenson, J. M., Roth, T. L., Lubin, F. D., Miller, C. A., Huang, I. C., Desai, P., et al. (2006). Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. Journal of Biological Chemistry, 281, 15763–15773.
Takizawa, T., Nakashima, K., Namihira, M., Ochiai, W., Uemura, A., Yanagisawa, M., et al. (2001). DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Developmental Cell, 1, 749–758.
Jafari, S., Hosseini, M. S., Hajian, M., Forouzanfar, M., Jafarpour, F., Abedi, P., et al. (2011). Improved In vitro development of cloned bovine embryos using s-adenosylhomocysteine, a Non-toxic epigenetic modifying reagent. Molecular Reproduction and Development, 78, 576–584.
Saavedra, O. M., Isakovic, L., Llewellyn, D. B., Zhan, L., Bernstein, N., Claridge, S., et al. (2009). SAR around (L)-S-adenosyl-L-homocysteine, an inhibitor of human DNA methyltransferase (DNMT) enzymes. Bioorganic & Medicinal Chemistry Letters, 19, 2747–2751.
Fang, M., Chen, D., & Yang, C. S. (2007). Dietary polyphenols may affect DNA methylation. Journal of Nutrition, 137, 223S–228S.
Singh, R. P., Shiue, K., Schomberg, D., & Zhou, F. C. (2009). Cellular epigenetic modifications of neural stem cell differentiation. Cell Transplantation, 18, 1197–1211.
Pogribny, I. P., Karpf, A. R., James, S. R., Melnyk, S., Han, T., & Tryndyak, V. P. (2008). Epigenetic alterations in the brains of Fisher 344 rats induced by long-term administration of folate/methyl-deficient diet. Brain Research, 1237, 25–34.
Ruan, Y., Peterson, M. H., Wauson, E. M., Waes, J. G., Finnell, R. H., & Vorce, R. L. (2000). Folic acid protects SWV/Fnn embryo fibroblasts against arsenic toxicity. Toxicology Letters, 117(3), 129–137.
Boot, M. J., Steegers-Theunissen, R. P., Poelmann, R. E., Van Iperen, L., Lindemans, J., & Gittenberger-de Groot, A. C. (2003). Folic acid and homocysteine affect neural crest and neuroepithelial cell outgrowth and differentiation in vitro. Developmental Dynamics, 227(2), 301–308.
Wang, X., & Fenech, M. (2003). A comparison of folic acid and 5-methyltetrahydrofolate for prevention of DNA damage and cell death in human lymphocytes in vitro. Mutagenesis, 18, 81–86.
Cheng, J. C., Matsen, C. B., Gonzales, F. A., Ye, W., Greer, S., Marquez, V. E., et al. (2003). Inhibition of DNA methylation and reactivation of silenced genes by zebularine. Journal of the National Cancer Institute, 95, 399–409.
Dote, H., Cerna, D., Burgan, W. E., Carter, D. J., Cerra, M. A., Hollingshead, M. G., et al. (2005). Enhancement of in vitro and in vivo tumor cell radiosensitivity by the DNA methylation inhibitor zebularine. Clinical Cancer Research, 11(12), 4571–4579.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 81072289, 81130053 and 30901192).
Author information
Authors and Affiliations
Corresponding author
Additional information
Suhui Luo and Xumei Zhang contributed equally to this study
Rights and permissions
About this article
Cite this article
Luo, S., Zhang, X., Yu, M. et al. Folic Acid Acts Through DNA Methyltransferases to Induce the Differentiation of Neural Stem Cells into Neurons. Cell Biochem Biophys 66, 559–566 (2013). https://doi.org/10.1007/s12013-012-9503-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9503-6