Abstract
We previously reported that the abnormal BTG2 expression was related to genesis/development of hepatocellular carcinoma (HCC). The aim of this study was to evaluate the BTG2 expression in HCC compared with p53, cyclin D1, and cyclin E. For this purpose, modified diethylnitrosamine (DEN)-induced primary HCC rat model was established. Target proteins and mRNAs were measured by western blot and RT-PCR/northern blot, respectively. In rat liver, expression of BTG2 and other proteins was determined by western blot, and BTG2 mRNA in HCC/normal tissues was detected by high-flux tissue microarray (TMA) and in situ hybridization (ISH). BTG2 mRNA/protein expression was increased in fetal liver, 7701, and LO2 cell lines but decreased in HepG2 cells. BTG2/p53 were expressed early after DEN treatment, peaked at 5 weeks and decreased gradually thereafter. Cyclin-D1/Cyclin-E expression increased significantly with the tumor progression. BTG2 mRNA was expressed in 71.19% HCC by ISH and correlated with differentiation. Expression of p53/cyclin D1/cyclin E was positive in 82.35/94.12/76.47% BTG2 mRNA-negative tissues, respectively. BTG2 protein expression was lost in 32.2% (19/59) HCC tissues, and the mRNA/protein expression correlated significantly with the increasing tumor grade (P < 0.05). In conclusion, BTG2 expression is commonly impaired in HCC which may be a factor involved in deregulation of cyclin-D1/cyclin-E expression during hepatocarcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The B-cell translocation gene-2 (BTG2) is an antiproliferative gene. It is an early growth response gene [1] and belongs to the BTG/Tob antiproliferative protein family [2, 3]. The rat and mouse homologs of BTG2 are designated as pheocromocytoma cell-3 (PC3) and 12-O-tetradecanoylphorbol-13-acetate (TPA) inducible sequences 21 (TIS21), respectively. The PC3 is an immediate early gene activated by nerve growth factor (NGF) during the onset of neuronal differentiation of PC12 cells which are derived from a tumor of the adrenal medulla [4]. TIS21 is isolated from SW3T3 cells treated with TPA [5]. BTG2 has been cloned from the chromosomal segment 1q32 and contains two exons, one intron, and a wild-type (wt) p53 response element in its 5′-flanking region (from 74 to 122) [6], confirming that BTG2 expression is dependent on wt p53 function [7, 8].
The BTG2 expression is known to correlate with cyclin-dependent kinases (CDKs). CDKs are the molecules responsible for phosphorylation and consequent inactivation of retinoblastoma protein (pRb) [9, 10]. BTG2 overexpression inhibits G1-S progression through the transcriptional regulation of cyclin D1 in the presence of pRb [11] whereas TIS21 downregulates expression of the cyclin E and CDK4 in the absence of pRb function [12]. It is known that BTG2 interacts with protein-arginine methyltransferase 1 (PRMT1) [13], transcription factor CAF1 [14], and homeogene HoxB9 [15] which is involved in cell cycle regulation and in cell differentiation. In addition, the genetic ablation of BTG2 prevents the G2-arrest in embryonic stem (ES) cells in a p53-dependent manner supporting the finding that BTG2 induces the G2-M arrest [7].
Only a few studies have investigated the potential role of BTG2 during carcinogenesis. However, the recent reports on reduced expression of BTG2 in different sets of human malignancies have been extended by a panel of matching tumor and normal tissue samples. These reports indicate that in a large proportion of breast and kidney carcinomas, BTG2 mRNA expression in the tumor tissues is significantly lower than that in the adjacent normal tissues. Further, it has also been observed that BTG2 expression is lost in primary tumors and cancer cell lines such as NCIH69 and A549 human lung cancer cells, thymic carcinoma tissues [16, 17], hyperproliferative epithelial cells, prostate cancer [18], and clear cell renal cell carcinoma [19]. These observations further confirm the significance of BTG2 deregulation in naturally occurring cancers. Interestingly, with regard to the above-mentioned cancers, BTG2 was found to be constitutively expressed in the corresponding normal tissues.
Low levels of BTG2 expression have been observed in normal hepatocytes [20]. Moreover, in our previous study on BTG2/PC3 gene expression in hepatocytes, we found that PC3 gene was rapidly induced after hepatocyte proliferation as detected by the representational difference analysis (RDA) in the rat model of two-third hepatectomy and the peak expression was observed within 1–2 h after surgery. PC3 has been demonstrated to be rapidly induced in a primary culture of rat hepatocytes by epidermal growth factor (EGF), hepatocyte growth factor (HGF), hepatopoietin (HPO), etc. This suggests that the BTG2/PC3 gene is involved in the regulation of liver regeneration, and that it is an immediate early gene closely related to liver regeneration and hepatocarcinogenesis [21–23].
In this study, hepatocellular carcinoma (HCC) was induced in rats by oral administration of diethylnitrosamine (DEN) in drinking water [24, 25], and the changes in the expression levels of BTG2, p53, cyclin D1, and cyclin E were determined over a certain period of time. Besides, we determined the expression of BTG2 mRNA in HCC as well as in HCC-surrounding normal liver tissues using a high-flux tissue microarray (TMA) technique combined with in situ hybridization (ISH). As well, in HCC patients’ population whose immunohistochemistry results indicated the absence of BTG2 mRNA expression, we analyzed the expression of p53, cyclin D1, and cyclin E. We also evaluated the expression of BTG2 in a large cohort of human HCC patients with varied histologies; its relationship with the clinicopathologic variables and with p53, cyclin D1, and cyclin E overexpression was also evaluated. In this study, we show that the loss of nuclear BTG2 expression in HCC correlates with the increasing tumor grade as well as with overexpression of cyclin D1 and cyclin E, suggesting that the loss of BTG2 expression may be a factor involved in the deregulation of cyclin D1 and/or cyclin E expression in human HCC.
Materials and Methods
Cell Culture and Fetal Liver Tissue
HepG2, 7701, and LO2 cells were plated at a density of 105 cells/cm2 in Dulbecco’s modified Eagle medium (DMEM; GIBCO, Invitrogen Life Technologies, CA, USA) containing 5% fetal calf serum (FCS) in 100-mm Petri dishes. The liver tissue samples from 4 and 7 months old fetuses were obtained from the Cryo-Tissue Bank of our hospital research center. The design and procedures of this study were approved by the human and animal research ethics committees of the 3rd Military Medical University, and written informed consent was obtained from all patients enrolled in this study.
Reagents and Antibodies
The Hank’s Buffered Salt Solution (HBSS) and DMEM were from Invitrogen (Invitrogen Life Technologies, CA, USA). The antibodies directed against BTG2, β-actin, p53, Cyclin D1, and Cyclin E were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated secondary antibody was purchased from Bio-Rad (Hercules, SA, USA).
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared using guanidinium thiocyanate (GITC) method (Promega Corp, WI, USA). The quality and integrity of RNA was verified by spectrophotometry and ethidium bromide agarose (1%) gel electrophoresis. For in vitro reverse transcription, briefly, total RNA (1 mg) was denatured at 72°C for 10 min and added to a final reaction volume of 20 μl containing nuclease-free water, 10× RT buffer (2 μl), 25 mmol/l MgCl2, 0.25 mmol/l dNTPs each, 0.5 U of RNasin, 100 U of AMVase and Oligo (dT) primers (Promega, Madison, WI, USA), and then incubated for 15 min at 42°C. After adding 100 μl of nuclease-free water, RT reactions were stored at −20°C until later use in PCR amplification reactions. The PCR primers used were as follows: BTG2 (sense) 5′-TAC CGC TGC ATT CGC ATC AAC-3′, (antisense) 5′-AGG GCC TAG CTG GAG ACT GC-3′, GAPDH (sense) 5′-CCC ATC ACC ATC TTC CAG GAG CG-3′, (antisense) 5′-AGA TGG AGG AGT GGG TGT CGC TGT-3′. The RT products (5 μl each) were amplified in PCR reaction of a 25 μl final volume containing 10× PCR buffer, 2.5 mmol/l dNTPs, 25 ρmol of each primer and 0.5 U of Taq DNA polymerase (Promega, Madison, WI, USA). Amplifications were carried out in a DNA thermal cycler (Perkin Elmer, Boston, MA, USA) using the following reaction profile: denaturation at 94°C for 60 s, primer annealing at 55°C for 60 s, and primer extension at 60°C for 60 s. The number of cycles was designed as to maintain the amplification reactions in the exponential phase which correspond to 30 cycles. PCR products were purified by electrophoresis on 1.5% agarose gel and analyzed by digital image analysis.
Northern Blot Analysis
Total RNA was extracted using TRIzol® method (Life Technologies, Gaithersburg, MD, USA) by following the manufacturer’s instructions and was fractionated on 1% agarose gel containing 0.6 mol/l formaldehyde, then transferred to GeneScreen Plus hybridization transfer membrane (PerkinElmer, MA, USA) and hybridized to 32P-labeled cDNA probes. Hybridization to all probes was carried out at 68°C in 6× standard saline citrate (SSC), 5× Denhardt’s solution, 1% SDS, and 100 μg/ml single-stranded (ss) DNA. The blots were washed in 2× SSC containing 0.05% SDS for 20 min at room temperature (RT) followed by 20 min washes for three times in 0.1× SSC containing 0.1% SDS at 50°C before exposure to X-ray film.
Western Blot Analysis
After the specified time intervals, fresh liver tissue (1 g) was homogenized in 3 ml of homogenization buffer (20 mM Tris, 137 mM NaCl, 10% glycerol, 1 mM NaVO4, 1 U/ml aprotinin, and 1 mM PMSF, pH 8.0). The homogenate was centrifuged at 9000×g for 20 min, and the supernatant fraction was collected. The protein concentration was determined using Bio-Rad protein assay (Life Science, CA, USA) by following the manufacturer’s instructions, and the samples were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). In brief, the samples (30 mg aliquots) were diluted in SDS sample buffer, boiled, and run immediately on 12 or 7.5% acrylamide gels. The fractionated proteins in gel were electroblotted to nitrocellulose, and the membranes were blocked in 5% non-fat milk, followed by incubation with specific primary antibodies (2 mg/ml each) for 3 h in Tris-buffered saline (TBS) containing 1 mg/ml of bovine serum albumin (BSA). Proteins were detected using ECL system (Amersham, Arlington Heights, IL, USA) with HRP-conjugated secondary antibody (1:5000 diluted) (Bio-Rad, Hercules, SA, USA). Densitometric analysis was carried out using Molecular Analyst 1 software (version 1.4; BIO-RAD Laboratories, CA, USA).
Animal Experiments
The research experiments on animals were carried out in accordance with the guidelines for care and use of laboratory animals under the Chinese Law and Statutory Regulations. The animals were carefully and humanely handled at all times and were subjected to no mutilation other than essential surgical procedures as permissible under the Law of Animal Research and Statutory Regulations. Sixty-four male Wistar rats (Japan Clea, Tokyo, Japan), weighing 130–160 g, were administered DEN at the rate of 99 μg/ml (Sigma Chemical Co., St. Louis, MO, USA) in drinking water ad libitum for 5 weeks, followed by plain water for 3 more weeks. After 8 weeks, the DEN administration as earlier described was restarted and continued until 16 weeks. Of the DEN-treated rats, 12 were sacrificed at 5, 8, 12, and 16 weeks. The control rats were given plain water without DEN, and four rats each were sacrificed at 5, 8, 12, and 16 weeks. This protocol of carcinogen treatment for different periods with carcinogen-free interval will allow regression of the non-specific changes associated with the carcinogen administration and is recommended in the literature. Animals were sacrificed at the end of the experiment. Livers were washed with phosphate buffered saline (PBS) by perfusion through the portal vein to eliminate blood cells and were removed, weighed, and used in western blot experiments.
Patients and TMA Construction
All tissue samples used in this study were procured from the archives of the Department of Pathology/Cryo-Tissue bank of the Daping and Xinan Hospitals, affiliated with the 3rd Military Medical University, China. The 59 HCC tissue samples and four normal tissue samples surrounding the morbid tissue were obtained from 59 patients (50 males and 9 females, aged 25–80 years with mean age of 50.7 years) by surgical resection. TMA was constructed from frozen 59 HCC tissues and 4 normal liver tissues (located far away from hepatoma). Sixteen HCCs were of grade 1, 28 of grade 2, and 15 of grade 3. All slides were independently evaluated by two pathologists blinded to the sample identification. In brief, normal and tumor tissues were embedded in paraffin, and hematoxylin and eosin (H&E) stained sections were examined to identify the morphologically representative areas of the specimen from which core biopsies were taken. The cores were transferred to the recipient block according to the array designed previously. Tissue cylinders with a diameter of 0.6 mm were then punched from the selected areas of each donor block and transferred to the recipient paraffin block, and 3–4 μm sections were cut from the tissue array block.
ISH
The digoxigenin (DIG)-labeled oligonucleotide BTG2 antisense probe sequence was as follows: 5′-CCA GTG GGG CCT CCT CGT ACA AGA CGC AGA TG-3′. ISH was performed using commercial kit (Tianjin Hao Yang Biological Manufacturing Corporation, China) and following the manufacturer’s instructions. In brief, pre-hybridization was carried out at 37°C for 1–2 h, followed by hybridization at 37°C for 4 h, and hybridization fluid without probe was used as control. The labeled and control tissue samples were examined by fluorescence microscopy. Negative or positive staining of tumor cells was assessed by color intensity as well as by area stained. Positive staining of tumor cells was represented as an amethyst area of staining. The scoring was as follows: no cell staining was taken as 0, <25% cell staining as 1; 25–50% cell staining as 2; and >50% cell staining as 3. When the average score was >2, we regarded it as positive staining; however, if the score was ≤2, we considered it as negative staining. Three researchers independently scored the samples and interpreted the results.
Immunohistochemical Analysis
Each paraffin-embedded tissue sample was cored eight times, i.e., four times from the periphery and four times from the center of the lesion. This represented the tumor’s peripheral and central regions. The cores were then transferred to a recipient paraffin block following the technique as described [26] and using an automated tissue microarrayer. Immunohistochemistry was performed on formalin-fixed, paraffin-embedded 5 μm tissue sections according to standard procedures as previously described [16]. All steps were performed at RT unless otherwise indicated. In brief, the tissue sections were deparaffinized and immersed in 0.01 M sodium citrate buffer (pH 6.0) in a microwave oven for two times, 5 min each, to enhance antigen retrieval. After washing, the slides were incubated with 0.3% hydrogen peroxide in PBS for 30 min at RT to quench endogenous peroxidase activity. Later, the sections were incubated overnight at 4°C with BTG2 antibody (1:200 diluted) (Santa Cruz Biotechnology, Inc, CA, USA) or with cyclin D1, p53, and cyclin E antibodies (each 1:100 diluted) (Santa Cruz Biotechnology, Inc, CA, USA) using DAKO EnVision system (DAKO Corp, Carpinteria, CA, USA) for detection. The negative control was processed by substituting PBS for the primary antibody, and the samples were microscopically examined. Immunohistochemical examination was performed using the following criteria on the basis of a comparison between cancer tissue and adjacent (normal) tissue [27]. The immunohistochemistry results using each antibody were classified as follows: similar intensity of staining in cancer tissue and normal bile duct epithelia or weaker staining intensity in <35% of cancer tissue was regarded as positive staining whereas weaker staining intensity in >30% of cancer tissue or no staining in cancer tissue was regarded as negative staining. Three researchers independently evaluated the samples and interpreted immunohistochemical results.
Statistical Analysis
All the data were processed using SPSS13.0 software. Chi-square (χ 2) test was performed to assess the significance of the association between expression of BTG2, p53, cyclin D1, cyclin E, and other tumor characteristics, e.g., tumor grade, size (≤5 or >5 cm), hepatitis B surface antigen (HBsAg), and metastasis. All P values <0.05 were considered as statistically significant.
Results
BTG2 Expression in Fetal Liver and Different Hepatoma Cell Lines
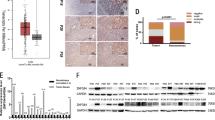
Expression of the BTG2 mRNA and protein was detected in fetal liver. As show in Fig. 1, there was no difference in the expression levels of BTG2 between 4- and 7-month-old tissues. Moreover, overexpression of the BTG2 mRNA and protein was observed in LO2 cells and hepatoma cell lines such as 7701 but not in HepG2 cells.
Expression of BTG2 mRNA and protein. Expression of the BTG2 mRNA was determined by a RT-PCR and b northern blot; while the protein expression was measured by c western blot, following protocols as described in “Materials and Methods” section. Both BTG2 mRNA and protein were overexpressed in fetal liver (4 and 7 months) as well as in cell lines (7701 and LO2); however, low expression was observed in HepG2 cells. GAPDH and β-actin were used as an endogenous control
Expression of BTG2, p53, Cyclin D1, and Cyclin E Proteins in DEN-Induced HCC
The expression of BTG2, p53, cyclin D1, and cyclin E proteins was determined by western blot. As shown in Fig. 2, the protein expression of BTG2 was found to be elevated that peaked at the 5th week of DEN treatment, then it gradually decreased and after 16 weeks, it was found to be even lower than that of untreated controls. Cyclin D1 protein was also found to be elevated at the 5th week of DEN treatment and the expression increased gradually with peak observed at the 16th week of the treatment. The DEN treatment also induced expression of cyclin E with peak expression observed at the 5th week whereas cyclin E expression was downregulated in untreated controls. Very low levels of p53 protein were detected in control livers; however, these levels increased dramatically and remained elevated until the 12th week of DEN treatment and decreased thereafter.
Expression of BTG2, p53, cyclin D1, and cyclin E proteins in DEN-induced rat HCC model at different stages. a The protein expression of BTG2, p53, cyclin D1, and cyclin E was measured by western blot at the stage of 5, 8, 12, and 16 weeks of DEN treatment for HCC induction. Each group comprised of 12 rats and the control group comprised of two rats. An increase in the BTG2 protein expression was observed at the early stage of DEN treatment that peaked at the 5th week and gradually decreased thereafter as observed at the subsequent time points of 8, 12, and 16 weeks, especially the expression was found to be very low at the 16th week. Also, an increase in the expression levels of cyclin D1 and cyclin E proteins was observed during hepatocarcinogenesis which was more evident at the 16th week of DEN treatment. A significant increase in the p53 expression was observed at the 5th week of DEN treatment as compared with that of untreated controls. The β-actin expression was used as an endogenous control. The representative blot from three independent experiments is shown. b The data (means ± SD), obtained from three independent experiments, represent relative band intensities of the proteins at different time points and were calculated as % expression relative to that of internal control. An asterisk (*) represents a statistically significant P value (<0.05) as compared with controls
Loss of BTG2 mRNA Expression in HCC TMA Correlates with Increasing Tumor Grade and Also with Increased Expression of p53, Cyclin D1, and Cyclin E Proteins
The positive BTG2 mRNA expression was detected, by ISH on TMA, in 71.19% (42/59) of HCC samples and in three of four normal liver tissue samples obtained from the region surrounding the morbid tissues obtained from HCC patients. BTG2 mRNA expression was predominantly localized in the cytoplasm of the tumor cells as indicated by hyacinthine staining of the cytoplasm (Fig. 3). The BTG2 mRNA expression correlated significantly with the degree of differentiation. The positivity rate of BTG2 mRNA expression in HCC was 93.75% (15/16) at high differentiation, 67.86% (19/28) at moderate differentiation, and 53.33% (8/15) at low differentiation. There were significant differences observed between high, moderate, and low rates of differentiation (Table 1).
BTG2 expression compared with other proteins in high-flux TMA/ISH. a Expression of the BTG2 mRNA in TMA (+++) (ISH ×40) is shown; b expression of the BTG2 protein in TMA (+++) (magnification, ×40) is shown; c the negative expression of BTG2 protein in TMA (−) (magnification, ×40) is shown; d the expression of BTG2, p53, cyclin D1, and cyclin E proteins in HCC is shown. A representative case of HCC displays loss of the BTG2 expression as compared with strong expression of p53, cyclin D1, and cyclin E proteins at the same location (magnification, ×400)
Since BTG2 expression is dependent on wt p53 function [7] and BTG2 suppresses the expression of cyclin D1 [11] and cyclin E [12], we analyzed whether the loss of BTG2 mRNA expression in HCC was related with the overexpression of cyclin D1, cyclin E, or the p53 mutant. We detected the expression of p53, cyclin D1, and cyclin E proteins in 17 patients who tested negative for BTG2 mRNA expression by immunohistochemistry. Cyclin D1 and p53 showed nuclear expression in the liver tumor cells. These proteins were also weakly expressed in the cytoplasm of these cells. Cyclin E, however, was expressed only in the nucleus of these cells (Fig. 3d). The p53, cyclin E, and cyclin D1 proteins were expressed in 82.35% (14 of 17), 76.47% (13 of 17), and 94.12% (16 of 17) HCC cases, respectively. All three proteins were coexpressed in 70.59% (12 of 17) HCC cases (Table 2).
Reduced BTG2 Protein Expression in HCC Correlates with the Overexpression of p53, Cyclin D1, and Cyclin E Proteins and the Tumor Grade
We evaluated BTG2 protein expression in HCC TMA samples by performing immunohistochemical analysis. Expression of the BTG2 protein was predominantly localized in the cytoplasm of the tumor cells with faint cytoplasmic staining of uninvolved epithelial cells of bile duct and part of liver cells (Fig. 3b). We also examined the BTG2 protein expression in ten normal liver tissue samples obtained from the region surrounding the HCC tissue which was found to be localized predominantly in the cytoplasm. BTG2 expression was absent in 32.2% (19 of 59) tumor samples. Expression of the BTG2 protein correlated significantly (P < 0.05; Table 2) with the degree of tumor differentiation. The positivity ratio of BTG2 protein expression in regard with various degrees of HCC differentiation was as follows: 87.5% (14/16) in high, 67.86% (19/28) in medium, and 46.67% (7/15) in low differentiation cases. There were significant differences observed between high, medium, and low differentiation.
To further evaluate whether the aberrant BTG2 protein expression in HCC correlated with the expression of p53, cyclin D1, and cyclin E and tumor characteristics, we examined the expression of these proteins in HCC TMA samples for which clinicopathologic and follow-up data were available. Of the 59 HCC tissue sections, 26 (44.07%) stained positively with p53 antibody and the positivity ratio in relation to degree of HCC differentiation was as follows: 31.25% (5/16) in high, 39.29% (11/28) in medium, and 66.67% (10/15) in low differentiation. Cyclin E protein was detected in 38.98% (23/59) HCC patients, and the positivity ratio regarding degree of tumor differentiation was as follows: 37.5% (6/16) in high, 32.14% (9/28) in medium, and 53.33% (8/15) in low differentiation. Positive staining for cyclin D1 protein was detected in 59.32% (35/59) HCC cases examined, and the positivity ratio with regard to degree of tumor differentiation was as follows: 31.25% (5/16) in high, 60.71% (17/28) in medium, and 86.67% (13/15) in low differentiation. Expression of cyclin D1 correlated significantly (P < 0. 05) with the tumor grade.
Discussion
Several lines of evidence suggest that BTG2 plays an important role in the suppression of carcinogenesis. Moreover, BTG2 expression was found to be undetectable in higher grade tumors and bone metastases [18]. Besides, our data show that BTG2 expression relates with the degree of tumor differentiation and hence correlates also with the clinical pathology of HCC (Table 1). These results imply that the abnormal BTG2 expression relates closely with the genesis and development of HCC. In this regard, it seems likely that HCC may be expressing certain factor(s) that facilitate the cytoplasmic retention and/or inhibit the nuclear localization of BTG2. Whether the loss of important protein(s) that regulate nuclear localization and/or the altered posttranslational modification of BTG2 are involved in the aberrant localization of BTG2 in HCC cells is still unclear and needs further studies.
It was recently reported that TIS21−/− mice show enhanced HCC development at the late stage of DEN-induced hepatocarcinogenesis [28]. Corroboratively, our results show inhibition of the BTG2 expression at the late stage of DEN-induced hepatocarcinogenesis in a rat model of HCC (Fig. 2a). The data from both studies thus support BTG2/TIS21 as tumor suppressors. Nonetheless, the possibility that BTG2 behaves as a tumor suppressor, though it remains speculative, is based on the rationale that BTG2 is involved in both tumor suppressor p53 and Rb pathways. Although the 1q32 region was shown to present with loss of heterozygosity in breast carcinomas with 25% frequency [17], it is still unclear whether the BTG2 gene undergoes mutations in tumors. We speculate that a possible mechanism of the loss of BTG2 expression in HCC may involve the BTG2 mutation such as tumor suppressor deletion. Although low levels of BTG2 expression have been reported in normal hepatocytes [20, 29], we previously reported [21–23] that BTG2 homologous gene PC3 was rapidly induced during hepatocyte proliferation as detected by RDA in the rat model of two-third hepatectomy and the peak expression was observed within 1–2 h after the surgery, suggesting that PC3/BTG2 gene is likely involved in the regulation of liver regeneration. The present data show that the expression of BTG2 is significantly increased at the early stage of DEN-induced hepatocarcinogenesis in the rat model of HCC which is also corroborated by the results of a recent study [28], further implying that the BTG2 expression is sensitive to acute hepatic injury.
In fact, BTG2 is intricately involved at G1 checkpoint of cell cycle as a p53-transactivated gene. We observed that in the rat model of DEN-induced hepatoma, p53 expression increased continuously during the early and middle stages of the induced cancer and decreased significantly at the advanced stages of the cancer. This pattern of p53 expression was similar to that observed in human tissues (Fig. 1a). Analysis of the array of HCC tissues indicated that there was a significant negative correlation between the expression of mutant p53 protein and BTG2. Further analysis of p53 protein in the HCC cases negative for BTG2 mRNA expression revealed that the p53 mutation was positively correlated with the deletion of BTG2 and an increase in the mutation of p53 gene resulted in a decrease in the expression of BTG2. This observation is consistent with the previous finding that activation of the BTG2 requires regulation through the wt p53-dependent pathway [8]. However, we detected the decreased BTG2 mRNA expression in HepG2 cells (expressing wt p53). The consistent staining pattern observed using anti-BTG2 antibody and the fact that p53 mutation expression was 44.07% in TMA of HCC suggest that the defects in the DNA damage-induced cytoprotective pathway in HCC may be independent of the p53 mutations. It has been recently reported that BTG2 is a potential target of cellular microRNAs (miRNAs) and BTG2 is regulated by miR-21 in human laryngeal carcinoma [30]. Interestingly, we already predicted using bioinformatics software that the BTG2 could possibly be a target gene of miR-18 whereas the expression of miR-18 in HepG2 cell line was reported as upregulated [31]. Arguably, it was suggested that the high expression of miR-18 in HepG2 cell line might be able to suppress expression of the BTG2 in these cells. Indeed, whether the overexpression of miRNAs that target posttranscriptional processing of BTG2 and/or protein–protein interactions with the cellular partners lead to aberrant regulation of BTG2 expression in HCC remains to be elucidated.
Using a modified rat model of DEN-induced primary HCC, we also observed that the expression levels of cyclin D1 and cyclin E proteins gradually increased during hepatocarcinogenesis. This suggests that the loss of BTG2 and the gain of cyclin D1 and cyclin E expression in HCC are likely to be the related events; and also that BTG2 may suppress the expression of cyclin D1 and/or cyclin E to inhibit the proliferation of HCC tumor cells. It is, however, unclear as to which pathway(s) is the key player in inducing this type of antiproliferative response. The BTG2-encoding protein is a secreted protein with a secreted peptide. It is closely related with cancer-inhibiting genes such as p53 and Rb. The dynamic pattern of BTG2 expression in HCC and its early loss in the tumorigenic process suggest that BTG2 expression is restricted in space and time to certain areas, and it may play an important role in the transformation and/or differentiation of hepatocytes. The interaction of BTG2 with the cell cycle proteins and its physical association with the p53 mutations make it an ideal candidate for restraining tumorigenesis. In global perspectives, it remains to be seen whether the coordinated inhibition of BTG2 and enhancement of cyclin D1 and/or cyclin E expression can increase the mutation frequency of wt p53 in hepatocarcinoma cells and thus serve as a molecular signature to identify patients at risk of developing hepatoma. In any case, further studies on the relationship between BTG2 expression and hepatoma formation will help in understanding the molecular mechanisms involved at the levels of cellular proliferation and differentiation that eventually lead to hepatoma development.
References
Sukhatme, V. P., Kartha, S., Toback, F. G., Taub, R., Hoover, R. G., & Tsai-Morris, C. H. (1987). A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Research, 1, 343–355.
Tirone, F. (2001). The gene PC3TIS21/BTG2, prototype member of the PC3/BTG/TOB family: Regulator in control of cell growth, differentiation, and DNA repair? Journal of Cellular Physiology, 187, 155–165.
Guehenneux, F., Duret, L., Callanan, M. B., Bouhas, R., Hayette, S., Berthet, C., et al. (1997). Cloning of the mouse BTG3 gene and definition of a new gene family (the BTG family) involved in the negative control of the cell cycle. Leukemia, 11, 370–375.
Bradbury, A., Possenti, R., Shooter, E. M., & Tirone, F. (1991). Molecular cloning of PC3, a putatively secreted protein whose mRNA is induced by nerve growth factor and depolarization. Proceedings of the National Academy of Sciences of the United States of America, 88, 3353–3357.
Fletcher, B. S., Lim, R. W., Varnum, B. C., Kujubu, D. A., Koski, R. A., & Herschman, H. R. (1991). Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. Journal of Biological Chemistry, 266, 14511–14518.
Duriez, C., Falette, N., Audoynaud, C., Moyret-Lalle, C., Bensaad, K., Courtois, S., et al. (2002). The human BTG2/TIS21/PC3 gene: Genomic structure, transcriptional regulation and evaluation as a candidate tumor suppressor gene. Gene, 282, 207–214.
Rouault, J. P., Falette, N., Guéhenneux, F., Guillot, C., Rimokh, R., Wang, Q., et al. (1996). Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nature Genetics, 14, 482–486.
Puisieux, A., & Magaud, J. P. (1999). Mechanisms of BTG2 activity, a transcriptional target of p53: Evidences and hypothesis. Bull Cancer, 86, 358–364.
DelSal, G., Loda, M., & Pagano, M. (1996). Cell cycle and cancer: Critical events at the G1-restriction point. Critical Reviews in Oncogenesis, 7, 127–142.
Sherr, C. J. (1996). Cancer cell cycles. Science, 274, 1672–1677.
Guardavaccaro, D., Corrente, G., Covone, F., Micheli, L., D’Agnano, I., Starace, G., et al. (2000). Arrest of G1-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Molecular and Cellular Biology, 20, 1797–1815.
Lim, I. K., Lee, M. S., Ryu, M. S., Park, T. J., Fujiki, H., Eguchi, H., et al. (1998). Overexpression of TIS21 in 293 cells induces growth inhibition by down-regulating the cyclin E and cyclin-dependent kinase 4 proteins. Molecular Carcinogenesis, 23, 25–35.
Lin, W. J., Gary, J. D., Yang, M. C., Clarke, S., & Herschman, H. R. (1996). The mammalian immediate early TIS21 protein and the leukemia associated BTG1 protein interact with a protein-arginine N-methyltransferase. Journal of Biological Chemistry, 271, 15034–15044.
Rouault, J. P., Prevot, D., Berthet, C., Birot, A. M., Billaud, M., Magaud, J. P., et al. (1998). Interaction of BTG1 and p53-regulated BTG2 gene products with mCaf1, the murine homolog of a component of the yeast CCR4 transcriptional regulatory complex. Journal of Biological Chemistry, 273, 22563–22569.
Prevot, D., Voeltzel, T., Birot, A. M., Morel, A. P., Rostan, M. C., Magaud, J. P., et al. (2000). The leukemia-associated protein Btg1 and the p53-regulated protein BTG2 interact with the homeoprotein Hoxb9 and enhance its transcriptional activation. Journal of Biological Chemistry, 275, 147–153.
Kawakubo, H., Carey, J. L., Brachtel, E., Gupta, V., Green, J. E., Walden, P. D., et al. (2004). Expression of the NF-kappaB-responsive gene BTG2 is aberrantly regulated in breast cancer. Oncogene, 23, 8310–8319.
Kawakubo, H., Carey, J. L., Brachtel, E., Hayashida, T., Yeo, G., Kish, J., et al. (2006). Loss of B-cell translocation gene-2 in estrogen receptor positive breast carcinoma is associated with tumor grade and overexpression of cyclin D1 protein. Cancer Research, 66, 7075–7082.
Ficazzola, M. A., Fraiman, M., Gitlin, J., Woo, K., Melamed, J., Rubin, M. A., et al. (2001). Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis, 22, 1271–1279.
Struckmann, K., Schraml, P., Simon, R., Elmenhorst, K., Mirlacher, M., Kononen, J., et al. (2004). Impaired expression of the cell cycle regulator BTG2 is common in clear cell renal cell carcinoma. Cancer Research, 64, 1632–1638.
Melamed, J., Kernizan, S., & Walden, P. D. (2002). Expression of B-cell translocation gene 2 protein in normal human tissues. Tissue and Cell, 34, 28–32.
Xu, W. X., Wang, S. Y., Wang, G., Wei, H., He, F., & Yang, X. (2000). Identification and characterization of differentially expressed genes in the early response phase during liver regeneration. Biochemical and Biophysical Research Communications, 278, 318–325.
Zhang, Z. M., Wang, G., Chen, C., Yang, Z. X., Jin, F., San, J. L., et al. (2009). Rapid induction of PC3/BTG2 gene by hepatopoietin or partial hepatectomy and its mRNA expression in hepatocellular carcinoma. Hepatobiliary and Pancreatic Diseases International, 8, 288–293.
Ge, W., En-ren, L., Lu, H., Wang, J., Leng, E. R., & Fang, D. C. (2002). Rapid Induction of mRNAs for PC3 genes by hepatopoietin and partial hepatectomy. Chinese Journal of Hepatology, 10, 256–259.
Sundarajan, M., Gupta, S., & Rao, K. V. K. (2002). Overexpression of cyclin D1 is associated with the decondensation of chromatin during DEN-induced hepatocarcinogenesis. Cell Biology International, 26, 699–706.
Fukumasu, H., Avanzo, J. L., Heidor, R., Silva, T. C., Atroch, A., Moreno, F. S., et al. (2006). Protective effects of guarana (Paullinia cupana Mart. var. sorbilis) against DEN-induced DNA damage on mouse liver. Food and Chemical Toxicology, 44, 862–867.
Kallioniemi, O. P., Wagner, U., Kononen, J., & Sauter, G. (2001). Tissue microarray technology for high-throughput molecular profiling of cancer. Human Molecular Genetics, 10, 657–662.
Onuma, K., Dabbs, D. J., & Bhargava, R. (2008). Mammaglobin expression in the female genital tract: Immunohistochemical analysis in benign and neoplastic endocervix and endometrium. International Journal of Gynecological Pathology, 27, 418–425.
Park, T. J., Kim, J. Y., Paul Oh, S., Kang, S. Y., Kim, B. W., Wang, H. J., et al. (2008). TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology, 47, 1533–1543.
Matsuda, S., Rouault, J., Magaud, J., & Berthet, C. (2001). In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Letters, 497, 67–72.
Liu, M., Wu, H., Liu, T., Li, Y., Wang, F., Wan, H., et al. (2009). Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Research, 19, 828–837.
LI, Q., Wang, G., & Zhang, Z. M. (2009). The relationship between microRNA-18 and BTG2 in the carcinogenesis of hepatocellular carcinoma [Article in Chinese]. Zhonghua Gan Zang Bing Za Zhi, 17, 42–45.
Acknowledgments
We thank the Chinese National Natural Science Foundation (Grants #30973457, #30901764) and the Academic Foundation for Authors of National Excellent Doctoral Dissertation of China (Award # 200261) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhimin Zhang and Chuan Chen contributed equally to this study as co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, Z., Chen, C., Wang, G. et al. Aberrant Expression of the p53-Inducible Antiproliferative Gene BTG2 in Hepatocellular Carcinoma is Associated with Overexpression of the Cell Cycle-Related Proteins. Cell Biochem Biophys 61, 83–91 (2011). https://doi.org/10.1007/s12013-011-9164-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9164-x