Abstract
Carbon monoxide poisoning (COP) may increase the risk of myocardial infarction. We conducted a study to investigate the effects of hyperbaric oxygen therapy (HBOT) on the risk. We used the Nationwide Poisoning Database in Taiwan to identify COP patients diagnosed between 1999 and 2012. We compared the risk for myocardial infarction between patients with and without HBOT by following up through 2013 and identified the independent predictors of myocardial infarction. The risk of myocardial infarction in the 7278 patients with HBOT was lower than in the 18,459 patients without HBOT, but this difference did not reach statistical significance [adjusted hazard ratio (AHR): 0.69; 95% confidence interval (CI) 0.45–1.07]. Stratified analyses showed that the reductions in the risk associated with HBOT for myocardial infarction reached statistical significance in male patients (AHR: 0.45; 95% CI 0.24–0.83) and during the first 2 weeks of follow-up (AHR: 0.22; 95% CI 0.05–0.96). In patients without HBOT, independent predictors of myocardial infarction were old age, male sex, and the underlying comorbidities of hypertension, diabetes, coronary artery disease, and congestive heart failure. In patients with HBOT, however, old age, male sex, and the underlying comorbidities of diabetes, coronary artery disease, and congestive heart failure were not independent predictors. HBOT was associated with a reduced risk of myocardial infarction in male patients and within 2 weeks following COP. These results may provide important reference for using HBOT in treating COP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon monoxide (CO) is an odorless and colorless gas emitted due to the incomplete combustion of organic compounds [1]. Carbon monoxide poisoning (COP) remains a very important issue in public health worldwide because it causes numerous accidental and intentional injuries and subsequent economic and social losses [1,2,3,4]. In the USA, around 50,000 exposures and 1000–2000 accidental deaths due to COP occur annually [2]. The primary toxicity of COP is due to its high affinity to hemoglobin, which is about 200–250 times than that of oxygen. This causes hypoxic injuries in all internal organs of the victims [1]. In addition to hypoxia, other mechanisms, including oxidative stress and inflammatory responses, are also responsible for subsequent organ damage and even death [1, 3, 5,6,7].
The heart and the brain are the organs most commonly damaged by COP because they have the highest oxygen demands [1, 8, 9, 12,13,14]. A study enrolling 230 patients with moderate to severe COP reported that 37% of patients had myocardial infarction following COP [12]. The risk for mortality in COP patients with myocardial infarction afterwards was higher than in the COP patients without myocardial infarction [15]. COP may also contribute to other cardiac dysfunction including arrhythmia and left ventricular systolic dysfunction [16]. Another study performing angiograms in COP patients with myocardial infarction reported that all the patients had normal coronary arteries, which suggested that COP-related myocardial infarction was caused not via coronary artery occlusion, but via hypoxia, oxidative stress, inflammatory response, and myocardial stunning [11]. Hyperbaric oxygen therapy (HBOT) is suggested as the preferred choice for treating severe COP because it reduces subsequent complications, especially neurological sequelae [1,2,3, 8,9,10]; however, it remains unclear whether HBOT reduces the risk for myocardial infarction, and therefore we conducted this study to clarify this issue. Our hypothesis was that HBOT may reduce the risk for myocardial infarction following COP.
Methods
Data Sources
The Nationwide Poisoning Database (NPD) is a sub-dataset of the National Health Insurance Research Database (NHIRD), which contains all poisoning cases, including instances of COP, diagnosed in Taiwan between 1999 and 2013. NHIRD, which is a large, computerized database derived by the National Health Insurance Administration (the former Bureau of National Health Insurance) and maintained by the National Health Research Institutes, provides subsets of data to scientists in Taiwan for research purposes [17].
Identification of Patients and Definitions of Variables
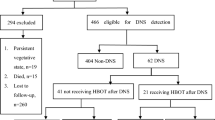
Patients diagnosed with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 986, E868, E952, or E982 during either admission or ambulance care between 1999 and 2012 were identified as having suffered from COP (Fig. 1). These COP patients were then divided into two cohorts “with HBOT” and “without HBOT.” The general criteria for the choice of HBOT in COP patients are carboxyhemoglobin level > 25%, pregnancy, cardiovascular dysfunction, and severe poisoning (e.g., unconsciousness, severe acidosis, or neurological signs) [18, 19]. The “with HBOT” cohort was defined as the patients who were labeled with 47054C, 9395, 59003B, 59004B, 59003A, or 59004A as their management codes during the index admission or emergency department care. In contrast, the “without HBOT” cohort was defined as the patients who were not labeled with any of the abovementioned management codes. Variables of demographic data and underlying comorbidities included for analysis were as follows: age, sex, hypertension (ICD-9-CM codes: 401–405), diabetes (ICD-9-CM code: 250), hyperlipidemia (ICD-9-CM code: 272), coronary artery disease (ICD-9-CM codes: 410–414), malignancy (ICD-9-CM codes: 140–208), congestive heart failure (ICD-9-CM code: 428), chronic obstructive pulmonary disease (ICD-9-CM code: 496), liver disease (ICD-9-CM codes: 570–576), renal disease (ICD-9-CM codes: 580–593), connective tissue disease (ICD-9-CM code: 710), HIV infection (ICD-9-CM codes: 042, 07953, V08), and alcoholism (ICD-9-CM codes: 291, 303, 305.0, 357.5, 425.5, 535.3, 571.0–571.3, V113). Age was divided into subgroups of < 20, 20–34, 34–50, 51–64, and ≥ 65 years [20]. Monthly income was divided into subgroups of < 19,999, 20,000–39,999, and ≥ 40,000 New Taiwan Dollars [21]. Concomitant conditions with COP during the index admission or emergency department care were defined as suicide (management codes: 94.0, 94.1 or ICD-9-CM codes: E950–E959), drug poisoning (ICD-9-CM codes: 960–989, exclusion of 986), acute respiratory failure (ICD-9-CM codes: 518.81, 518.84 or management codes: 960, 9601, 9602, 9603, 9604, 9605, 9390, 9391, 311), myocardial infarction (ICD-9-CM code: 410), acute hepatitis (ICD-9-CM code: 573.3), and acute renal failure (ICD-9-CM codes: 584 or management code: 339.5).
Comparison of the Risk for Myocardial Infarction Between Patients With and Without HBOT
We compared the risk for myocardial infarction between the two cohorts by following up until 2013 (Fig. 1). Stratified analyses by age subgroups, sex, underlying comorbidities, monthly income, concomitant conditions of suicide, drug poisoning, and acute respiratory failure, and follow-up periods were also performed. Investigation of the independent predictors for the risk of myocardial infarction in patients with and without HBOT was also performed.
Ethics Statement
This study was approved by the Institutional Review Board at Chi-Mei Medical Center and conducted according to the Declaration of Helsinki. Informed consents were waived because data in the NPD are de-identified, and the welfare of the participants is not affected.
Statistical Analysis
For comparisons of demographic data, underlying comorbidities, monthly income, and concomitant conditions between the two cohorts and the two sexes, we used the two-sample t test and the Chi-square test for continuous variables and categorical variables, respectively. Cox proportional hazard regression analyses were used for comparing the risks of myocardial infarction and for investigating independent predictors. The Kaplan–Meier method and log-rank test were also used to compare the cumulative incidence rates for myocardial infarction during follow-up periods. We used SAS 9.4 for Windows (SAS Institute, Cary, NC, USA) for all statistical analyses and set the significance level at 0.05 (two-tailed).
Results
In total, 25,737 COP patients were identified, including 7278 patients with HBOT (28.28%) and 18,459 patients without HBOT (71.72%) (Table 1). The mean age ± standard deviation was 36.0 ± 16.5 years, and patients with HBOT were younger than the patients without HBOT (34.9 ± 14.7 vs. 36.4 ± 17.1 years, p < 0.001). Both cohorts had a nearly equal sex ratio. Patients with HBOT had lower prevalence rates of underlying comorbidities of hypertension, diabetes, hyperlipidemia, coronary artery disease, malignancy, congestive heart failure, chronic obstructive pulmonary disease, liver disease, renal disease, and alcoholism than patients without HBOT. The monthly income was not different between the two cohorts. In the comparison of concomitant conditions, there were higher percentages of suicide, acute respiratory failure, and acute renal failure in patients with HBOT than in patients without HBOT. The prevalence of myocardial infarction during the index admission or emergency department care was lower in patients with HBOT than in patients without HBOT (0.36% vs. 0.67%, p = 0.003).
Overall, patients with HBOT were at a lower risk for myocardial infarction than patients without HBOT after adjusting for age, sex, hypertension, diabetes, hyperlipidemia, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, liver disease, renal disease, connective tissue disease, HIV infection, alcoholism, monthly income, suicide, drug poisoning, and respiratory failure [adjusted hazard ratio (AHR): 0.69]. However, this difference did not reach statistical significance [95% confidence interval (CI) 0.45–1.07] (Table 2). The Kaplan–Meier method and log-rank test showed a lower risk for myocardial infarction in patients with HBOT than in patients without HBOT during the follow-up (Fig. 2). The stratified analysis revealed that male patients with HBOT had a significantly lower risk for myocardial infarction than male patients without HBOT (AHR: 0.45; 95% CI 0.24–0.83), but females patients with HBOT had a similar risk to female patients without HBOT (AHR: 1.04; 95% CI 0.55–1.96). Patients with HBOT had a significantly lower risk for myocardial infarction in the first 2 weeks following COP than patients without HBOT following COP (AHR: 0.22; 95% CI 0.05–0.96). In patients with the underlying comorbidity of coronary artery disease, HBOT was associated with a lower risk for myocardial infarction (AHR: 0.51), but the difference did not reach statistical significance (95% CI 0.25–1.05). In the analyses stratified by age subgroups, underlying comorbidities, monthly income, and concomitant conditions of suicide, drug poisoning, acute respiratory failure, and follow-up periods at > 2 weeks, no differences between the two cohorts reached statistical significance.
In patients with HBOT, independent predictors for the risk of myocardial infarction were hypertension (AHR: 4.68; 95% CI 1.64–13.39) and chronic obstructive pulmonary disease (AHR: 5.74; 95% CI 1.60–20.57) (Table 3). In patients without HBOT, independent predictors for the risk of myocardial infarction were older age, male sex, hypertension, diabetes, coronary artery disease, and congestive heart failure.
Comparisons of age, underlying comorbidities, monthly income, and concomitant conditions among male and female COP patients with and without HBOT were shown in the Table 4. The Kaplan–Meier method and log-rank test showed a lower risk of myocardial infarction in male patients with HBOT than in male patients without HBOT during the follow-up. However, the difference between patients with and without HBOT was not significant in the female population (Fig. 3).
Discussion
Overall, this study demonstrated that HBOT showed a trend toward reducing the risk for myocardial infarction in COP patients. Stratified analyses showed that HBOT reduced the risk for myocardial infarction in male patients for a follow-up period of < 2 weeks. After 2 weeks following COP, there was no significant difference in the risk of myocardial infarction between patients with and without HBOT. In patients with HBOT, independent predictors of myocardial infarction were hypertension and chronic obstructive pulmonary disease. In patients without HBOT, independent predictors of myocardial infarction were older age, male sex, hypertension, diabetes, coronary artery disease, and congestive heart failure.
Acceleration of the elimination of carboxyhemoglobin and COP-related direct disruption of cellular oxidative processes by HBOT are probably the mechanisms involved in reducing myocardial infarction. To our knowledge, there is no human study addressing this issue in the literature. A meta-analysis of randomized controlled trials showed that oxygen inhalation did not benefit patients with acute myocardial infarction when performed with normal oxygen saturation; however, this study did not include HBOT for the analyses [22]. Some animal studies have shown that HBOT can limit infarct size in rabbit hearts and that the earlier the initiation of treatment, the better the outcome [23, 24], which are compatible with our finding that HBOT reduced acute myocardial infarction within 2 weeks following COP. The standard treatment for COP is the administration of 100% oxygen at atmospheric pressure (normobaric oxygen), which can shorten the half-life of carboxyhemoglobin approximately fivefold faster than room air [1]. HBOT can further hasten the elimination of carboxyhemoglobin [1], and therefore it is theoretically used to treat COP, especially in patients with a high risk for subsequent complications. The most common and concerning complications after COP are the neurological sequelae [1, 3, 25]. Whether HBOT is better than normobaric oxygen in reducing the neurological sequelae is still being debated [1, 3, 25]. One of the best known studies supporting HBOT in reducing the neurological sequelae is the double-blind, randomized trial by Weaver et al. in 2002, which showed that HBOT reduced neurological sequelae at 6 weeks and 12 months [25]. However, several studies have challenged the positive effect of HBOT. For example, a 2011 review by Cochrane reported that existing and randomized trials did not establish whether HBOT reduces the incidence of neurological sequelae in COP patients [1]. Although their findings did not support the use of HBOT for COP, the authors of the review suggested that an additional study, such as a multicenter randomized controlled trial, is needed to clarify this issue [1].
Our stratified analyses showed that HBOT reduced the risk of myocardial infarction in male patients, but not in female patients. A study reported that male COP patients have more comorbidities and are at a higher risk for subsequent mortality than female COP patients [4]. Therefore, HBOT may have more benefits for the male population. The lower risk of developing myocardial infarction in females as compared to males is a well-known fact, and the difference was also observed in patients with COP [14]. The protecting effect of estrogen is generally recognized as a major factor contributing to the sex difference [26]. We speculate that the protecting effect of estrogen was a main reason why the beneficial effect of HBOT against myocardial infraction following COP was smaller in women. To our knowledge, however, there is no study comparing the differences in the impact of HBOT on myocardial infarction between the two sexes. The independent predictors for patients with and without HBOT were different, which suggested that HBOT has modification effects on the impacts of certain risk factors for myocardial infarction. Specifically, in patients without HBOT, the independent predictors of myocardial infarction were similar to the conventional risk factors for myocardial ischemia, including older age, male sex, hypertension, diabetes, coronary artery disease, and congestive heart failure, but in patients with HBOT only hypertension and chronic obstructive pulmonary disease appeared to be independent predictors. HBOT may have a greater impact on reducing myocardial infarction following COP in the patients with older age, male sex, diabetes, coronary artery disease, and congestive heart failure.
The generally applied criteria for the choice of HBOT in the COP patients do not take comorbidities, except cardiovascular dysfunction, into account, and many differences in comorbidities between groups that got HBOT versus not were observed in our study. This might affect the incidence of myocardial infarction. Nonetheless, after adjusting for these comorbidities, we still observed a decreased risk in the patients who received HBOT.
This study has the strength of being the first, large, and nationwide study on the effect HBOT on subsequent myocardial infarction following COP. However, it has some limitations. First, we might underestimate the number of patients with myocardial infarction by using the ICD-9-CM code to identify patients. However, the misclassification should be non-differential because the diagnosis of myocardial infarction was unlikely to be affected by the administration of HBOT. Second, we did not include certain socioeconomic variables, such as smoking, obesity, family history, diet, physical activity, and stress, which are risk factors for myocardial infarction and possible confounding factors in this study. However, we have included several underlying comorbidities that may be surrogates for these unavailable variables in our database, and therefore the impact may be minimal. Third, the modification effect on the impacts of older age, male sex, diabetes, coronary artery disease, and congestive heart failure in this study is novel. Validation of the finding and clarification of the mechanisms are also needed in the future. Lastly, although this was a nationwide population-based study, the results may not be generalizable to other nations because of the differences in race, culture, and medical resources.
Conclusions
This nationwide population-based cohort study demonstrated that HBOT shows a trend toward reducing subsequent risk for myocardial infarction following COP. The effect of HBOT in reducing the risk for myocardial infarction was significant in males and in the acute follow-up period of < 2 weeks. These results provide us with an important reference for the treatment of COP patients in the future. However, further studies are warranted to delineate the underlying mechanisms of this process and to validate our findings.
Abbreviations
- COP:
-
Carbon monoxide poisoning
- HBOT:
-
Hyperbaric oxygen therapy
- AHR:
-
Adjusted hazard ratio
- CI:
-
Confidence interval
- CO:
-
Carbon monoxide
- NPD:
-
National Poisoning Database
- NHIRD:
-
National Health Insurance Research Database
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- HIV:
-
Human immunodeficiency virus
References
Buckley, N. A., Juurlink, D. N., Isbister, G., Bennett, M. H., & Lavonas, E. J. (2011). Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Systematic Review,4, CD002041.
Hampson, N. B., & Weaver, L. K. (2007). Carbon monoxide poisoning: A new incidence for an old disease. Undersea and Hyperbaric Medicine,34(3), 163–168.
Weaver, L. K. (2009). Clinical practice. Carbon monoxide poisoning. New England Journal of Medicine,360(12), 1217–1225.
Huang, C. C., Chung, M. H., Weng, S. F., Chien, C. C., Lin, S. J., Lin, H. J., et al. (2014). Long-term prognosis of patients with carbon monoxide poisoning: A nationwide cohort study. PLoS ONE,9(8), e105503.
Thom, S. R., Bhopale, V. M., Fisher, D., Zhang, J., & Gimotty, P. (2004). Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proceedings of the National Academy of Science USA,101(37), 13660–13665.
Thom, S. R., Bhopale, V. M., & Fisher, D. (2006). Hyperbaric oxygen reduces delayed immune-mediated neuropathology in experimental carbon monoxide toxicity. Toxicology and Applied Pharmacology,213(2), 152–159.
Huang, C. C., Ho, C. H., Chen, Y. C., Lin, H. J., Hsu, C. C., Wang, J. J., et al. (2017). Demographic and clinical characteristics of carbon monoxide poisoning: Nationwide data between 1999 and 2012 in Taiwan. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine,25(1), 70.
Zou, J. F., Guo, Q., Shao, H., Li, B., Du, Y., Liu, M., et al. (2014). A positive Babinski reflex predicts delayed neuropsychiatric sequelae in Chinese patients with carbon monoxide poisoning. BioMed Research International,2014, 814736.
Zou, J. F., Guo, Q., Shao, H., Li, B., Du, Y., Liu, M., et al. (2015). Lack of pupil reflex and loss of consciousness predict 30-day neurological sequelae in patients with carbon monoxide poisoning. PLoS ONE,10(3), e0119126.
Huang, C. C., Ho, C. H., Chen, Y. C., Lin, H. J., Hsu, C. C., Wang, J. J., et al. (2017). Hyperbaric oxygen therapy is associated with lower short- and long-term mortality in patients with carbon monoxide poisoning. Chest,152(5), 943–953.
Kalay, N., Ozdogru, I., Cetinkaya, Y., Eryol, N. K., Dogan, A., Gul, I., et al. (2007). Cardiovascular effects of carbon monoxide poisoning. American Journal of Cardiology,99(3), 322–324.
Satran, D., Henry, C. R., Adkinson, C., Nicholson, C. I., Bracha, Y., & Henry, T. D. (2005). Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. Journal of the American College of Cardiology,45(9), 1513–1516.
Huang, C. C., Ho, C. H., Chen, Y. C., Wang, Y. F., Lin, H. J., Hsu, C. C., et al. (2018). Impact of hyperbaric oxygen therapy on subsequent neurological sequelae following carbon monoxide poisoning. Journal of Clinical Medicine,7(10), 349.
Huang, C. C., Ho, C. H., Chen, Y. C., Lin, H. J., Hsu, C. C., Wang, J. J., et al. (2019). Risk of myocardial infarction after carbon monoxide poisoning: A nationwide population-based cohort study. Cardiovascular Toxicology,19(2), 147–155.
Henry, C. R., Satran, D., Lindgren, B., Adkinson, C., Nicholson, C. I., & Henry, T. D. (2006). Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA,295(4), 398–402.
Rose, J. J., Wang, L., Xu, Q., McTiernan, C. F., Shiva, S., Tejero, J., et al. (2017). Carbon monoxide poisoning: Pathogenesis, management, and future directions of therapy. American Journal of Respiratory and Critical Care Medicine,195(5), 596–606.
National Health Insurance Administration, Ministry of Health and Welfare, Taiwan, R.O.C. (2014). National Health Insurance Annual Report 2014–2015.
Huang, C. C., Lee, J. C., Lin, K. C., Lin, H. J., Su, S. B., Hsu, C. C., et al. (2019). Exposure duration and history of hypertension predicted neurological sequelae in patients with carbon monoxide poisoning. Epidemiology,30(Suppl 1), S76–S81.
Clardy, P. F., Manaker, S., Perry, H. Carbon monoxide poisoning. Retrieved July 3, 2019, from https://www.uptodate.com/contents/carbon-monoxide-poisoning#H1884832241.
Huang, C. C., Ho, C. H., Chen, Y. C., Lin, H. J., Hsu, C. C., Wang, J. J., et al. (2017). Increased risk for diabetes mellitus in patients with carbon monoxide poisoning. Oncotarget,8(38), 63680–63690.
Lee, C. C., Lee, M. T., Chen, Y. S., Lee, S. H., Chen, Y. S., Chen, S. C., et al. (2015). Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Internal Medicine,175(11), 1839–1847.
Li, W. F., Huang, Y. Q., & Feng, Y. Q. (2018). Oxygen therapy for patients with acute myocardial infarction: A meta-analysis of randomized controlled clinical trials. Coronary Artery Disease,29(8), 652–656.
Thomas, M. P., Brown, L. A., Sponseller, D. R., et al. (1990). Myocardial infarct size reduction by the synergistic effect of hyperbaric oxygen and recombinant tissue plasminogen activator. American Heart Journal,120(4), 791–800.
Sterling, D. L., Thornton, J. D., Swafford, A., et al. (1993). Hyperbaric oxygen limits infarct size in ischemic rabbit myocardium in vivo. Circulation,88(4 Pt 1), 1931–1936.
Weaver, L. K., Hopkins, R. O., Chan, K. J., Churchill, S., Elliott, C. G., Clemmer, T. P., et al. (2002). Hyperbaric oxygen for acute carbon monoxide poisoning. New England Journal of Medicine,347(14), 1057–1067.
Pedersen, L. R., Frestad, D., Michelsen, M. M., Mygind, N. D., Rasmusen, H., Suhrs, H. E., et al. (2016). Risk factors for myocardial infarction in women and men: A review of the current literature. Current Pharmaceutical Design,22(25), 3835–3852.
Funding
This study was supported by Grant MOST 108-2638-B-006-001-MY2 from the Ministry of Science and Technology and Grants CMFHR10677 and CMFHR10734 from the Chi-Mei Medical Center.
Author information
Authors and Affiliations
Contributions
C-CH and H-RG designed and conceived this study and wrote the manuscript. H-CH and Y-CC performed the statistical analysis and wrote the manuscript. H-JL, C-CH, J-JW, and S-BS provided professional suggestions and wrote the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
This study was conducted in strict accordance with the Declaration of Helsinki and was approved by the Institutional Review Board at the Chi-Mei Medical Center. The two databases used in this study consisted of depersonalized information, and so the informed consent was waived as the study did not affect the welfare of the participants.
Additional information
Handling Editor: Y. James Kang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, CC., Ho, CH., Chen, YC. et al. Effects of Hyperbaric Oxygen Therapy on Acute Myocardial Infarction Following Carbon Monoxide Poisoning. Cardiovasc Toxicol 20, 291–300 (2020). https://doi.org/10.1007/s12012-019-09552-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-019-09552-7