Abstract
Macrophages as a principal component of immune system play an important role in the initiation, modulation, and final activation of the immune response against pathogens. Upon stimulation with different cytokines, macrophages can undergo classical or alternative activation to become M1 or M2 macrophages, which have different functions during infections. Although chitotriosidase is widely accepted as a marker of activated macrophages and is thought to participate in innate immunity, particularly in defense mechanisms against chitin containing pathogens, little is known about its expression during macrophages full maturation and polarization. In this study we analyzed CHIT-1 modulation during monocyte-to-macrophage maturation and during their polarization. The levels of CHIT-1 expression was investigated in human monocytes obtained from buffy coat of healthy volunteers, polarized to classically activated macrophages (or M1), whose prototypical activating stimuli are interferon-γ and lipopolysaccharide, and alternatively activated macrophages (or M2) obtained by interleukin-4 exposure by real-time PCR and by Western blot analysis. During monocyte–macrophage differentiation both protein synthesis and mRNA analysis showed that CHIT-1 rises significantly and is modulated in M1 and M2 macrophages.Our results demonstrated that variations of CHIT-1 production are strikingly associated with macrophages polarization, indicating a different rule of this enzyme in the specialized macrophages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macrophages play a crucial role in regulating the initiation, amplification, and resolution of innate immune responses. Several diseases including atherosclerosis, diabetes, cancer, and rheumatoid arthritis are associated with a deficiency or alteration in macrophage function [1]. Macrophages are a heterogeneous population of cells that exhibit a wide range of functions depending on their physical location, as well as external cues received from the tissue microenvironment [1, 2]. In this context, it is essential to distinguish between the processes of “macrophage differentiation” and “macrophage polarization.” The term “macrophage differentiation” describes the changes that occur after monocytes have entered the arterial wall and transform from free floating, spherical cells into adherent cells of irregular shape that take up antigen and migrate within the arterial wall. The term “macrophage polarization” describes the propensity of fully differentiated macrophages to respond to external stimuli by changing their phenotypic and functional characteristics. The M1 or “classically” polarized macrophages are the most commonly described subset of macrophages. These macrophages are activated by proinflammatory cytokines and pathogen-associated molecular patterns that result in stimulation of pattern recognition receptors including the family of TLRs. The “M1 macrophages” produce a plethora of proinflammatory cytokines such as IL-1β, IL-6, IL-8, or TNF-α, and an array of cytotoxic molecules that aid in the clearance of invading pathogens and stimulate the acquired immune response.
Human chitotriosidase (CHIT-1), a phagocyte-specific chitinase, is thought to play an important role in the mammalian innate immune response against fungi, bacteria, and other pathogens [3, 4]. CHIT-1 is selectively expressed and highly regulated in activated macrophages and it is also present in human neutrophil specific granules which release it upon stimulation [5]. As a result CHIT-1 activity is currently used as a biochemical marker of macrophage activation in some lysosomal diseases. The sources of secreted chitotriosidase was first described in abnormal lipid-laden macrophages formed in tissues of Gaucher disease patients [6]. Gaucher cells can be classified as a variation of alternatively activated macrophages; they express CD68, C14, HLA class II, CD163, CCL18, and IL-1-receptor antagonist, but do not express CD11b, CD40, and proinflammatory cytokines such as TNF-α and MCP1 [7]. Subsequently the levels of this enzyme was found to be elevated in other pathological conditions including β-thalassemia [8], sarcoidosis [9], multiple sclerosis [10], and in parasitic infections such as Plasmodium falciparum malaria [11]. Interestingly, other investigations have suggested that CHIT-1 could have a crucial role even in pathological conditions, such as atherosclerosis, coronary artery disease [12], acute ischemic stroke [13], cerebrovascular dementia, and Alzheimer’s disease [14]. Furthermore, it has been shown that CHIT-1 may be involved in the progression of nonalcoholic steatohepatitis [15, 16]. Overall, these data underline the idea that CHIT-1 acts as an important factor in inflammation [17]. A 24-bp duplication in exon 10 in the CHIT-1 gene activates a cryptic 39 splice site in the same exon, generating an abnormally spliced mRNA with an in-frame deletion of 87 nucleotides. The spliced mRNA encodes an enzymatically inactive protein that lacks an internal stretch of 29 amino acids; the resulting phenotype is an asymptomatic Chit activity deficiency. A mild enzymatic activity has been detected in heterozygous subjects for the duplication [18]. Recently it has been found that this common natural genetic variation within the CHIT-1 gene was strongly associated with human longevity and was also associated with several phenotypes of healthy aging [19]. It was shown that genetic variants of this allele, severely compromising chitotriosidase activity, correlate with a higher risk for Gram-negative infection in children undergoing therapy for acute myeloid leukemia [20].
Although several reports indicated the stimulatory effect of proinflammatory stimuli on CHIT-1 expression [21], little is known about its expression during macrophages full maturation and polarization. In order to deepen the possible roles of CHIT-1 in the immune system we analyzed CHIT-1 modulation during monocyte-to-macrophage transition and during polarization of the primary human macrophage into classically activated (M1) macrophages and alternative activated (M2) macrophages.
Materials and Methods
Cells
Human monocyte-derived macrophages were isolated from fresh buffy coat of healthy volunteers as previously described [22]. The buffy coat were diluted with phosphate-buffered saline (PBS) supplemented with 2.5 mM EDTA and layered onto Ficoll–Hypaque gradients (Gibco, Invitrogen, Milan, Italy). After 30 min of centrifugation at 400×g at room temperature, the mononuclear cells were collected, washed twice with PBS, and placed in plastic Petri dishes at a concentration of 1 × 106–2 × 106 cells/cm surface areas in Iscove’s medium supplemented with 2 mM glutamine, and 50 mg/ml of penicillin/streptomycin. After 2 h incubation, the non-adherent cells were washed out using PBS. The adherent cells (monocytes) were cultured in Iscove’s medium supplemented with rHuman M-CSF 5 ng/ml (Peproteck, BDA, Italy), 10 % fetal bovine serum (FBS), 2 mM glutamine, and 1 % of penicillin/streptomycin (Invitrogen, Milan, Italy).
Macrophages Differentiation
Macrophages were obtained by culturing monocytes for 7 days in Iscove’s medium supplemented with 10 % FBS 2 mM glutamine, 1 % of penicillin/streptomycin (Invitrogen), and 5 ng/ml M-CSF in Petri dishes at a density 1 × 106–2 × 106 cells/cm2. Macrophage polarization was obtained as previously described [23]. Briefly, macrophage polarization was obtained by removing the culture medium and culturing cells for an additional 18 h in RPMI 1640 supplemented with 5 % FBS and LPS (50 ng/ml) plus IFN-γ (100 μg/ml) (for M1 polarization) or IL-4 (20 ng/ml) (for M2 polarization) (Peproteck, Milan, Italy). Five different cell types were generated: freshly isolated monocytes (Mono T0), cells at intermediate differentiation (3 days of culture: Mono T3), resting fully differentiated macrophages (7 days of culture: Macrophages), classical activated macrophages (M1), and alternative activated macrophages (M2) (Fig. 1a). Macrophage polarization was confirmed by real-time PCR for two M1 markers (TNF-α and CXCL11) and two M2 markers (CCL18 and CD206) (Fig. 1a).
a Real-time PCR confirming macrophage polarization performed for two M1 markers (TNF-α and CXCL11) and two M2 markers (CCL18 and CD206). Statistical analysis was performed by Student’s t test. b Microphotographs of monocytes during differentiation and polarization into macrophages and analysis by cytofluorimeter of marker CD68. The monocytes (Mono T0) have a spherical appearance with prominent surface ruffles and blebs and 9–15 μm of diameter. In the differentiated macrophages, the diameter (20–25 μm) and number of cytoplasmic granules increase. Both the classical activated macrophages (M1) and the alternative activated macrophages (M2) show a similar morphology to differentiated macrophages
Gene Expression Analysis by Real-Time PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Milan, Italy). For reverse transcription-polymerase chain reaction (RT-PCR), 2 μg of total RNA was reverse-transcribed with high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Monza, Italy) in a 20 ml reaction solution. Real-time fluorescence PCR, based on SYBR Green, was carried out in a 30 ml final volume containing 1 SYBR Green PCR Master Mix (Applied Biosystems, Monza, Italy), 200 nM forward and 200 nM reverse primers (Table 1), and 20 ng of cDNA. Thermal cycling was performed for each gene in triplicate on cDNA samples in MicroAmp Optical 96-well reaction plate (Applied Biosystems, Monza, Italy) with MicroAmp optical caps (Applied Biosystems) using the ABI PRISM 7700 sequence detection system (Applied Biosystems, Monza, Italy). Amplification was carried out with the following conditions: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles each of 95 °C for 15 s and 60 °C for 1 min. Data are presented as mean % ±SD of at least three independent experiments. Differences were analyzed by Student t test, with p < 0.05 being considered statistically significant.
Western Blot
Cells were harvested by trypsinization, and total proteins were extracted using a cell lysis buffer. The lysates were collected for Western blot analysis. Protein levels were visualized by immunoblotting with antibodies against human CHIT-1(R&D systems-DBA-ITALY). Briefly, 20 μg of lysate supernatant was separated by SDS/polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham, Milan, Italy) using a semidry transfer apparatus (Bio-Rad, Hercules, CA). The membranes were incubated with 5 % milk in 10 mM Tris–HCl (pH 7.4), 150 mM NaCl, and 0.05 % Tween 20 (TBST) buffer for 1 h at room temperature with constant shaking. After washing with TBST, the membranes were incubated with a 1:2,000 dilution of anti-CHIT-1 or anti-Beta Actin (Abcam) antibodies at 4 °C overnight. The filters were then washed and probed with horseradish peroxidase-conjugated donkey anti-rabbit IgG (Amersham) at a dilution of 1:2,000. Chemiluminescence detection was performed with the Amersham ECL Detection Kit according to the manufacturer’s instructions.
Flow Cytometry
Flow cytometric measurements were performed using a four color FacsCalibur (BD Biosciences, San Jose, CA). Cells were fixed and permeabilized using Cytofix/Cytoperm Kit (BD Biosciences, San Jose, CA), as per manufacturer’s instruction. Intracellular staining for CD68 was performed using PE-conjugated mouse anti-human CD68 (BD Biosciences, San Jose, CA). Data were analyzed using Cyflogic software, version 1.2.1
Immunofluorescence
Cells were grown directly on coverslips before indirect immunofluorescence. After washing with PBS, cells were fixed in methanol–acetone (1:1) for 10 min at −20 °C and were then washed three times for 5 min each in PBS. Subsequently, coverslips were incubated with the primary antibody anti-chitotriosidase (sc-99033; Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted 1:300 in PBS containing 0.1 % Tween 20 and 1 % BSA) overnight at +4 °C. Cells were washed as above before incubation with the goat anti-rabbit secondary antibody, DyLightTM 488 conjugated (Thermo Scientific, Rockford, IL, USA), diluted 1:500 in PBS and 0.1 % Tween 20 and 1 % BSA, then washed once more as above. Finally, coverslips were mounted on microscope slides with UltracruzTM anti-fade medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Santa Cruz Biotechnology).
Microphotography
Microphotography was done with Optika microscope XDS1R (OPTIKA Microscopes, Italy), OPTIKAM pro 3 LT digital camera (OPTIKA Microscopes, Italy), and software (OPTIKA Microscopes, Italy).
Statistical Analysis
Data are expressed as mean ± standard error (SE). Significance was assessed by one-way analysis of variance (ANOVA). P < 0.05 was considered to be statistically significant.
Results
Levels of TNF-α and Superoxide Dismutase (SOD2) During In Vitro Differentiation and Polarization of Peripheral Blood Monocytes
In order to verify that monocytes/macrophages were not activated during differentiation, we detected the transcriptional levels of TNF-α and superoxide dismutase SOD2. During the first 7 days of differentiation, the levels of both TNF-α and SOD2 were significantly down regulated, confirming a non-activation of the monocyte. In particular, the decrement of TNF-α expression varied from 50 %, during the first 5 days, to 80 % on the 7th day (p = 0.015) whereas SOD2 levels ranged from −70 to −68 % on the 7th day (p = 0.005) (Fig. 2a, b). In contrast, the polarization of macrophages in M1 induced by LPS (50 ng/ml) and IFN-γ (100 μg/ml) resulted in an increase of both TNF-α and SOD2 expression (1,550 %, p = 0.009; 119 %, p = 0.006; respectively) (Fig. 2a, b).
Real-time PCR confirming the non-activation of the monocyte/macrophages. a Variation of TNF-α expression and b SOD2 expression on monocyte/macrophages at day 0, 3, 5, 7 (M Φ), classical activated macrophages (M1), alternative activated macrophages (M2). All RT-PCR values are given as ΔCt values, which correspond to the difference of the maximum number of RT-PCR cycles (40) and the value obtained for each individual sample. Statistical analysis was performed by Student’s t test. *P < 0.05; **p < 0.001 of differentiated or polarized cells versus day 0 cells
Differential mRNA Expression of CHIT-1 During In Vitro Differentiation and Polarization of Peripheral Blood Monocytes
The modulation of CHIT-1 expression was investigated using quantitative real-time RT-PCR on the primary human monocyte-to-macrophage maturation and subsequent polarization into M1 or M2 cells. The trend of CHIT-1 mRNA expression during the differentiation of monocytes into macrophages and subsequent macrophages polarization into M1 and M2 underwent significant changes. In the first 3 days of culture no significant variations were observed (56.68 %) compared to the day 0 (Fig. 3). Whereas at 5th day an increase of 1,412.15 % (p = 0.04) was observed compared to the day 0 (Fig. 3). On the 7th day, when monocytes were completely differentiated into macrophages as confirmed by cytoflorometric data (Fig. 1b), CHIT-1 levels increased by 1,475 % in comparison with day 0 (p = 0.0017). As M1 polarization occurred, CHIT-1 expression increased by 562.50 % as opposed to the day 0, but in comparison with day 7 it decreased significantly (57.93 %, p = 0.012). Concerning CHIT-1 expression in M2, it rose by 651.90 % compared to the day 0 macrophages and, in comparison with day 7, a reduction of 52.25 % (p = 0.011) was detected (Fig. 3). Although CHIT-1 levels were lower in M2 compared with macrophages at 7 days, its levels were higher than M1 macrophages. Our analysis showed that macrophage differentiation and polarization were characterized by distinct patterns of CHIT-1 expression (Fig. 3).
Real-time PCR of CHIT-1 expression during human monocyte/macrophages differentiation and polarization. T0, T3, T5 (monocyte/macrophages at day 0, 3, 5); M Φ 7 (macrophages at day 7), M1 classical activated macrophages, M2 alternative activated macrophages. All RT-PCR values are given as ΔCt values, which correspond to the difference of the maximum number of RT-PCR cycles (40) and the value obtained for each individual sample. Statistical analysis was performed by Student’s t test. *P < 0.05 and **p < 0.001 of differentiated or polarized cells versus day 0 cells
Differential Protein CHIT-1 Synthesis During In Vitro Differentiation and Polarization of Peripheral Blood Monocytes
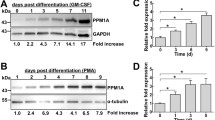
In order to assess whether the increase in CHIT-1 mRNA was associated with the elevation in CHIT-1 protein, Western blot analysis was performed on cell lysates obtained from the primary human monocyte-to-macrophage maturation and subsequent polarization into M1 or M2 cells. Using CHIT-1 antibodies that recognize both human and rat CHIT-1 protein, we demonstrated that macrophage differentiation and polarization were characterized by distinct patterns of CHIT-1 protein synthesis.
In the first 3 days of culture no significant variations were observed compared to the day 0 (5.53-fold increases) (Fig. 4a). On the contrary, at 5th day an increase of 8.47 fold was observed compared to the day 0 (Fig. 4a). On the 7th day, CHIT-1 levels increased by 14.87 fold p = 0.001 (Fig. 4a) in comparison with day 0 (p = 0.001). As M1 polarization occurred, CHIT-1 protein level declined by 8.68 fold relative to day 7. Regarding M2 macrophages, CHIT-1 expression rose by 10.90 fold compared to the day 0 macrophages, but in comparison with day 7 a reduction of 3.97 fold was detected. Quantitative evaluation of CHIT-1 protein was measured by scanning densitometry. A similar amount of β-actin proteins was observed during the different stages of macrophages differentiation. These results confirmed that CHIT-1 enhancement is a mechanism operant with macrophages differentiation and polarization.
a Western blot analysis of CHIT-1 expression during human monocyte/macrophages differentiation and polarization. Monocyte/macrophages at day 0, 3, 5, 7 (M Φ), M1 classical activated macrophages, M2 alternative activated macrophages. Level of CHIT-1 protein normalized to beta-actin. Statistical analysis was performed by Student’s t test. *P < 0.05 and **p < 0.001 of differentiated or polarized cells versus day 0 cells. b Immunofluorescence analysis of chitotriosidase (green) in macrophages at 3, 5, and 7 days of culture. Most of the cells in T5 and T7 immunostaining for chitotriosidase show a characteristic distribution pattern, the signal being localized in the inner margin of the cell with a characteristic spotted pattern at plasma membrane level (panel T7 b, arrows). Blue represents DAPI nuclear counterstain. Scale bar 15 μm (Color figure online)
Chitotriosidase Distribution
The expression of chitotriosidase in macrophages at 3, 5, and 7 days of culture was evaluated by immunofluorescence analysis (Fig. 4b). Many cells showed a characteristic distribution pattern, the immunostaining signal being mainly localized in the inner margin of the cell. Moreover, many cells at 7 days of culture showed a chitotriosidase immunolabeling mainly concentrated in a spotted budding area at plasmalemma level.
Effect of LPS and IFN-γ on the Expression of CHIT-1 in Monocytes
In order to verify the action carried out by LPS (50 ng/ml) and IFN-γ (100 μg/ml) on the expression of CHIT-1, we treated monocytes at different time points (2, 4, 8, and 24 h) maintaining the concentration used for the polarization in M1. We found that the treatment with LPS did not induce significant increases in the CHIT-1 expression at 2 and 24 h (15 %). In contrast the treatment with IFN-γ enhanced the expression of CHIT-1 at 2 and 24 h (59 %, p = 0.04 and 58 % p = 0.04) compared to the control. These results confirmed the decrement of CHIT-1 expression in M1 polarized macrophages compared with monocyte at 7 days (Fig. 5).
Effect of IL-4 on the Expression of CHIT-1 in Monocytes
In order to check the progress of CHIT-1 expression under induction by IL-4, we treated monocytes at different time points (2, 4, 8, and 24 h) with the same concentration used for the polarization of macrophages in M2. After 2 and 4 h, we found that the expression levels underwent a significant increase (430 and 447.46 %, respectively, with a p = 0.0034 and p = 0.0079), showing thereafter at 24 h a significant decrease (82.35 %, p = 0.02). The data confirmed the behavior observed during the polarization of macrophage toward M2. The capability of macrophage M2 to attack pathogenic organisms provided with coatings of chitin would explain the increase of the messenger during the first hours of treatment (Fig. 6).
Discussion
The results reported here show for the first time that CHIT-1 expression during the differentiation of monocytes into macrophages and subsequent macrophages polarization into M1 and M2 undergoes significant changes. Macrophages are a heterogeneous population of cells that exhibit a wide range of functions depending on their physical location, as well as external cues received from the tissue microenvironment [1, 2]. Moreover, differentiated and polarized macrophages are accompanied by important changes in gene expression as demonstrated in a transcriptome analysis [24]. Our results show that during the effective maturation of monocytes into macrophages, CHIT-1 levels exponentially increase by the over time, showing a peak of expression between the fifth and the seventh day of culture. Therefore, the detection of CHIT-1 is particularly elevated in the later inflamed stages. Numerous evidences report the central role of CHIT-1 in the expanding spectrum of disorders ranging from granulomatous disease such as sarcoidosis [9] to a large amount of infections including mycobacterial diseases such as tuberculosis [25, 26] and leprosy [27]. In this contest, a great attention must be given to the fact that this enzyme is produced by differentiated macrophages themselves, products from these cells can also be damaging to host tissues and are implicated in the progression of a number of chronic inflammatory diseases. Additionally, our finding demonstrating that the variations of CHIT-1 production are associated with the macrophages polarization indicates a different rule of this enzyme in the specialized macrophages. Overproduction of CHIT-1 exerts deleterious effect in many degenerative disorders [14–16]. This concept is also sustained by our recent findings in which we observed that genetic variation within the CHIT-1 gene was strongly associated with human longevity and with several phenotypes of healthy aging [19], and that a functional polymorphism in the CHIT-1 gene protects from NAFLD progression [28]. Inflammatory and healing macrophages play key roles in the induction and resolution of inflammation and are a key target population for treating common inflammatory disorders such as inflammatory bowel disease, rheumatoid arthritis, and atherosclerosis. In the light of these findings the possibility of dampening inflammation and enhancing resolution by regulating CHIT-1 expression is an attractive one. In our study, verifying the effect that LPS or IFN-γ individually exerts on mRNA CHIT-1 expression, we found that treatment with LPS did not induce significant changes in CHIT-1 levels, instead IFN-γ enhanced CHIT-1 levels. Previously we reported that IFN-γ and LPS induced the expression of CHIT-1 in monocyte-derived macrophages [21]. This difference on CHIT-1 expression may depend on the time of CHIT-1 detection during the maturation state of the monocyte or macrophage and paradoxically confirm the long-range contribution exerted by CHIT-1 operant with macrophages differentiation and polarization. Macrophages at the different stages of differentiation have a plastic gene expression profile that is influenced by the type, concentration, and longevity of exposure to the stimulating agents [29]. Many of these activities appear to be opposing in nature: pro- versus anti-inflammatory effects, immunogenic versus tolerogenic activities, and tissue destruction versus tissue repair [29]. The proinflammatory properties of classically activated M1 macrophages directed to promote inflammation and kill the invading pathogens are in clear contrast with the anti-inflammatory activities of alternatively activated M2 macrophages, which provide regulatory signals to protect the host from a plethora of inflammatory responses [29, 30]. Interestingly, in our M0 monocytes IL-4 treatment induced a significant increase on mRNA CHIT-1 expression. The Th2 cytokine IL-4 promotes immune responses to parasites. This finding set straight why CHIT-1 increased secretion is closely associated with pathophysiological conditions dominated by T-helper type 2 cells including infections with fungal pathogens and malaria parasites, fibrosis, allergy, and asthma [31]. Additionally, IL-4 is well-appreciated antagonist of the M1 response and macrophage pro-inflammatory properties [29]. IL-4 can also promote fibrosis through TGFβ production [30]. Importantly, IL-4 also activates PPAR-γ [32] and PPAR-δ [33] to promote monocyte/macrophage alternative activation. Of interest, IL-4 promotes a M2 phenotype in regressive atherosclerotic lesions [34], providing in such a way a potential explanation of chitotriosidase activity associated with the presence of atherosclerosis [12]. Nevertheless, since macrophages are paradoxically involved in both generation of fibrosis and its resolution and since M2 polarization generates a positive feedback loop during resolution of inflammation, it is unclear what are the events influencing M2 differentiation and interrupting tissue repair/remodeling as well fibrotic outcomes. This finding could support the idea that increased levels of CHIT-1 in this stage could be involved in the modulation of the extracellular matrix affecting cell adhesion and migration during the tissue remodeling processes that take place in fibrogenesis [15, 16]. These same fibrotic changes occur over time in the lung fibrosis as a result of chronic and acute inflammation. Moreover, it has been suggested that chitinases play a role in tissue remodeling responses in models of infection and Th2-driven inflammation [35]. We thus forecast that chitinase inhibition might have beneficial effects on the expression of genes associated with tissues remodeling. In conclusion, our results further confirm that CHIT-1 is up-regulated in response to inflammatory stimuli in innate response but, as macrophages shift toward the M1–M2 phenotype, CHIT-1 become involved in phagocytosis, bacterial killing, and initiation of the adaptive immune response. Moreover, since M2 cells scavenge debris, phagocytize apoptotic cells after inflammatory injury, and orchestrate tissue remodeling and repair through the production of extracellular matrix proteins, the findings presented here emphasize that CHIT-1 may exert an additional role during Th2-driven immune response. In conclusion, these new findings on CHIT-1 expression during the diverse stages—maturation and polarization of monocyte/macrophages underscore that CHIT-1 can be regarded not only as a marker of macrophages activation but also as an important regulator of the immune and of inflammatory functions. Additionally, our study confirms that CHIT-1 overexpression could be deleterious in some disorders and open the indication for further investigations.
References
Ross, R., Ross, X. L., Ghadially, H., Lahr, T., Schwing, J., Knop, J., et al. (1999). Mouse langerhans cells differentially express an activated T cell attracting CC chemokine. Journal of Investigative Dermatology, 113, 991–998.
Martinez, F. O., Sica, A., Mantovani, A., & Locati, M. (2008). Macrophage activation and polarization. Frontiers in Bioscience, 13, 453–461.
van Eijk, M., van Roomen, C. P., Renkema, G. H., Bussink, A. P., Andrews, L., Blommaart, E. F., et al. (2005). Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. International Immunology, 17, 1505–1512.
Malaguarnera, L. (2006). Chitotriosidase: The yin and yang. Cellular and Molecular Life Sciences, 63, 3018–3029.
van Eijk, M., Voorn-Brouwer, T., Scheij, S. S., Verhoeven, A. J., Boot, R. G., & Aerts, J. M. (2010). Curdlan-mediated regulation of human phagocyte-specific chitotriosidase. FEBS Letters, 584, 3165–3169.
Bussink, A. P., van Eijk, M., Renkema, G. H., Aerts, J. M., & Boot, R. G. (2006). The biology of the Gaucher cell: The cradle of human chitinases. International Review of Cytology, 252, 71–128.
Boven, L. A., van Meurs, M., Boot, R. G., Mehta, A., Boon, L., Aerts, J. M., et al. (2004). Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. American Journal of Clinical Pathology, 122, 359–369.
Barone, R., Bertrand, G., Simporè, J., Malaguarnera, M., & Musumeci, S. (2001). Plasma chitotriosidase activity in beta-thalassemia major: A comparative study between Sicilian and Sardinian patients. Clinica Chimica Acta, 306, 91–96.
Bargagli, E., Maggiorelli, C., & Rottoli, P. (2008). Human chitotriosidase: A potential new marker of sarcoidosis severity. Respiration, 76, 234–238.
Comabella, M., Domínguez, C., Rio, J., Martín-Gallán, P., Vilches, A., Vilarrasa, N., et al. (2009). Plasma chitotriosidase activity in multiple sclerosis. Clinical Immunology, 131, 216–222.
Malaguarnera, L., Simporè, J., Prodi, D. A., Angius, A., Sassu, A., Persico, I., et al. (2003). 24-bp duplication in exon 10 of human chitotriosidase gene from the sub-Saharan to the Mediterranean area: role of parasitic diseases and environmental conditions. Genes and Immunity, 4, 570–574.
Artieda, M., Cenarro, A., Gañán, A., Lukic, A., Moreno, E., Puzo, J., et al. (2007). Serum chitotriosidase activity, a marker of activated macrophages, predicts new cardiovascular events independently of C-reactive protein. Cardiology, 108, 297–306.
Palasik, W., Fiszer, U., Lechowicz, W., Czartoryska, B., Krzesiewicz, M., & Lugowska, A. (2005). Assessment of relations between clinical outcome of ischemic stroke and activity of inflammatory processes in the acute phase based on examination of selected parameters. European Neurology, 53, 188–193.
Di Rosa, M., Dell’Ombra, N., Zambito, A. M., Malaguarnera, M., Nicoletti, F., & Malaguarnera, L. (2006). Chitotriosidase and inflammatory mediator levels in Alzheimer’s disease and cerebrovascular dementia. European Journal of Neuroscience, 23, 2648–2656.
Malaguarnera, L., Di Rosa, M., Zambito, A. M., dell’Ombra, N., Di Marco, R., & Malaguarnera, M. (2006). Potential role of chitotriosidase gene in nonalcoholic fatty liver disease evolution. American Journal of Gastroenterology, 101, 2060–2069.
Malaguarnera, L., Di Rosa, M., Zambito, A. M., dell’Ombra, N., Nicoletti, F., & Malaguarnera, M. (2006). Chitotriosidase gene expression in Kupffer cells from patients with non-alcoholic fatty liver disease. Gut, 55, 1313–1320.
Kzhyshkowska, J., Gratchev, A., & Goerdt, S. (2007). Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomarker Insights, 2, 128–146.
Boot, R. G., Renkema, G. H., Verhoek, M., Strijland, A., Bliek, J., de Meulemeester, T. M., et al. (1998). The human chitotriosidase gene. Nature of inherited enzyme deficiency. Journal of Biological Chemistry, 273, 25680–25685.
Malaguarnera, L., Ohazuruike, L. N., Tsianaka, C., Antic, T., Di Rosa, M., & Malaguarnera, M. (2010). Human chitotriosidase polymorphism is associated with human longevity in Mediterranean nonagenarians and centenarians. Journal of Human Genetics, 55, 8–12.
Lehrnbecher, T., Bernig, T., Hanisch, M., Koehl, U., Behl, M., Reinhardt, D., et al. (2005). Common genetic variants in the interleukin-6 and chitotriosidase genes are associated with the risk for serious infection in children undergoing therapy for acute myeloid leukemia. Leukemia, 19, 1745–1750.
Malaguarnera, L., Musumeci, M., Di Rosa, M., Scuto, A., & Musumeci, S. (2005). Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. Journal of Clinical Laboratory Analysis, 19, 128–132.
Di Rosa, M., Zambito, A. M., Marsullo, A. R., Li Volti, G., & Malaguarnera, L. (2009). Prolactin induces chitotriosidase expression in human macrophages through PTK, PI3-K, and MAPK pathways. Journal of Cellular Biochemistry, 2009(107), 881–889.
Fagone, P., Di Rosa, M., Palumbo, M., De Gregorio, C., Nicoletti, F., Malaguarnera, L. (2012, Jun 16). Modulation of heat shock proteins during macrophage differentiation. Inflammation Research.
Martinez, F. O., Gordon, S., Locati, M., & Mantovani, A. (2006). Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. Journal of Immunology, 177, 7303–7311.
Cakır, G., Gumus, S., Ucar, E., Kaya, H., Tozkoparan, E., Akgul, E. O., et al. (2012). Serum chitotriosidase activity in pulmonary tuberculosis: Response to treatment and correlations with clinical parameters. Annals of Laboratory Medicine, 32, 184–189.
Bargagli, E., Margollicci, M., Nikiforakis, N., Luddi, A., Perrone, A., Grosso, S., et al. (2007). Chitotriosidase activity in the serum of patients with sarcoidosis and pulmonary tuberculosis. Respiration, 74, 548–552.
Iyer, A., van Eijk, M., Silva, E., Hatta, M., Faber, W., Aerts, J. M., et al. (2009). Increased chitotriosidase activity in serum of leprosy patients: Association with bacillary leprosy. Clinical Immunology, 131, 501–509.
Di Rosa, M., Mangano, K., De Gregorio, C., Nicoletti, F., & Malaguarnera, L. (2012). Association of chitotriosidase genotype with the development of nonalcoholic fatty liver disease. Hepatitis Research. doi:10.1111/j.1872-034X.2012.01063.x.
Martinez, F. O., Helming, L., & Gordon, S. (2009). Alternative activation of macrophages: an immunologic functional perspective. Annual Review of Immunology, 27, 451–483.
Wynn, T. A., & Barron, L. (2010). Macrophages: master regulators of inflammation and fibrosis. Seminars in Liver Disease, 30, 245–257.
Stein, M., Keshav, S., Harris, N., & Gordon, S. (1992). Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. Journal of Experimental Medicine, 176, 287–292.
Berry, A., Balard, P., Coste, A., Olagnier, D., Lagane, C., Authier, H., et al. (2007). IL-13 induces expression of CD36 in human monocytes through PPAR gamma activation. European Journal of Immunology, 37, 1642–1652.
Kang, K., Reilly, S. M., Karabacak, V., Gangl, M. R., Fitzgerald, K., Hatano, B., et al. (2008). Adipocyte-derived Th2 cytokines and myeloid PPAR delta regulate macrophage polarization and insulin sensitivity. Cell Metabolism, 2008(7), 485–495.
Pourcet, B., Feig, J. E., Vengrenyuk, Y., Hobbs, A., Kepka-Lenhart, D., Garabedian, M., et al. (2011). LXR{alpha} regulates macrophage arginase 1 through PU.1 and interferon regulatory factor 8. Circulation Research, 109, 492–501.
Lee, C. G. (2009). Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Medical Journal, 50, 22–30.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Rosa, M., Malaguarnera, G., De Gregorio, C. et al. Modulation of Chitotriosidase During Macrophage Differentiation. Cell Biochem Biophys 66, 239–247 (2013). https://doi.org/10.1007/s12013-012-9471-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9471-x