Abstract
In the present study, we evaluated whether stem cell-to-tenocyte differentiation could be evaluated via measurement of the mechanical properties of the cell. We used mechanical uniaxial cyclic stretching to induce the differentiation of human bone marrow mesenchymal stem cells into tenocytes. The cells were subjected to cyclic elongation of 10 or 15 % at a cyclic frequency of 1 Hz for 24 or 48 h, and differentiation was assessed by real-time PCR (rtPCR) determination of messenger RNA expression levels for four commonly used markers of stem cell-to-tenocyte differentiation: type I collagen, type III collagen, tenascin-C, and scleraxis. The rtPCR results showed that cells subjected to 10 % cyclic elongation for 24 or 48 h differentiated into tenocytes. Atomic force microscopy (AFM) was then used to measure the force curves around the cell nuclei, and the AFM data were used to calculate the elastic moduli of the cell surfaces. The elastic modulus values of the control (non-stretched) cells differed significantly from those of cells stretched at 10 % for 24 or 48 h (P < 0.01). Confocal fluorescence microscopic observations of actin stress fibers suggested that the change in elastic modulus was ascribable to the development of the cellular cytoskeleton during the differentiation process. Therefore, we conclude that the atomic force microscopic measurement of the elastic modulus of the cell surface can be used to evaluate stem cell-to-tenocyte differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendons are a type of connective tissue which directly attaches muscles to skeletal structures, permitting locomotion and enhancing joint stability [1–3]. Tendon injuries, which can range from repetitive strain injury to complete rupture, are often seen in athletes and other active people, and the reduced functionality of the injured tendon tissue can be devastating to everyday life. Tendon injuries are difficult to manage; although spontaneous healing can occur, it often results in the formation of scar tissue which differs morphologically, biochemically, and biomechanically from healthy tendon tissue [4]. Repaired tendon tissue is impaired in its dynamic properties and strength [5–9]; thus, current treatments for tendon injury, both conservative and surgical, can be said to be limited in their effectiveness [9].
The current lack of effective treatments for tendon injury suggests a need for tendon tissue engineering, in which a functional tissue replacement is produced in vitro and then implanted into the body. In one approach, cells capable of forming tendons are combined with a scaffold to produce a construct that is then implanted into the injury site to promote the formation of new tendon tissue. Explanted tendon tissue is not an optimal source of suitable cells for this procedure; however, the relatively acellular nature of tendons means that only small numbers of cells can be obtained from explanted tendon tissue [10]. Furthermore, cells that are obtained from explanted tendon tissue tend to be terminally differentiated with a very limited proliferative capacity [11]. On the other hand, bone marrow mesenchymal stem cells (BMSCs) offer a high proliferative capacity and pluripotency [12–16] and are thus more commonly used.
Chemical stimulation with growth factors, such as CTGF [17], GDF-5/BMP-14 [18, 19], and GDF-6/BMP-13 [20], have been often attempted to be used to enhance tendogenesis. However, the use of chemical stimulation to induce mesenchymal stem cell (MSC)-to-tenocyte differentiation poses major difficulties, including the necessity for optimization of the types and quantities of growth factors and the possibility of immunologic rejection.
During everyday movement of the body, tendons are subjected to numerous types of mechanical strain. Tendons respond to mechanical forces by adapting their metabolism and structural and mechanical properties [2]. Mechanical stretching appears to influence human tendon fibroblast proliferation [21] and to increase the production of collagen, the primary constituent of tendon tissue [22–24]. Furthermore, the application of mechanical stretch appears to stimulate MSCs to proliferate and differentiate into tenocytes [25–27]. Therefore, mechanical stimulation is being investigated as a simpler and safer differentiation-inducing technique than chemical stimulation.
Cell differentiation is commonly assessed by measuring gene expression levels for differentiation marker proteins by real-time PCR (rtPCR). For the differentiation of MSCs into tenocytes, the pathognomonic differentiation markers are type I collagen (Col I), type III collagen (Col III), tenascin-C (Tnc), and scleraxis (Scx), all of which are key constituents of tendon tissue [28, 29]. However, the measurement of gene expression levels is a complicated procedure which depletes the number of available samples, despite its utility for the evaluation of cell differentiation.
In this study, we examined the potential usefulness of a different approach for the evaluation of cell differentiation: the use of atomic force microscopy (AFM) to measure the mechanical properties of the cell. AFM allows accurate measurement of the mechanical properties of individual living cells. Although many researchers have investigated the mechanical properties of cells [30–37], there have been no reports from the perspective of cell differentiation evaluation. Here, we used AFM to measure the elastic moduli of MSCs induced to differentiate by mechanical stimulation (specifically, a 1-Hz sinusoidal cyclic stretch at 10 or 15 % elongation over 24 or 48 h) and compared the MSC-to-tenocyte differentiation results to those obtained by rtPCR as described above. This analysis demonstrated a statistically significant difference in the elastic moduli of the 10 %-stretched, differentiated cells and the control (non-stretched) cells. These findings, together with cytoskeletal observations obtained by confocal fluorescence microscopy, demonstrate that MSC-to-tenocyte differentiation can be evaluated by AFM.
Materials and Methods
Cells and Culture with Mechanical Cyclic Stretching
Human BMSCs (hBMSCs; cell line RCB2154; Riken Cell Bank, Tsukuba, Japan) were maintained in low-glucose Dulbecco’s modified Eagle’s medium (Wako Pure Chemical Industries, Ltd., Osaka, Japan) containing 10 % calf serum, 0.5 % GlutaMax, and 0.05 % gentamicin (all from Invitrogen, Carlsbad, CA, USA) at 37 °C under 5 % CO2 in a humidified incubator (Astec, Fukuoka, Japan). The medium was replaced with fresh medium every 3 days. At near-confluence, which occurred every 7–10 days, cells were detached from culture dishes with trypsin (Wako) and seeded into new culture dishes. All hBMSCs used in this study were at or before 28th passage to insure high proliferative capacity.
A simple stretching device (STB-140; Strex, Osaka, Japan) fitted with elastic, silicon rubber chambers (Fig. 1a) was used to apply uniaxial, cyclic deformation. The elastic chambers have a transparent bottom (20 × 20 mm2) which is 100-μm thick and walls that are 9.9-mm tall (Fig. 1b). The chamber is deformable by up to 20 % along a single direction by the use of a grappling-hook device made of stainless steel. Uniaxial deformation of the elastic substrate is accompanied by a small degree of subsidiary deformation in the orthogonal direction because the sides of the elastic substrate are allowed to deform. Although the orthogonal deformation is suppressed by the thick walls of the elastic chamber, a 10 % elongation induces up to an approximately 4.9 % perpendicular retraction.
The elastic chambers were incubated in distilled water in an ultrasonic bath for 15 min, placed in sterile phosphate-buffered saline (PBS; Cosmo Bio, Tokyo, Japan), and sterilized by exposure to ultraviolet light in a sterile hood for 30 min. They were then coated with fibronectin (50 μg/ml in PBS; BD Biosciences, Franklin Lakes, New Jersey, USA) for 3 h. Trypsinized hBMSCs were plated onto the bottom of the chambers at a density of 1.0 × 105 to 1.3 × 105 cells/ml and cultured as described above for 2 days without cyclic stretching. Finally, 1-Hz uniaxial cyclic stretching of 10 or 15 % elongation was applied to the hBMSCs in the culture environment over 24 or 48 h. The experimental conditions are summarized in Table 1. Control cells were treated similarly, but were not subjected to cyclic stretching. Cellular morphology was observed using a phase-contrast microscope (IX71; Olympus, Tokyo, Japan).
Quantitative rtPCR
When the cyclic stretching period was completed, a portion of the cells was lysed, and their total RNA was isolated using an RNeasy Mini Kit (Qiagen, Düsseldorf, Germany). The purity and concentration of the RNA was assessed by determination of the absorbance ratio at 260 and 280 nm. Reverse transcription was completed using a High Capacity RNA-to-cDNA Kit (Applied Biosystems, Carlsbad, California, USA). Gene expression levels for glyceraldehyde 3-phosphate dehydrogenase (GAPDH; internal control), Col I, Col III, Tnc, and Scx were analyzed using pre-designed minor groove binder probes (Applied Biosystems), TaqMan PCR Master Mix (Applied Biosystems) and a Light Cycler apparatus (ABI 7300; Applied Biosystems). Gene expression levels were calculated by the standard curve method and normalized to gene expression levels for GAPDH.
Col I molecules self-assemble into highly organized fibrils which form collagen fibers [38]. Cross-linking of these fibers in the extracellular matrix (ECM) gives them a high tensile strength and provides mechanical strength for tendon tissue [39]. Col III forms smaller, less organized fibrils [40]. Tnc is thought to be involved in ECM formation, contributing to the mechanical stability of tendon tissue through its interactions with collagen fibrils and decorin, a proteoglycan [41]. Scx is a transcription factor specifically expressed in tendons and ligaments. It is involved in the activation of the proα1(I) collagen gene in tendon fibroblasts [39].
AFM Measurement of Elastic Modulus
A commercial atomic force microscope (NanoMan VS-1N; Bruker AXS, Madison, Wisconsin, USA) fitted with a silicon-nitride cantilever (spring constant, 0.06 N/m; DNPS-10; Bruker AXS) was used to obtain the elastic modulus of individual cell surfaces. The geometry of the tip was pyramidal with an opening angle of 25°. After AFM was used to measure the force curves of a cell, the elastic modulus of the cell was calculated by fitting the force curves to the Hertz model assuming the contact area to be a semi-infinite medium [42]. The elastic modulus E of the cell was obtained from the following equation:
where F is the force (=0.05–0.3 nN) of the cantilever, δ is the indentation (=2–300 nm) of the cantilever; α is the opening angle (=25°) of the tip of the cantilever; and ν is the Poisson’s ratio of the cell (=0.5 when the cells are considered incompressible [43]). The range of fit of the force curves was 0.05–0.3 nN, where the typical indentation of the cells was 2–300 nm, corresponding to less than 10 % of the cell thickness.
To obtain the elastic modulus of living cells, the walls of the elastic chambers were cut and the chambers were immersed in culture solution. Then, the AFM measurement was performed on the cells in the solution. The temperature of the culture solution was maintained at 37 °C using a heater (Bruker AXS) during the measurement. Since the values of elastic modulus change significantly at different position of the cell, the force curves were acquired approximately at the same position around the nucleus of each cell (within a few micrometers of the nucleus periphery) to prevent measurement errors in this study.
Confocal Fluorescence Microscopy
The internal architectures of the control and experimental (stretched) cells were observed under a confocal fluorescence microscope (A1Rsi-N; Nikon Instech. Co., Ltd., Tokyo, Japan). For observation of the internal cytoskeleton and nucleus, cells were fixed with 4 % paraformaldehyde (Wako) for 15 min, permeabilized in 0.5 % Triton X-100 (Wako) in PBS for 15 min, rinsed with PBS, and stained with FITC (Enzo Life Sciences, New York, USA)- or DAPI (Calbiochem, Darmstadt, Germany)-tagged antibodies, respectively.
Statistical analysis
The means and standard errors are reported for three repeat samples in the rtPCR and fluorescence observation, and nine repeat samples in the AFM measurement. The paired Student’s t test was used for statistical analyses, and P < 0.01 and 0.05 were taken to indicate statistical significance.
Results
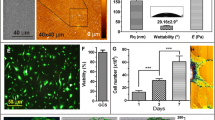
Phase-contrast images showing the cellular morphologies of the control cells and the 10 % (24 h)-, 10 % (48 h)-, 15 % (24 h)-, and 15 % (48 h)-stretched cells are shown in Fig. 2a–e, respectively. These images show a tendency of cell proliferation with increasing time in culture with particularly high proliferation occurring in the 10 %-stretched cells (Fig. 2b, d). There were, however, no statistically significant differences in terms of the quantitative proliferation data (data not shown). Although the application of sinusoidal cyclic stretching did not cause substantial changes in cell morphology, it did induce marked changes in cell orientation; the control cells remained randomly oriented (Fig. 2a), whereas the stretched cells began to orient perpendicular to the stretch direction. The tendency toward perpendicular orientation increased as the amount of elongation or length of the cyclic stretching period increased (Fig. 2b–e).
The time-related changes in gene expression for the MSC-to-tenocyte differentiation markers Col I, Col III, Tnc, and Scx are shown in Fig. 3; in this figure, the expression level of each gene is shown relative to the expression level of that gene in the control cells (defined as 1.0). All the marker genes exhibited significant upregulation in the 10 %-stretched cells. In particular, Scx gene expression in 10 %-stretched cells increased to relative levels of 1.6 and 2.5 at 24 and 48 h, respectively. On the other hand, all the maker genes indicated slight upregulation for the 15 %-stretched cells after 24 h although no significant differences in the gene expression were observed compared with the control cells. Furthermore, all marker gene expression levels were lower in the 15 %-stretched cells than in the control cells after 48 h.
Time-related changes in Col I, Col III, Tnc, and Scx mRNA levels in cells subjected to 10 or 15 % cyclic uniaxial stretching at 1 Hz for 24 or 48 h as indicated. Data are normalized to the corresponding mRNA levels in non-stretched cells (defined as 1). *P < 0.05 relative to control, **P < 0.01 relative to control
A least-squares fit (blue line) of representative AFM force data to Eq. 1 is shown in Fig. 4. The experimental data (red squares) agreed well with the Hertz model and showed a typical dependence of applied force on the depth of indentation. The elastic modulus results (mean ± SEM) obtained from the force curves are shown in Fig. 5. The elastic modulus values for both of the 10 %-stretched groups were significantly greater than that of the control group (P < 0.01), but no statistically significant changes were observed for the 15 %-stretched groups although the data trends mirrored those of the 10 %-stretched groups.
A representative curve showing the AFM-measured force around a cell nucleus. Red squares experimental points, blue line least-squares fit of the data to Eq. 1 (Color figure online)
Fluorescence images of control and 10 %-stretched cells are shown in Fig. 6. In the control cells, the appearance of the actin stress fibers (green) and nuclei (blue) indicated the immaturity of the cytoskeleton (Fig. 6a). Cyclic 10 % stretching was associated with development of the cytoskeleton (Fig. 6b), and cytoskeletal development increased as the duration of the cyclic stretching period increased (Fig. 6c).
Discussion
A familiar phenomenon in studies of cell mechanics is the orientation of the major cellular axis perpendicular to the direction of cyclic applied stretch, as shown in Fig. 2 [44–47]. Neidlinger-Wilke et al. [46] suggested that this cell orientation response occurs primarily because the cell reorients to avoid major axial surface strains would induce a change in cell length. Our results are consistent with those of Zhang et al. [47] and Chen et al. [48], who observed that the degree of orientation increases as the duration and force of the strain increases, and that stretched cells become slightly longer and more slender.
Messenger RNAs (mRNAs) for four MSC-to-tenocyte differentiation markers (Col I, Col III, Tnc, and Scx) were expressed at higher levels in 10 %-stretched cells than in control cells (Fig. 3), but the effects of lengthening the experimental stretching period from 24 to 48 h varied among the marker mRNAs. Although Col I and Col III mRNA levels increased during the first 24 h of 10 %-stretching, they remained constant or decreased over the next 24 h, suggesting a gradual decrease in the mechanical stress experienced by the cells as they became oriented perpendicular to the cyclic stretch axis [49]. On the other hand, mRNA levels for Tnc and Scx continued to increase from 24 to 48 h despite the decreased mechanical stress at 48 h. These results suggest that the response of Tnc/Scx expression to decreased mechanical stress lags behind that Col I/Col III expression, as also suggested by Zhang et al. [47].
Unlike the 10 %-stretched cells, although there were no meaningful differences in differentiation marker mRNA levels after 24 h between the 15 %-stretched cells and the control cells, all maker gene expression levels exhibited slight upregulation. By 48 h, the 15 %-stretched cells exhibited marked downregulation of marker mRNA gene expression. Costa et al. [50] noted previously that a large degree of elongation (>15 %) can destroy some cellular functions. Taking all these results into consideration, we conclude that hBMSCs subjected to 10 % elongation differentiate into tenocytes and are not functionally damaged. The differentiation of hBMSCs into tenocytes subjected to 15 % elongation cannot be completely denied, but the effect would not be prominent. The induction of hBMSC-to-tenocyte differentiation by 10 % stretching has been previously reported [25, 48, 51].
In our experiments, the elastic modulus of cells subjected to stretching ranged from 3.7 to 24.5 kPa. Other researchers using AFM or scanning probe microscopy to measure the elastic moduli of various cells have reported values ranging from a few kPa to approximately 100 kPa [30–37]. For example, Titushkin and Cho [34], using AFM, obtained an elastic modulus value of 3.2 ± 1.4 kPa for human MSCs, and Haga et al. [37], using AFM, obtained elasticity values ranging from 4 to 100 kPa for the cellular surface of living fibroblasts. Therefore, the elastic modulus values obtained in our study seem reasonable.
Using AFM, we were able to observe statistically significant elevation of the elastic modulus in 10 %-stretched cells, but not in 15 %-stretched cells, after 24 and 48 h of cyclic stretching (Fig. 5). We note that the elastic modulus values increased from 0 to 24 and from 24 to 48 h in both the 10- and 15 %-stretched groups although the differences between the 24- and 48-h time points were not statistically significant. This observation suggests the possibility that the elevation of the elastic modulus results directly from the mechanical cyclic stretching and not from cell differentiation. Admittedly, Mizutani et al. [35] reported that mechanical cyclic stretching can increase the cellular elastic modulus in the absence of cell differentiation. However, because we failed to see a significant increase in the elastic modulus of the 15 %-stretched cells in our experiment, we conclude that our elastic modulus results derive from cell differentiation.
Because tenocytes are fibroblasts, the differentiation of hBMSCs into tenocytes involves the development of the cytoskeleton [52]. Therefore, our observations of actin stress fibers, a major cytoskeletal constituent, via confocal fluorescence microscopy (Fig. 6), provide an explanation for the differentiation-associated increase in elastic modulus. The actin stress fibers around the nuclei of 10 %-stretched cells appeared denser and longer after 24 h, consistent with differentiation, than those in control cells (compare Fig. 6a, b). After 48 h, the actin stress fibers in the 10 %-stretched cells were even denser (Fig. 6c). We confirmed that the growth of the actin stress fibers was constricted in 15 %-stretched cells (data not shown). Thus, cytoskeletal development increased the cellular elastic modulus. This result indicates that the cellular elastic modulus is a valid measure of stem cell-to-tenocyte differentiation and suggests that AFM might be applicable to the evaluation of stem cell differentiation into other types of cells.
The application of sinusoidal cyclic stretching of 10 or 15 % elongation at 1 Hz over 24 or 48 h induced the differentiation of hBMSCs into tenocytes. Differentiation of the 10 %-stretched cells into tenocytes was confirmed by rtPCR evaluation of mRNA expression levels for the MSC-to-tenocyte differentiation markers Col I, Col III, Tnc, and Scx. AFM measurements of the elastic modulus of 10 %-stretched cells after 24 or 48 h of cyclic stretching showed clear elevation of the elastic modulus relative to that of the control (non-stretched) cells. Observations of the cells by confocal fluorescence microscopy enabled us to attribute the increase in elastic modulus to the growth and development of the cellular cytoskeleton as the cells differentiated into tenocytes. We conclude in this paper that AFM measurement of the cellular elastic modulus is a valid method for monitoring the differentiation of stem cells into tenocytes.
References
Wolfman, N. M., Hattersley, G., Cox, K., Celeste, A. J., Nelson, R., Yamaji, N., et al. (1997). Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. Journal of Clinical Investigation, 100(2), 321–330.
Wang, J. H. C. (2006). Mechanobiology of tendon. Journal of Biomechanics, 39(9), 1563–1583.
Goh, J. C. H., Ouyang, H. W., Teoh, S. H., Chan, C. K. C., & Lee, E. H. (2003). Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Engineering, 9(1), S31–S44.
Sharma, P., & Maffulli, N. (2006). Biology of tendon injury: Healing, modeling and remodeling. Journal of Musculoskeletal and Neuronal Interactions, 6(2), 181–190.
Sharma, P., & Maffulli, N. (2005). Current concepts review tendon injury and tendinopathy: Healing and repair. Journal of Bone and Joint Surgery American Volume, 87A(1), 187–202.
Miyashita, H., Ochi, M., & Ikuta, Y. (1997). Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Archives of Orthopaedic and Trauma Surgery, 116(8), 454–462.
Tohyama, H., Yasuda, K., Kitamura, Y., Yamamoto, E., & Hayashi, K. (2003). The changes in mechanical properties of regenerated and residual tissues in the patellar tendon after removal of its central portion. Clinical Biomechanics, 18(8), 765–772.
Chan, B. P., Fu, S. C., Qin, L., Rolf, C., & Chan, K. M. (1998). Pyridinoline in relation to ultimate stress of the patellar tendon during healing: An animal study. Journal of Orthopaedic Research, 16(5), 597–603.
Bagnaninchi, P. O., Yang, Y., El Haj, A. J., & Maffulli, N. (2007). Tissue engineering for tendon repair. British Journal of Sports Medicine, 41(8), e10.
Bullough, R., Finnigan, T., Kay, A., Maffulli, N., & Forsyth, N. R. (2008). Tendon repair through stem cell intervention: Cellular and molecular approaches. Disability and Rehabilitation, 30(20–22), 1746–1751.
Hampson, K., Forsyth, N. R., El Haj, A., & Maffulli, N. (2008). Tendon tissue engineering. In N. Ashammakhi, R. Reis & F. Chiellini (Eds.), Topics in tissue engineering, Chapter 3 (Vol. 4), Expertissues, E-book.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Caplan, A., & Bruder, S. P. (2001). Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends in Molecular Medicine, 7(6), 259–264.
Giovannini, S., Brehm, W., Mainil-Varlet, P., & Nesic, D. (2008). Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation, 76(2), 118–129.
Awad, H., Butler, D. L., Boivin, G. P., Smith, F. N. L., Malaviya, P., Huibregtse, B., et al. (1999). Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Engineering, 5(3), 267–277.
Friedens, A. J., Petrakov, K. V., Kuroleso, A. I., & Frolova, G. P. (1968). Heterotopic transplants of bone marrow—analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation, 6(2), 230–247.
Lee, C. H., Moioli, E. K., & Mao, J. J. (2006). Fibroblastic differentiation of human mesenchymal stem cells using connective tissue growth factor. Conference Proceedings of IEEE Engineering in Medicine and Biological Society, 1, 775–778.
Koch, H., Jadlowiec, J. A., Fu, F. H., Nonn, J., Merk, H. R., Hollinger, J. O., et al. (2004). The effect of growth/differentiation factor-5 (GDF-5) on genotype and phenotype in human adult mesenchymal stem cells. Zeitschrift für Orthopädie und ihre Grenzgebiete Deutsche Orthopädische Gesellschaft, 142(2), 248–253.
Park, A., Hogan, M. V., Kesturu, G. S., James, R., Balian, G., & Chhabra, A. B. (2010). Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Engineering, Part A, 16(9), 2941–2951.
Haddad-Weber, M., Prager, P., Kunz, M., Seefried, L., Jakob, F., Murray, M. M., et al. (2010). BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy, 12(4), 505–513.
Zeichen, J., van Griensven, M., & Bosch, U. (2000). The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. American Journal of Sports Medicine, 28(6), 888–892.
Tanaka, H., Manske, P. R., Pruitt, D. L., & Larson, B. J. (1995). Effect of cyclic tension on lacerated flexor tendons in-vitro. Journal of Hand Surgery American Volume, 20A(3), 467–473.
Nöth, U., Schupp, K., Heymer, A., Kall, S., Jakob, F., Schutze, N., et al. (2005). Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy, 7(5), 447–455.
Yang, G. G., Crawford, R. C., & Wang, J. H. C. (2004). Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. Journal of Biomechanics, 37(10), 1543–1550.
Zhang, L., Tran, N., Chen, H. Q., Kahn, C. J. F., Marchal, S., Groubatch, F., et al. (2008). Time-related changes in expression of collagen types I and III and of tenascin-C in rat bone mesenchymal stem cells under co-culture with ligament fibroblasts or uniaxial stretching. Cell and Tissue Research, 332(1), 101–109.
Kuo, C. K., & Tuan, R. S. (2008). Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Engineering, Part A, 14(10), 1615–1627.
Altman, G. H., Horan, R. L., Martin, I., Farhadi, J., Stark, P. R. H., Volloch, V., et al. (2001). Cell differentiation by mechanical stress. FASEB Journal, 15(14), 270.
Farng, E., Urdaneta, A. R., Barba, D., Esmende, S., & McAllister, D. R. (2008). The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clinical Orthopaedics and Related Research, 466(8), 1930–1937.
Xu, B., Song, G., Ju, Y., Li, X., Song, Y., & Watanabe, S. (2012). RhoA/ROCK, cytoskeletal dynamics and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. Journal of Cell Physiology, 227(6), 2722–2729.
Mahaffy, R. E., Park, S., Gerde, E., Kas, J., & Shih, C. K. (2004). Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophysical Journal, 86(3), 1777–1793.
Takai, E., Costa, K. D., Shaheen, A., Hung, C. T., & Guo, X. E. (2005). Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Annals of Biomedical Engineering, 33(7), 963–971.
Costa, K. D., Sim, A. J., & Yin, F. C. P. (2006). Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. Journal of Biomechanical Engineering: The ASME, 128(2), 176–184.
Simon, A., Cohen-Bouhacina, T., Porte, M. C., Aime, J. P., Amedee, J., Bareille, R., et al. (2003). Characterization of dynamic cellular adhesion of osteoblasts using atomic force microscopy. Cytometry, Part A, 54A(1), 36–47.
Titushkin, I., & Cho, M. (2007). Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophysical Journal, 93(10), 3693–3702.
Mizutani, T., Haga, H., & Kawabata, K. (2004). Cellular stiffness response to external deformation: Tensional homeostasis in a single fibroblast. Cell Motility and the Cytoskeleton, 59(4), 242–248.
Goldmann, W. H., Galneder, R., Ludwig, M., Xu, W. M., Adamson, E. D., Wang, N., et al. (1998). Differences in elasticity of vinculin-deficient F9 cells measured by magnetometry and atomic force microscopy. Experimental Cell Research, 239(2), 235–242.
Haga, H., Sasaki, S., Kawabata, K., Ito, E., Ushiki, T., & Sambongi, T. (2000). Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy, 82(1–4), 253–258.
Eyre, D. R., Paz, M. A., & Gallop, P. M. (1984). Cross-linking in collagen and elastin. Annual Review of Biochemistry, 53, 717–748.
Lejard, V., Brideau, G., Blasis, F., Salingcarnboriboon, R., Wagner, G., Roehrl, M. H. A., et al. (2007). Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. Journal of Biological Chemistry, 282(24), 17665–17675.
Lapiere, C. M., Nusgens, B., & Pierard, G. E. (1977). Interaction between collagen type-1 and type-3 in conditioning bundles organization. Connective Tissue Research, 5(1), 21–29.
Elefteriou, F., Exposito, J. Y., Garrone, R., & Lethias, C. (2001). Binding of tenascin-X to decorin. FEBS Letters, 495(1–2), 44–47.
Sneddon, I. N. (1965). The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. International Journal of Engineering Science, 3(1), 47–57.
Radmacher, M. (2002). Measuring the elastic properties of living cells by the atomic force microscope. Methods in Cell Biology, 68, 67–90.
Wang, H. C., Ip, W., Boissy, R., & Grood, E. S. (1995). Cell orientation response to cyclically deformed substrates: Experimental validation of a cell model. Journal of Biomechanics, 28(12), 1543–1552.
Naruse, K., Yamada, T., & Sokabe, M. (1998). Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. American Journal of Physiology: Heart and Circulatory Physiology, 274(5), H1532–H1538.
Neidlinger-Wilke, C., Grood, E. S., Wang, J. H. C., Brand, R. A., & Claes, L. (2001). Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. Journal of Orthopaedic Research, 19(2), 286–293.
Zhang, L., Kahn, C. J. F., Chen, H. Q., Tran, N., & Wang, X. (2008). Effect of uniaxial stretching on rat bone mesenchymal stem cell: Orientation and expressions of collagen types I and III and tenascin-C. Cell Biology International, 32(3), 344–352.
Chen, Y. J., Huang, C. H., Lee, I. C., Lee, Y. T., Chen, M. H., & Young, T. H. (2008). Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connective Tissue Research, 49(1), 7–14.
Park, J. S., Chu, J. S. F., Cheng, C., Chen, F. Q., Chen, D., & Li, S. (2004). Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnology and Bioengineering, 88(3), 359–368.
Costa, K. D., Hucker, W. J., & Yin, F. C. P. (2002). Buckling of actin stress fibers: A new wrinkle in the cytoskeletal tapestry. Cell Motility and the Cytoskeleton, 52(4), 266–274.
Xu, B., Song, G., & Ju, Y. (2011). Effect of focal adhesion kinase on the regulation of realignment and tenogenic differentiation of human mesenchymal stem cells by mechanical stretch. Connective Tissue Research, 52(5), 373–379.
Kannus, P., Jozsa, L., & Jarvinnen, M. (2000). Basic science of tendons. In W. J. Garrett, K. Speer, & D. Kirkendall (Eds.), Principles and practice of orthopaedic sports medicine (pp. 21–37). Philadelphia: Lippincott Williams and Wilkins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morita, Y., Mukai, T., Ju, Y. et al. Evaluation of Stem Cell-to-Tenocyte Differentiation By Atomic Force Microscopy to Measure Cellular Elastic Moduli. Cell Biochem Biophys 66, 73–80 (2013). https://doi.org/10.1007/s12013-012-9455-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9455-x