Abstract
Enterococcus hirae grow well under anaerobic conditions at alkaline pH (pH 8.0) producing acids by glucose fermentation. Bacterial growth was shown to be accompanied by decrease of redox potential from positive values (~+35 mV) to negative ones (~−220 mV). An oxidizer copper (II) ions (Cu2+) affected bacterial growth in a concentration-dependent manner (within the range of 0.05 mM to 1 mM) increasing lag phase duration and decreasing specific growth rate. These effects were observed with the wild-type strain ATCC9790 and the atpD mutant strain MS116 (with absent β subunit of F1 of the FoF1 ATPase) both. Also ATPase activity and proton–potassium ions exchange were assessed with and without N,N′-dicyclohexylcarbodiimide (DCCD), inhibitor of the FoF1 ATPase. In both cases (DCCD ±), even low Cu2+ concentrations had noticeable effect on ATPase activity, but with less visible concentration-dependent manner. Changes in the number of accessible SH-groups were observed with E. hirae ATCC9790 and MS116 membrane vesicles. In both strains Cu2+ markedly decreased the number of SH-groups in the presence of K+ ions. The addition of ATP increased the amount of accessible SH-groups in ATCC9790 and decreased this number in MS116; Cu2+ blocked ATP-installed increase in SH-groups number in ATCC9790. H+–K+-exchange of bacteria was markedly inhibited by Cu2+, but stronger effects were detected together with DCCD. Moreover, discrimination between Cu2+ and other bivalent cation—Ni2+ was shown. It is suggested that Cu2+ ions inhibit E. hirae cell growth by direct affect on the FoF1 ATPase leading to conformational changes in this protein complex and decrease in its activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enterococcus hirae grow well under anaerobic conditions which is accompanied by acidification of the medium and changes in environment redox potential (Eh). It is known that positive values of Eh inhibit the anaerobic bacteria growth (for reviews, see [10, 38]) while Eh negative values are required for bacterial growth [4, 7, 22]. The latter can be inhibited by oxidizers, which maintain Eh on positive level [4, 15], and stimulated by reducers, which decrease Eh to negative values [14]. Moreover, Eh affects proton-motive force by changing pH gradient across the membrane [31].
Low concentrations of oxidizing Cu2+ ions are required for Escherichia coli and the other bacteria (for reviews, see [11, 29, 32]) whereas in considerably higher concentrations they can cause a number of toxic cellular effects inhibiting E. coli cell growth [15, 39], which can be explained by alteration of H+ flux through the FoF1 ATPase as installed by Kirakosyan and Trchounian [15] and inhibition of hydrogenase activity associated with the FoF1 ATPase as determined by Kirakosyan et al. [16], by increase in surface charge density as shown by Volodina et al. [39] as well as by changes in membrane permeability as suggested for this bacterium by Lebedev et al. [18]. The change in H+-permeability and the other properties of bacterial membrane may be related to membrane proteins re-organization or changed functional activity, and that might depend on proteins thiol groups’ state and distribution. In accordance with these ideas, it is suggested that Cu2+ can break disulfides in membrane proteins [18], increasing the number of accessible SH-groups [16]. However, a role of E h in bacterial growth, effects of oxidizers and reducers on bacterial growth would be clarified; the appropriate mechanisms for Cu2+ uptake and intracellular handling are still not clear.
Kirakosyan et al. [16] have shown that in E. coli membrane vesicles, prepared from anaerobically grown cells at slightly alkaline pH, Cu2+ ions alone increase the level of SH-groups but block ATP-stimulated increase of these groups when added together with ATP. The obtained effects might be the result of Cu2+ action on E h or a direct effect of these ions on proteins in bacterial membrane, probably on the FoF1 ATPase. It is suggested that the energy of ATP can be transferred through a dithiol–disulfide interchange between the FoF1 ATPase and the other membrane proteins [5, 16], which are forming the protein–protein associations within the membrane [6, 22, 36]. And the marked increase in the number of FoF1 ATPase SH-groups by ATP but not by ADP has been shown with membrane vesicles [23]. Furthermore, Cu2+ can break these interactions thus increasing the level of accessible SH-groups. The latter is proposed to be another way which also depends on E h. In our laboratory it has been established [15] that the addition of 0.1 and 2 mM Cu2+ into the E. coli growth medium results in a delayed decrease of E h although a drop in E h is less for 2 mM than for 0.1 mM of these ions. All these findings can be taken into consideration to explain the action mechanisms of oxidizers effects on E. coli growth.
A closed relationship of the FoF1 ATPase with other membrane proteins is also assumed in case of E. hirae [37]. This idea results from two findings at least: H+–K+-exchange with the fixed stoichiometry through the FoF1 ATPase and via the K+ uptake system [24, 37] and N,N′-dicyclohexylcarbodiimide (DCCD)-inhibited ATPase activity depended on K+ ions [37]. Moreover, the marked increase in the number of FoF1 ATPase SH-groups by ATP and nicotinamide adenine dinucleotides has been determined with E. hirae membrane vesicles [27]. However, mechanisms of such relationship and thiol groups’ role in the E. hirae FoF1 ATPase activity remain unclear. At present there are no data on Cu2+ effects with E. hirae.
Our present data show that Cu2+ affect E. hirae cell growth by increasing lag phase duration and decreasing specific growth rate. These ions have noticeable effect on H+–K+-exchange and ATPase activity and lower the number of accessible SH-groups in a concentration-dependent manner; discrimination between Cu2+ and Ni2+ is shown. The results with data obtained by using the E. hirae atpD mutant strain MS116 with defective FoF1 ATPase might indicate direct effects of Cu2+ on this ATPase.
Materials and Methods
Bacterial Strains and Growth, Whole Cells, Membrane Vesicles, Redox Potential
The wild-type strain E. hirae ATCC9790 [17] and the atpD mutant strain MS116 (having absent β subunit of F1 of the FoF1 ATPase) [3, 37] were used in this study. MS116 strain expresses the FoF1 ATPase to the level as wild-type one [3], but it has significantly lowered H+ efflux [2] and ATPase activity [3, 37]. The strains were kindly supplied by Prof. H. Kobayashi (Department of Biochemistry, Chiba University, Chiba 263, Japan).
Bacteria were grown under anaerobic conditions at 37°C in a 0.2% glucose containing growth medium (1% tryptone, 0.5% yeast extract, 1% K2HPO4; pH 8.0) as described earlier [2, 24, 26, 27, 37]. The medium pH was measured by a pH-meter with selective pH-electrode (HJ1131B, Hanna Instruments, Portugal) and adjusted by means of 0.1 M NaOH or HCl. Bacterial growth was assessed by measuring optical density (OD) changes in bacterial suspension using a Spectro UV–vis Auto spectrophotometer (Labomed, USA) at wavelength of 600 nm. To study the effects of Cu2+, 0.05 mM, 0.1 mM, or 1 mM CuCl2 were added in bacterial suspension, when mentioned. The latent (lag) growth phase duration was determined as described before [14]. The specific growth rate was calculated over the interval, where the logarithm of OD for the culture increased linearly with time, and it was expressed as lg2 (lg2 = 0.693)/doubling time.
Whole cells [2, 15, 25] were prepared and right-side-out membrane vesicles [16, 27, 37] were isolated as described except that the buffers lacked K+.
Eh was measured by a platinum (Pt) (EPB-1, GSEEE, or PT42BNC, Hanna Instruments, Portugal) or titanium-silicate (Ti–Si) (EO-02, Electrometer Equipment State Enterprise, Gomel, Belarus) electrode as described elsewhere [4–7, 14, 16, 22]. When the Pt-electrode was used together with a Ti–Si-electrode, which, unlike the former, is insensitive to molecular oxygen and molecular hydrogen, no significant differences in electrode readings were detected.
It was detected that E h value in the conditions used was changed on 35–40 mV only by ~8–10-fold alteration of bacterial count. It was also determined that E h value was not changed more than on 25–30 mV by ~sixfold change of Cu2+ concentration within the concentration range used. So the significant decrease of E h during bacterial growth (see “Results and Discussion” section) does not depend on either bacterial count or Cu2+ ions count change.
Accessible SH-Groups and ATPase Assays
Accessible SH-groups of membrane vesicles were determined by Ellmann’s reagent (5,5′-dithiobis-2-nitrobenzoic acid) [30] using spectroscopic method as described [16, 23, 27] and glutathione as a standard. Membrane vesicles were treated with the reagent and OD was measured after ~1.5–2 h (OD became constant), corrections were made for blanks without membrane vesicles and with different reagents used. The level of SH-groups was expressed in nmol per mg protein. Using this reagent gave the same data for the number of SH-groups as the other reagents as shown [16, 23, 28].
ATPase activity was measured by amount of inorganic phosphate (Pi) liberated after adding 5 mM ATP (Tris salt) [22, 28]. Pi was determined by the spectrophotometric method of Taussky and Shorr [35], corrections were made for blanks without ATP or membrane vesicles. ATPase activity was expressed in nmol Pi per μg protein in 1 min.
The assay mixture was of 200 mM Tris-phosphate (pH 8.0) containing 0.4 mM MgSO4, 1 mM NaCl and 1 mM KCl; for ATPase activity determination 50 mM Tris–HCl (pH 8.0), containing 0.4 mM MgSO4 with or without 100 mM KCl was used. When used, membrane vesicles were pre-incubated with Cu2+ or DCCD for 10 min.
Proton and Potassium Ions Transport Study
H+ and K+ fluxes through the bacterial membrane in whole cells were measured using appropriate selective electrodes (HJ1131B, Hanna Instruments, Portugal; and PVC membrane type, Cole Parmer Instruments Co., USA, or E-031, “Niko Analit” Sci.-Prod. Co., Russia) as described elsewhere [7, 15, 22, 26]. Electrode readings were calibrated by titration of the assay medium (200 mM Tris-phosphate buffer (pH 8.0) containing 0.4 mM MgSO4, 1 mM NaCl and 1 mM KCl) with 0.01 N HCl and 0.02 mM KCl. Ions transport was determined after addition of glucose into the assay medium. Ion fluxes are expressed as the change in external activity of the ion in mM/min per number of cells in a unit of medium volume (ml). For DCCD inhibition studies, cells were treated with this reagent at 0.1 mM for 10 min prior assays; during treatment with DCCD bacterial count was not changed. Note, Cu2+ ions had no effects on proton and potassium electrode readings.
Other and Chemicals
Bacterial count was determined by counting colony-forming units grown on solid media with glucose (after plating of diluted bacterial suspension). Protein was measured by the method of Lowry et al. [20] using bovine serum albumin as a standard. All assays were routinely carried out under anaerobic conditions and all measurements were done at 37°C. At least three independent measurements were made; standard errors were not more than 3% if not represented. The Student’s validity criteria (p) was calculated to show statistically reliable difference between changed values and control [16].
Trypton, yeast extract were from Roth (Germany), ATP, DCCD, Ellmann’s reagent, glucose and glutathione were from Sigma (USA) and other reagents of analytical grade were used in experiments. For metal ions appropriate chloride salts were used.
Results and Discussion
Bacterial Growth and Redox Potential in the Presence of Copper (II) Ions
The wild-type strain E. hirae ATCC9790 and the atpD mutant strain MS116 with defective FoF1 ATPase are known to grow well under anaerobic conditions at pH 8.0 [2, 3, 26, 37].
The addition of Cu2+ into the bacterial growth medium resulted in an increased lag phase duration and decreased specific growth rate (Fig. 1). With low concentrations of Cu2+ (0.05 mM) no statistically reliable bacterial growth differences were observed (in comparison with control samples, P > 0.05). In contrast, a higher concentration of Cu2+ (0.1 mM and 1 mM) notably prolonged lag phase duration and significantly decreased the specific growth rate (P < 0.05).
Effects of Cu2+ ions in different concentrations on the E. hirae ATCC9790 and MS116 cell growth. a Lag phase duration, b specific growth rate. CuCl2 of 0.05 mM to 1 mM was added (if specified, see x-axis) to the growth medium before inoculation of bacteria; control was bacterial growth in the growth medium without CuCl2 added. For the others, see “Materials and Methods” section
The influence of Cu2+ on MS116 cell growth was less noticeable than that on ATCC9790 (see Fig. 1). The lag phase duration with this atp mutant strain is ~4.5-fold higher than that with wild-type strain but specific growth rate are almost the same (see Fig. 1). These findings point out that the FoF1 ATPase is not essential for E. hirae growth at alkaline pH. This contradicts with a common idea that the FoF1 ATPase of bacteria is a main membrane enzyme of bioenergetic meaning that is responsible for generation of H+-motive force under anaerobic conditions (for review, see [36]). However, this seems to be in favor with data of Kobayashi with co-workers [17, 24] that E. hirae can grow at alkaline pH in the presence of a protonophore which almost completely dissipates the H+-motive force. However, the requirement of H+-motive force for E. hirae growth and mechanisms of its generation at alkaline pH if any are not clear yet. In addition, the obtained results with Cu2+ ions are in accordance with those of Kirakosyan et al. [16] reported for E. coli.
Furthermore, the suppression of E. hirae growth at alkaline pH can be resulted by Cu2+ direct effect on bacterial membrane or on E h.
During E. hirae ATCC9790 growth E h measured with Pt-electrode drops from positive values (35 ± 10 mV) to negative ones (−220 ± 10 mV) (Fig. 2a). In the case of MS116 the initial E h value is 5 ± 10 mV which drops to negative values (−170 ± 10 mV) as the culture passes to the stationary growth phase (Fig. 2b). This drop of E h indicates that there are many reduction processes taking place at anaerobic growth [14, 38]. At the stationary phase, after 24 h of growth, E h increases, but does not reach the initial values (not shown).
Changes in redox potential during E. hirae ATCC9790 growth in the presence of Cu2+ (a) and Cu+ ions (c), and MS116 growth in the presence of Cu2+ ions (b). Redox potential was measured with a Pt-electrode. For details, see the legends in Fig. 1 and “Materials and Methods” section
Changes in E h during E. hirae growth were also observed in the presence of Cu2+. These ions were shown to affect E h values changes in a concentration-dependent manner (Fig. 2). The effects of Cu2+ were more expressed in case of wild-type strain E. hirae ATCC9790 if comparing with MS116 (Fig. 2a, b). To have clear data with Cu2+, we have detected that copper (I) ions had a little bit less noticeable effect on E h change (see Fig. 2c). This proves that Cu2+ more than Cu+ ions have significant action on E h changes during E. hirae growth, although Cu+ rather than Cu2+ could be accumulated in spite of some balance between influx and efflux systems for these ions [32]. These results may be related to changes in the number of SH-groups which depends on E h and redox-regulated H+-translocating ATPase activity [8, 38]. Besides, changes in E h decrease upon bacterial growth are suggested could be related with changes in bacterial growth [14, 31].
Effects of Cu2+ on Accessible SH-Groups Number, Its Changes, and ATPase Activity
Accessible SH-groups were determined in membrane vesicles from E. hirae ATCC9790 and MS116 both. As it is supposed there is an energy transduction within the bacterial membrane between the FoF1 ATPase and secondary transport systems [16, 36, 37], and if the accessible SH-groups in E. hirae membrane vesicles are the FoF1 ATPase cysteine residues [27], their number can be affected by ATP. In fact, the E. hirae FoF1 ATPase is a typical FoF1 ATPase but its gene structure is not identical to that of other bacterial FoF1 ATPases [33], and there are no data about cysteine residues localization in the subunits of E. hirae FoF1 ATPase. However, the findings with E. coli that there are two cysteine residues in the b subunit of Fo sector of the FoF1 ATPase, which are probably accessible to ATP and are responsible for an appropriate changes in SH-groups number by ATP [23] may be employed in the study with E. hirae. Indeed, in this study, ATP (3 mM) was shown to markedly (~1.3-fold) increase the number of accessible SH-groups in E. hirae ATCC9790 membrane vesicles in the presence of 100 mM K+ (P < 0.05) (Fig. 3.). Such effect was not detected in MS116 membrane vesicles (Fig. 3). These results confirm data of Poladyan and Trchounian [27] that the increase in the number of SH-groups by ATP might be associated with the FoF1 ATPase; some effect of ATP itself is not ruled out.
Effects of Cu2+ and ATP on the number of accessible SH-groups in E. hirae ATCC9790 and MS116 membrane vesicles. The assay medium contains 100 mM K+, 3 mM ATP and/or 0.05 mM to 1 mM of CuCl2 were added into the assay medium when specified. The levels of SH-groups in these strains were 1; relative changes by Cu2+ and ATP were calculated. For details, see “Materials and Methods” section
Note, this bacterium has the other—Na+-transporting ATPase belonging to the vacuolar (V)-type ATPase [25], and both genes for F- and V-type ATPases are functionally expressed in one bacterial cell [34]; however it is likely that the FoF1 ATPase is mainly responsible for the changes in the number of SH-groups (see Fig. 3) which are accessible in our growth and assay conditions.
We have determined the effect of Cu2+ on SH-groups number in ATCC9790 and MS116 membrane vesicles both. The addition of Cu2+ decreased the number of SH-groups in a concentration-dependent manner which was less at a higher concentration (Fig. 3). In addition, Cu2+ inhibited the effect of ATP in E. hirae ATCC9790 membrane vesicles by blocking the ATP-stimulated increase in the number of SH-groups (see Fig. 3). We have shown that only when ATP was added there was a marked difference between SH-groups numbers in ATCC9790 and MS116 (see Fig. 3). The addition of elevated concentrations of Cu2+ had no significant effect on SH-groups number difference independent on ATP (see Fig. 3). We can assume that the FoF1 ATPase has a major role in these processes especially at a low Cu2+ concentration. These ions might cause some conformational change in the FoF1 ATPase disturbing its activity. The results obtained seems to be in favor with an idea that oxidizing Cu2+ might protect the activity of membrane enzymes from inactivation by SH-reagents presumably by forming a disulfide [19]; decrease in SH-groups level was observed. The breakage of disulfides in membrane proteins when Cu2+ ions are reduced on cell surface [18] is less probable with E. hirae. Moreover, the results are in agreement with data reported earlier by Kirakosyan et al. [14] who showed that in the case of E. coli the addition of ATP increase the number of SH-groups, but when ATP is added together with Cu2+ the latter block the ATP-stimulated increase in these groups number as in the case of E. hirae ATCC9790.
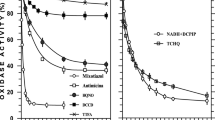
Furthermore, we can suggest that Cu2+ might affect directly the FoF1 ATPase. The action of Cu2+ on ATPase activity of E. hirae membrane vesicles was assessed with and without DCCD (0.1 and 0.2 mM), inhibitor of the FoF1 ATPase [1, 27, 36], in the presence of 100 mM K+. Abrams and Baron [1] had shown many years ago that when a sample of 0.25 mg dry weight of enterococcal membranes (membrane preparations were obtained by osmotic lysis of E. hirae protoplasts) were incubated with 0.2 mM DCCD for 15 min, the inhibition of membrane ATPase was 89%. This was the maximum inhibition that can be obtained since some of the membrane ATPase is resistant to inhibition by DCCD (Fig. 4) and, moreover, the other ATPase different from the FoF1 ATPase might express its activity [34]. In our experiments even with 0.2 mM DCCD used, the inhibition of ATPase activity on ~60% was detected (see Fig. 4). This difference might be due to different growth and assay conditions, the FoF1 ATPase level, DCCD preparations and different treatment with DCCD applied. In all cases (DCCD ±) even low Cu2+ concentrations had noticeable effect on ATPase activity, but with less visible concentration-dependent manner. Thus, the effect of Cu2+ on the number of SH-groups and the FoF1 ATPase activity can be explained by destruction of dithiol–disulfide interchange in membrane proteins, especially with the FoF1 ATPase.
ATPase activity of membrane vesicles of E. hirae ATCC9790 in K+ containing medium. 0.1 and 0.2 mM DCCD was present in the assay medium when indicated. For details, see “Materials and Methods” section
Effect of Cu2+ on Proton-Coupled Transport
When grown in a glucose-containing medium, E. hirae excrete protons and accumulate potassium ions and such H+–K+-exchange has been studied earlier [26]. The FoF1 ATPase associated with potassium uptake by TrkA-like system namely KtrI [13] is suggested to be responsible for such an exchange in bacteria [36, 37].
We have shown that DCCD and Cu2+ affected each of these ions transport markedly decreasing the fluxes of these ions (Table 1). Moreover, stronger effects were observed when these substances were present together in the medium. The influence of Cu2+ on ion fluxes had a concentration-dependent manner (see Table 1). The effect of Cu2+ on H+-coupled transport could be explained by a direct effect of Cu2+ on FoF1 ATPase those might cause some conformational changes in FoF1 destroying dithiol–disulfide interchange between the FoF1 ATPase and TrkA-like (KtrI) system. Direct action of Cu2+ on the latter system is less probable.
Discrimination Between Cu2+ and Ni2
If Cu2+ ions have specific effect on bacterial cells, especially on the number of SH-groups and ATPase activity, there must be discrimination between Cu2+ and the other bivalent cations, for instance, nickel (II) ions (Ni2+). The latter is required for all organisms as an enzyme cofactor, and nickel-containing enzymes can account for several percent of the cellular proteins in bacteria such as E. coli [12, 21]. Free Ni2+ can be toxic to cells by binding nonspecifically to biological macromolecules or by displacing other metals from their native binding sites. It is also known that Ni2+ can be absorbed on E. hirae cell walls [9]. To study effects of Ni2+ ions, NiCl2 (0.05, 0.1, and 1 mM) was added in ATCC9790 wild-type strain bacterial suspension. It was established that Ni2+ ions did not cause so significant changes in the number of SH-groups of E. hirae membrane vesicles if compared with Cu2+: the decrease in SH-groups number by 0.05 mM Ni2+ was ~fourfold lower than that with the same concentration of Cu2+ (Fig. 5a). Furthermore, Ni2+ ions did not also affect ATPase activity: if even low Cu2+ ions concentrations (0.05 mM CuCl2) had noticeable effect on ATPase activity (P < 0.01), much higher Ni2+ concentrations (1 mM NiCl2) did not have marked influence on ATPase activity (Fig. 5b).
Changes in the number of SH-groups (a) and ATPase activity (b) of membrane vesicles of E. hirae ATCC9790 in K+ containing medium in the presence of Ni2+ ions. NiCl2 was added into the assay medium when indicated. For details, see the legends in Figs. 3 and 4, and “Materials and Methods” section
Thus, the effect of Cu2+ is specific among bivalent metal ions, not for one protein, as we have shown that the same concentration of Ni2+ ions has lower effect on SH-groups number but has no marked effect on ATPase activity (comp. data on Fig. 5).
Conclusions
In this study, Cu2+ ions as oxidizers are shown to inhibit E. hirae cell growth at alkaline pH affecting lag phase duration and decreasing specific growth rate. These effects have a concentration-dependent manner, those are less visible in atpD mutant MS116 (absence of the β subunit of F1) and are suggested to be intermediated through E h (see Fig. 2).
In E. hirae ATCC9790 wild-type strain membrane vesicles from anaerobically grown cells, ATP increases the level of accessible SH-groups (see Fig. 3) confirming data reported previously [27]. The addition of Cu2+ ions decreases the number of SH-groups in a concentration-dependent manner and these ions block the ATP-stimulated increase in the number of these groups (see Fig. 3). The obtained effects on ATPase activity and SH-groups number are specific for Cu2+ (see Fig. 5). These results novel for E. hirae are in accordance with those obtained with E. coli [14] except opposite effects on the number of SH-groups. Therefore, it is most likely to propose action mechanisms for E. hirae.
Such Cu2+ ions influence may be resulted by action of these ions on E h (see Fig. 2a) or by their direct effect on membrane proteins. Cu2+ ions are defined to affect directly the FoF1 ATPase modulating its activity (see Fig. 4) and to change H+-efflux and H+-coupled transport (see Table 1). Cu2+ is suggested can cause some conformational changes in the FoF1 ATPase. Revealing action mechanisms with E. hirae, in contrast to E. coli, oxidizing Cu2+ might participate in formation a disulfide [19], changing interaction of the FoF1 ATPase with Trk-like (KtrI) system [26, 36, 37]; decrease in SH-groups level is observed (see Figs. 3, 5). The breakage of disulfides in membrane proteins when Cu2+ ions are reduced on cell surface [18] is less probable.
So Cu2+ might affect SH-groups of the FoF1 ATPase in E. hirae through disturbing a disulfide–dithiol interchange between this ATPase and the other membrane proteins that is installed by ATP and changing activity of this ATPase.
The effects of oxidizing Cu2+ ions on E. hirae cell growth and activity of the FoF1 ATPase have a significance to regulate bacteria in environment and their application in biotechnology.
References
Abrams, A., & Baron, C. (1970). Inhibitory action of carbodiimide on bacterial membrane ATPase. Biochemical and Biophysical Research Communications, 41, 858–861.
Akopyan, K., & Trchounian, A. (2005). Membrane proton conductivity and energy-dependent proton fluxes in Enterococus hirae in media with different pH. Biophysics (Moscow), 50, 595–598.
Arikado, E., Ishihara, H., Ehara, T., Shibata, C., Saito, H., Kakegawa, T., et al. (1999). Enzyme level of enterococcal FoF1-ATPase is regulated by pH at the step of assembly. European Journal of Biochemistry, 259, 262–268.
Bagramyan, K., Galstyan, A., & Trchounian, A. (2000). Redox potential is a determinant in the Escherichia coli anaerobic growth and survival: Effects of impermeable oxidant. Bioelectrochemistry, 51, 151–156.
Bagramyan, K. A., & Martirosov, S. M. (1989). Formation of an ion transport supercomplex in Escherichia coli. An experimental model of direct transduction of energy. FEBS Letters, 246, 149–152.
Bagramyan, K., Mnatsakanyan, N., Poladyan, A., Vassilian, A., & Trchounian, A. (2002). The role of hydrogenases 3 and 4, and the FoF1-ATP synthase in H2 production by Escherichia coli at alkaline pH. FEBS Letters, 516, 172–178.
Bagramyan, K. A., & Trchounian, A. A. (1997). Decrease of redox potential in the anaerobic growing Escherichia coli suspension and proton-potassium exchange. Bioelectrochemistry and Bioenergetics, 43, 129–134.
Bald, D., Noji, H., Yoshida, M., Hirono-Hara, Y., & Hisabori, T. (2001). Redox regulation of the rotation of F1-ATP synthase. Journal of Biological Chemistry, 276, 39505–39507.
Bossrez, S., Remacle, J., & Coyette, J. (1999). Adsorption of nickel on Enterococcus hirae cell walls. Journal of Chemical Technology and Biotechnology, 70, 45–50.
Breznak, J. A., & Costilow, R. H. (1994). Physicochemical factors in growth. In P. Gerhardt, R. G. Nurrey, W. A. Wood, & N. R. Krieg (Eds.), Methods for general and molecular bacteriology (pp. 137–155). Washington, DC: ASM Press.
Cooksey, D. A. (1993). Copper uptake and resistance in bacteria. Molecular Microbiology, 7, 1–5.
Ermler, U., Grabarse, W., Shima, S., Goubeaud, M., & Thauer, R. K. (1998). Active sites of transition-metal enzymes with a focus on nickel. Current Opinion on Structural Biology, 8, 749–758.
Kawano, M., Igarashi, K., & Kakinuma, Y. (2002). Isolation of Enterococcus hirae mutant deficient in low-affinity potassium uptake at alkaline pH. Bioscience, Biotechnology, Biochemistry, 66, 1597–1600.
Kirakosyan, G., Bagramyan, K., & Trchounian, A. (2004). Redox sensing by Escherichia coli: effects of dithiothreitol, a redox reagent reducing disulphides, on bacterial growth. Biochemical and Biophysical Research Communication, 325, 803–806.
Kirakosyan, G., & Trchounian, A. (2007). Redox sensing by Escherichia coli: Effects of copper ions as oxidizers on proton-coupled membrane transport. Bioelectrochemistry, 70, 58–63.
Kirakosyan, G., Trchounian, K., Vardanyan, Z., & Trchounian, A. (2008). Copper (II) ions affect Escherichia coli membrane vesicles’ SH-groups and a disulfide-dithiol interchange between membrane proteins. Cell Biochemistry and Biophysics, 51, 45–50.
Kobayashi, H., Suzuki, T., Kinoshita, N., & Unemoto, T. (1984). Amplification of the Streptococcus faecalis proton-translocating ATPase by a decrease in cytoplasmatic pH. Journal of Bacteriology, 158, 1157–1160.
Lebedev, V. S., Volodina, L. A., EYu, Deinega., & YuI, Fedorov. (2005). Structural modifications of the surface of Escherichia coli bacteria and copper induced permeability of plasma membrane. Biofizika, 50, 107–113. (in Russian).
Letelier, M. E., Lepe, A. M., Faundez, M., Salazar, J., Martin, R., Aracena, P., et al. (2005). Possible mechanisms underlaying copper-induced damage in biological membranes leading to cellular toxicity. Chemico-Biological Interactions, 151, 71–82.
Lowry, N. O., Rosenbrough, N. J., Farr, A. C., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 263–275.
Maroney, M. J. (1999). Structure/function relationships in nickel metallo-biochemistry. Current Opinion in Chemistry and Biology, 3, 188–199.
Mnatsakanyan, N., Bagramyan, K., Vassilian, A., Nakamoto, R., & Trchounian, A. (2002). FO cysteine, bCys21, in the Escherichia coli ATP synthase is involved in regulation of potassium uptake and molecular hydrogen production in anaerobic conditions. Bioscience Reports, 22, 421–430.
Mnatsakanyan, N., Poladian, A., Bagramyan, K., & Trchounian, A. (2003). The number of accessible SH-groups in Escherichia coli membrane vesicles is increased by ATP and by formate. Biochemical and Biophysical Research Communications, 308, 655–659.
Mugikura, S., Nishikawa, M., Igarashi, K., & Kobayashi, H. (1990). Maintenance of a neutral cytoplasmic pH is not obligatory for growth of Escherichia coli and Streptococcus faecalis at an alkaline pH. Journal of Biochemistry, 108, 86–91.
Murata, T., Yamato, I., & Kakinuma, Y. (2005). Structure and mechanism of vacuolar Na+-transporting ATPase from Enterococcus hirae. Journal of Bioenergetics and Biomembranes, 37, 411–413.
Poladyan, A., & Trchounian, A. (1999). Stoichiometry of the proton-potassium exchange in Enterococcus hirae grown at high pH values. Biophysics, 44, 472–474.
Poladyan, A., & Trchounian, A. (2006). The increase in the number of accessible SH-groups in the Enterococcal membrane vesicles by ATP and nicotinamide adenine dinucleotides. Current Microbiology, 52, 300–304.
Poladyan, A., Trchounian, К., Tadevosyan, L., & Trchounian, A. (2008). Effects of Ellman’s and the other thiol reagents on ion transport and ATPase activity in anaerobically grown Escherichia coli. Biochemistry (Moscow): A Membrane and Cell Biology, 2, 1–7.
Rensing, C., & Grass, G. (2003). Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiology Reviews, 27, 197–213.
Riddles, P., Blakeley, R., & Zerner, B. (1983). Reassessment of Ellman’s reagent. Methods of Enzymology, 91, 49–60.
Riondet, C., Cachon, R., Wache, Y., Alcarez, G., & Divies, C. (1999). Changes in the proton-motive force in Escherichia coli in response to external oxidoreduction potential. European Journal of Biochemistry, 262, 595–599.
Rosen, B. P. (2002). Transport and detoxication systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comparative Biochemistry and Physiology. A Molecular and Integrative Physiology, 133, 689–693.
Shibata, C., Ehara, T., Tomura, K., Igarashi, K., & Kobayashi, H. (1992). Gene structure of Enterococcus hirae (Streptococcus faecalis) FoF1-ATPase, which functions as a regulator of cytoplasmic pH. Journal of Bacteriology, 174, 6117–6124.
Takase, K., Yamato, I., & Kakinuma, Y. (1993). Cloning and sequencing of the genes coding for the A and B subunits of vacuolar-type Na+-ATPase from Enterococcus hirae. Coexistence of vacuolar- and FoF1 ATPases in one bacterial cell. Journal of Biological Chemistry, 268, 11610–11616.
Taussky, H., & Shorr, E. (1953). A microcolorimetric method for the determination of inorganic phosphorus. Journal of Biological Chemistry, 202, 675–685.
Trchounian, A. (2004). Escherichia coli proton-translocating FoF1 ATP synthase and its association with solute secondary transporters and/or enzymes of anaerobic oxidation-reduction under fermentation. Biochemical and Biophysical Research Communications, 315, 1051–1057.
Trchounian, A., & Kobayashi, H. (1998). Relationship of K+-uptaking system with H+-translocating ATPase in Enterococcus hirae, growth at a high or low alkaline pH. Current Microbiology, 36, 114–118.
Vassilian, A., & Trchounian, A. (2009). Environment oxidation-reduction potential and redox sensing by bacteria. In A. Trchounian (Ed.), Bacterial membranes (pp. 163–195). Kerala (India): Research Signpost.
Volodina, L. A., Zhigach, A. N., Leypunsky, I. O., YuI, Fedorov., & Glushenko, N. N. (2009). On the mechanism of toxic effect of copper nanoparticles on bacteria Escherichia coli. Biofizika, 54, 1060–1065. (in Russian).
Acknowledgments
We thank Prof. H. Kobayashi for supplying E. hirae strains and valuable advices as well as Drs. Anna Poladyan and Gayane Kirakosyan for help in some experiments and useful comments. The study was supported by the Grant (#1012-2008) from the Ministry of Education and Science of the Republic of Armenia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vardanyan, Z., Trchounian, A. The Effects of Copper (II) Ions on Enterococcus hirae Cell Growth and the Proton-Translocating FoF1 ATPase Activity. Cell Biochem Biophys 57, 19–26 (2010). https://doi.org/10.1007/s12013-010-9078-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-010-9078-z