Abstract
The objective of this study was to analyze the influence of TNF-α on rat mesenchymal stem cells (MSCs) and to assess feasibility of MSC transplantation to repair ischemic injury. In this study, adhesion molecules and cell specific surface markers on MSCs were measured after exposure to different concentrations of TNF-α. MSCs stimulated with varying concentrations of TNF-α were cultured with aortic endothelial cells, and the adhesion rate was measured. MSCs were then stimulated with an optimum concentration of TNF-α as determined in vitro, and injected intravenously into rats with ischemic hind limb injury. The number of MSCs in muscle samples from the ischemic area was counted. The results showed that (1) TNF-α induced a concentration-dependent increase in VCAM-1 expression in MSCs, whereas the expression of L-selectin, ICAM-1 and VLA-4 did not change significantly. Expression of MSC-specific antigens was unchanged. (2) MSCs pretreated with 10 ng/ml TNF-α showed significantly increased adhesion to endothelial cells in vitro, and accumulated to a greater extent in the areas of ischemic damage in rat hind limbs. We were able to conclude that TNF-α has no effect on expression of MSC-specific markers, but can increase the expression of VCAM-1 on rat MSCs. Suitable concentrations of TNF-α can promote MSC adhesion to endothelial cells and migration to damaged tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs) have broad application potential in the treatment of heart, brain, and necrotic limb disease as well as gene therapy [1–3] due to their pluripotency and low immunogenicity [4–6]. However, in clinical application studies, few MSCs settled in local tissue or differentiated after bone marrow MSC transplantation, and tissue repair could not be achieved. Therefore, the most crucial issue is to devise a mechanism to increase the number of transplanted MSCs that migrate to areas of tissue damage. Compared to numerous recent studies on chemokine-mediated migration of MSCs to areas of tissue damage, studies of adhesion molecules in MSCs are rare [7, 8]. In our study, we explored the expression of adhesion molecules on MSCs in response to different concentrations of TNF-α, and the means to promote MSC migration to tissue lesions.
Materials and Methods
Cell Culture
MSC Isolation and Culture
MSCs were isolated from bone marrow of 4-week-old Wistar rats (Experimental Animal Center of Shandong University) as described in previous reports [9]. Cells were cultured in l-DMEM medium (Gibco) containing 10% fetal bovine serum (HyClone) and passaged every 3–4 days. The third passage of MSCs was used in the experiments.
TNF-α Stimulation
Cells were stimulated for 24 h with TNF-α concentrations of 2, 5, 10, or 20 ng/ml. Control cells were incubated in parallel without TNF-α. Cells pre-treated with TNF-α were used for subsequent experiments.
Aortic Endothelial Cells Isolation and Culture
Aortic endothelial cells were isolated and cultured from 4-week-old Wistar rats as previously described [10].
Flow Cytometry
TNF-α treated and control MSCs, adjusted to a cell density of 106/ml, were stained with the following antibodies (all purchased from BD): FITC-ICAM-1, FITC-VLA-4, FITC-l-Selectin, PE-VCAM-1, FITC-CD29, and FITC-CD90. Cells were incubated with the appropriate antibody for 20 min in the dark at room temperature, washed with PBS, and resuspended in 500 μl PBS. The fluorescence was measured by flow cytometry. All the experiments were repeated three times each.
MSCs and Aortic Endothelial Cell in Vitro Adhesion Assay
MSCs were harvested after pretreatment with varying concentrations of TNF-α. The cell density was adjusted to 1 × 106/ml, and the cells were fluorescently stained by adding 0.15 μl DiI working solution as described by the manufacturer (Invitrogen). After incubation at 37°C for 20 min, the MSC cells were maintained in 10% FBS-DMEM. The second passage of cultured rat aortic endothelial cells was seeded into 24-well culture plates at a density of 104 per well. When the endothelial cells reached confluency, 6 × 104 fluorescently labeled cultured rat MSCs were added per well and incubated for 12 h at 37°C. After washing with PBS, the fluorescence in five randomly selected wells was counted by fluorescence microscopy.
Rat Bone Marrow MSCs Transplantation in a Rat Hind Limb Ischemia Model
Rat Autologous Clot Particles
Nine adult rats weighing about 250 g were chosen. A total volume of 10 ml of blood was obtained from each animal by jugular vein puncture, 3–4 ml at a time with 1 week between samples. The blood was allowed to coagulate and then dried. The resulting blood clots were crushed and sifted under sterile conditions to obtain particles with a diameter of 50–60 μm. These particles were mixed with sterile saline to form a 4% w/v solution that was used as the embolizing agent for creating the rat hind limb ischemic injury model.
Rat Hind Limb Ischemia Model
Hind limb ischemia was induced in nine adult rats under 10% chloral hydrate intraperitoneal anesthesia. 0.6 ml of 4% w/v blood clot particle solution was injected into the femoral artery. MSC transplantation was performed 24-h post ischemia.
Bone Marrow MSCs Transplantation into Rats
Rats treated in 4.2 were divided into three groups of three rats each: (1) infusion of DiI-labeled rat bone marrow MSCs pre-treated for 24 h with 10 ng/ml TNF-α, (2) infusion of DiI labeled control MSCs, (3) infusion of an equal volume PBS alone. MSCs were infused as follows: MSCs were first filtered with a 40-μm filter and then 1.5 × 106 MSCs were diluted in 0.5 ml PBS. The cell suspensions were injected via the tail vein. The inner thigh and calf gastrocnemius muscles were excised under anesthesia 24 h after infusion of the MSCs and immersed in hydroxymethyl cellulose. The tissues were immediately placed in a −80°C freezer to produce frozen sections. Ten consecutive tissue slides were obtained from each tissue sample, three microscope fields of each slide were randomly selected under a fluorescence microscope, and the numbers of MSCs were counted.
Statistics
SPSS10.0 software was used for paired t tests and data analysis. Data are shown as mean ± standard error, differences of P < 0.05 were considered to be statistically significant.
Results
Expression of Cell Surface Markers
In control MSCs, the expression of ICAM-1 was high, whereas the expression of VCAM-1, l-selectin, and VLA-4 were relatively low. Treatment of MSCs for 24 h with 2, 5, 10, or 20 ng/ml TNF-α resulted in a concentration-dependent increase in the expression of VCAM-1 in MSCs. This increase was found to be statistically significant (P < 0.05) when compared to the control group. The expression of the adhesion molecules ICAM-1, l-selectin and VLA-4, and the MSC markers, CD29 and CD90, did not change significantly, as shown in Figs. 1 and 2.
The effect of different concentrations of TNF-α on the expression of adhesion molecules (a), and of MSC markers (b). MSC cells were treated for 24 h with a range of TNF-α concentrations, and the expressions of the adhesion molecules, VCAM-1, ICAM-1, L-selectin, and VLA-4, as well as the MSC markers, CD29 and CD90, were quantified by immunostaining and flow cytometry. Mean values of triplicate samples are shown; error bars indicate standard error
The effect of 10 μg/ml TNF-α on expressions of adhesion molecules and MSC markers. MSC cells were incubated for 24 h in the absence (left) or the presence (right) of 10 ng/ml TNF-α, and expressions of the adhesion moleculess VCAM-1, ICAM-1, l-selectin, and VLA-4, as well as the MSC markers, CD29 and CD90, were quantified by immunostaining and flow cytometry. In untreated cells, VCAM-1, ICAM-1, l-selectin, VLA-4, CD29, and CD90 were expressed on 5.9, 99.8, 2.0, 0.5, 96.4, and 96.6% of cells, respectively. In MSCs pre-treated with 10 ng/ml TNF-α, the expression rates of VCAM-1, ICAM-1, l-selectin, VLA-4, CD29, and CD90 were 80.9, 100, 3.5, 1.1, 93.7, and 96.1%, respectively. Note the substantial increase in VCAM-1 expression
In Vitro Adhesion of Bone Marrow MSCs to Endothelial Cells
MSC cells were incubated in a range of concentrations of TNF-α for 24 h, fluorescently labeled with DiI, and then incubated with rat endothelial cells for 12 h. The numbers of MSCs adhering to endothelial cells were counted by fluorescence microscopy (Fig. 3). MSCs incubated in 20 or 10 ng/ml TNF-α adhered to endothelial cells in significantly greater numbers than untreated control cells (P < 0.05), but MSC cells treated with 5 or 2 ng/ml did not differ significantly from the controls.
Dose-dependent effect of TNF-α on the MSC adhesion to endothelial cells in vitro. MSC cells were incubated in a range of concentrations of TNF-α for 24 h, fluorescently labeled, and then incubated with rat endothelial cells for 12 h. The numbers of MSCs adhering to endothelial cells was assessed by fluorescence microscopy. Photographs of untreated MSC (a), and cells treated with 10 ng/ml TNF-α (b) are shown. Fluorescent MSC in five randomly selected wells at each TNF-α concentration were counted. Results are shown as the mean; error bars indicate standard error; Asterisks indicates statistically significant differences, P < 0.01
Migration of Bone Marrow MSCs to Tissue Lesions After Transplantation
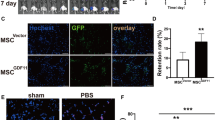
Rats were subjected to ischemic hind limb damage as described in “Materials and Methods” section, and fluorescently labeled MSCs were injected into the tail vein. The number of MSCs accumulating in hind limb muscle tissue was determined by counting the number of fluorescent cells in frozen tissue sections. Compared to control cells, MSCs pre-treated with 10 ng/ml TNF-α accumulated in muscle tissue in significantly higher numbers(P < 0.01) as shown in Fig. 4.
Accumulation of TNF-α stimulated MSC in ischemic muscle tissue in vivo. MSC cells were pretreated with 10 ng/ml TNF-α, fluorescently labeled, and then injected into the tail vein of rats that had ischemic injury to the hind limbs. The number of accumulated MSC in frozen sections of muscle tissue was determined by fluorescence microscopy. Untreated control MSC (a) did not accumulate as well as TNF-α treated MSC (b). The number of fluorescently labeled cells in control and treated samples was counted (c), the mean of each group, with triplicate fields from ten sections per sample, is shown. Error bars indicate standard error, and asterisks indicates a statistically significant difference, P < 0.01
Discussion
Adhesion molecules are widely distributed on the cell surface and in the extracellular matrix. Inflammatory cytokines released upon inflammation or ischemic injury, such as TNF-α and IL-1β, [11] usually stimulate white blood cells and endothelial cells to express adhesion molecules. Adhesion molecules, by binding to their receptors, mediate cell–cell or cell–matrix contacts and are involved in cell activation and migration. They play an important role in the processes of inflammation, ischemic injury and wound healing. In recent years, studies of MSC applications in heart, brain, and other ischemic diseases have shown that MSCs have a “homing” feature toward ischemic or injured tissue [12–15]. Inflammatory cytokines such as TNF-α and IL-1β released from the injured tissue [16] stimulate increased expression of adhesion molecules in vascular endothelial cells [17]. Some researchers have found that inflammatory factors contribute to the adhesion of local MSCs to vascular endothelial cells or homing to injured myocardial tissue [18]. Antibody blockade of TNF-α in myocardial ischemia has been shown to increase the myocardial damage, suggesting that TNF-α plays a pivotal role in MSC homing to ischemic or injured tissue [19]. In our study, different concentrations TNF-α were used in transient pre-treatment of MSCs. We first measured the expression of the adhesion molecules l-selectin, ICAM-1, VCAM-1, and VLA-4 and the MSC stem cell markers, CD29 and CD90, in rat MSCs in the presence or the absence of TNF-α using flow cytometry. We then tested whether TNF-α pretreatment would increase the ability of MSC cells to adhere to aortic endothelial cells in vitro. Based on the results from the in vitro experiments, we selected a suitable pre-treatment concentration of TNF-α to prepare MSCs for stem cell transplantation in our rat hind limb ischemia model. The results showed that baseline expression of ICAM-1 in rat MSCs was high whereas levels of l-selectin, VCAM-1, and VLA-4 were very low. Pretreatment with TNF-α for 24 h resulted in a dose-dependent, statistically significant increase in VCAM-1 expression but did not significantly affect other adhesion molecules such as l-selectin, ICAM-1 and VLA-4.
Selectins mediate leukocyte initial adhesion or rolling along vascular endothelium. A study of the adhesion mechanism of human bone marrow MSCs has found that P-selectin mediated initial adhesion of MSCs to cord blood endothelial cells [20], but the role of l-selectin was rarely discussed. Our results show that l-selectin levels were very low in both TNF-α stimulated and control MSCs, suggesting that l-selectin may not play a role on the initial steps of the interaction between MSCs and endothelial cells. We found that ICAM-1 levels were high in both TNF-α stimulated and control MSCs. One study has shown that blockade of ICAM-1 by monoclonal antibody does not affect the adhesion of MSCs to endothelial cells [18]. The ligand of ICAM-1 is LFA-1 which is expressed on white blood cells [21]. These observations suggest that ICAM-1 may not play a role in the adhesion process between MSCs and endothelial cells.
The ligand of VCAM-1 is VLA-4 [22]. Levels of VCAM-1 and VLA-4 are low under normal conditions. After MSCs are stimulated by TNF-α, VCAM-1 expression is significantly increased in a dose dependent manner, but VLA-4 expression does not change significantly. The expression levels of VCAM-1 in MSCs and in other types of tissue are similar. VCAM-1 is only expressed when it is activated by inflammatory factors. Although the expression of VLA-4 is not high in MSCs, MSCs and vascular endothelial cells both express detectable levels of VLA-4. Studies have reported that the use of anti-VCAM-1 blocking monoclonal antibody significantly reduces the adhesion of MSCs to vascular endothelial cells [18]. These results suggest that VCAM-1 plays a major role in MSCs adhesion to vascular endothelial cells after stimulation with inflammatory cytokines.
In vitro adhesion assays showed that although all concentrations of TNF-α were effective in inducing VCAM-1 expression in MSCs, only the two highest doses, 10 and 20 ng/ml, significantly increased adhesion to aortic endothelial cells in vitro. In our study, we chose the lower dose, 10 ng/ml of TNF-α, to pre-treat MSCs for stem cell transplantation in a rat model of limb ischemia. We found that the number of MSCs adhering to vascular endothelial cells 24 h after infusion was significantly increased when the MSCs were pre-treated with TNF-α. This suggests that TNF-α may increase MSC adhesion to vascular endothelial cells by up-regulating VCAM-1 expression on the cell surface, which facilitates the migration of MSCs into the damaged tissue. TNF-α is an inflammatory factor in injured tissues. Pretreatment of MSCs with small doses of TNF-α can increase expression of adhesion molecule VCAM-1, whereas the expressions of stem cell markers CD29 and CD90 were not significantly altered. This suggests that pretreatment of MSCs with TNF-α might be of use in MSC transplantation by increasing the expressions of cell surface molecules affecting homing and adhesion without affecting the stem cell properties of MSCs
References
Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105, 1815–1822.
Kotobuki, N., Hirose, M., Takakura, Y., et al. (2004). Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artificial Organs, 28(1), 33–39.
Deans, R. J., & Moseley, A. B. (2000). Mesenchymal stem cells: biology and potential clinical uses. Experimental Hematology, 28(8), 875–884.
Koh, S. H., Kim, K. S., Choi, M. R., et al. (2008). Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Research, 1229, 233–248.
Wu, K. H., Zhou, B., Yu, C. T., et al. (2007). Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Annals of Thoracic Surgery, 83(4), 1491–1498.
Wu, K. H., Zhou, B., Mo, X. M., et al. (2007). Therapeutic potential of human umbilical cord-derived stem cells in ischemic diseases. Transplant Proceedings, 39(5), 1620–1622.
Honczarenko, M., Le, Y., Swierkowski, M., et al. (2006). Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells, 24, 1030–1041.
Sasaki, M., Abe, R., Fujita, Y., et al. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. Journal of Immunology, 180, 2581–2587.
Philippe, T., Danièle, N., Nadine, P., et al. (2004). Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Experimental Cell Research, 295(2), 395.
Xiao, Q., Fang, Y. H., Xu, Y., et al. (2008). A new method for effectively isolating rat aortic endothelial cells. Modern General Surgery Development of China, 11(02), 111–113.
Caplan, A. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9(5), 641–650.
Rombouts, W. J., & Ploemacher, R. E. (2003). Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia, 17, 160–170.
Shake, J. G., Gruber, P. J., Baumgartner, W. A., et al. (2002). Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Annals of Thoracic Surgery, 73, 1919–1925.
Chapel, A., Bertho, J. M., Bensdhoum, M., et al. (2003). Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. Journal of Gene Medicine, 5, 1028–1038.
Paul, B., Morgan, D., Wechsler, A., et al. (2004). Chemokine induced migration of human mesenchymal stem cells: a strategy for directing cardiac repair. Journal of the American College of Surgeons, 199, 33–38.
Frangogiannis, N. G., Smith, C. W., & Entman, M. L. (2002). The inflammatory response in myocardial infarction. Cardiovascular Research, 53, 31–47.
Mantovani, A., Bussolino, F., & Dejana, E. (1992). Cytokine regulation of endothelial cell function. FASEB Journal, 6, 2591–2599.
Segers, V. F., van-Riet, I., Andries, L. J., et al. (2006). Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. American Journal of Physiology Heart Circulatory Physiology, 290(4), 1370–1377.
Mann, D. L. (2002). Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circulation Research, 91(11), 988–998.
Ruster, B., Gottig, S., Ludwig, R. J., et al. (2006). Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood, 12(108), 3938–3944.
Makgoba, M. W., Sanders, M. E., & Ginther Luce, G. E. (1988). ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature, 331(6151), 86–88.
Chan, P. Y., & Aruffo, A. (1993). VLA-4 integrin mediates lymphocyte migration on the inducible endothelial cell ligand VCAM-1 and the extracellular matrix ligand fibronectin. Journal of Biological Chemistry, 268(33), 24655–24664.
Acknowledgments
This study was supported by the Natural Science foundation of Shandong Province (ZR2009CZ014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, Q., Wang, Sk., Tian, H. et al. TNF-α Increases Bone Marrow Mesenchymal Stem Cell Migration to Ischemic Tissues. Cell Biochem Biophys 62, 409–414 (2012). https://doi.org/10.1007/s12013-011-9317-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9317-y