Abstract
Bone mesenchymal stem cells (BMSCs) transplantation has been recognized as an effective method for the treatment of myocardial infarction (MI). However, its efficacy is always restricted by the low survival of transplanted BMSCs in the ischemic myocardium. The aim of this study was to investigate the effect of catalpol pre-treatment on the survival and vascular endothelial growth factor (VEGF) secretion of BMSCs under oxygen glucose deprivation (OGD) condition and their role in myocardial repair in a rat model of MI. According to our results, pre-treatment with catalpol enhanced VEGF secretion and survival of OGD-treated BMSCs. Moreover, the apoptosis of BMSCs induced by OGD was restrained by catalpol as evidenced by increased level of B-cell lymphoma-2 (Bcl-2) and decreased levels of BCL2-associated X (Bax) and cleaved caspase-3. In vivo study suggested that the survival of transplanted BMSCs was improved by catalpol pre-treatment. The myocardial fibrosis and apoptosis was further inhibited in catalpol pre-treated BMSCs group. Cardiac function detected by echocardiography was obviously improved by catalpol pre-treated BMSCs transplantation. Finally, angiogenesis and VEGF expression in the ischemic myocardium were significantly promoted in catalpol pre-treated BMSCs group. In conclusion, catalpol pre-treatment may facilitate the survival and VEGF secretion of BMSCs and improve their therapeutic effect on MI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI), as a type of coronary heart disease, has severely threatened the human health. Unlike other organs, after MI the impaired heart does not have the ability to repair itself by regeneration of cardiac muscle cells [1]. The fibrous scar may replace the necrotic myocardium, which results in left ventricular remodeling and cardiac dysfunction [2]. Bone marrow-derived stem cells (BMSCs) transplantation has been recognized as a safe and effective measure to regenerate cardiac muscle cells [3, 4]. Growing evidence has demonstrated the curative effect of BMSCs transplantation on MI [5, 6]. However, its efficacy is restricted by low survival rate of transplanted cells caused by ischemia, anoxia, and inflammation [7, 8]. Therefore, promoting the proliferation and survival of BMSCs plays important roles in the improvement of efficacy of BMSCs transplantation.
Vascular endothelial growth factor (VEGF) is a pro-angiogenesis factor that facilitates the proliferation, migration, and survival of endothelial cells [9]. It has been suggested that VEGF could increase blood flow and improve cardiac function after MI [10]. Interestingly, VEGF also plays pivotal roles in BMSCs transplantation after MI [11]. So regulation of the secretion of VEGF may affect the therapeutic effect of BMSCs transplantation.
Catalpol is an active compound isolated from a Chinese medicine Radix rehmanniae, which has been reported to have wide biological activities, including hypoglycemic action [12], neuroprotection [13], anti-aging [14], and anti-tumor [15]. In addition, a previous study has shown that catalpol could promote VEGF-mediated angiogenesis in rats’ stroke model [16]. Studies also indicated that catalpol could promote survival and inhibit apoptosis in various cell models [17, 18]. According to the above background, we speculate that catalpol may enhance the survival and VEGF secretion of the transplanted BMSCs in the treatment of MI.
In the present study, we explored whether catalpol could protect BMSCs against hypoxic–ischemic damage and thereby promote their curative effect in a rat model of MI.
Methods
Isolation and Identification of BMSCs
The BMSCs were isolated from bone marrow derived from the tibias and femurs of 4-week-old Wistar rats (Liaoning Changsheng Biotechnology Co. Ltd., Benxi, China) according to a previous study [19]. The isolated BMSCs were cultured in DMEM/F12 (Gibco, USA) supplemented with 10% FBS (Hyclone, USA) at 37 °C containing 5% CO2. When at high confluence (> 80%), the BMSCs were digested by 0.2% trypsin and subcultured. Three passages later, BMSCs were identified by incubation with primary antibodies against CD44 (eBioscience, USA), CD90 (eBioscience, USA), CD45 (eBioscience, USA), CD34 (Abcam, USA) via flow cytometry and used for the following experiments.
Catalpol Pre-treatment and Oxygen Glucose Deprivation (OGD)
The BMSCs were pre-treated with different concentrations (12.25 µg/ml (L), 24.5 µg/ml (M), or 49 µg/ml (H)) of catalpol (Solarbio Science & Technology Co. Ltd., Beijing, China, diluted in DMSO) for 24 h. After that, the BMSCs were subjected to OGD for 12 h. To induce OGD, the BMSCs were cultured in glucose-, serum- and sodium pyruvate-free DMEM/F12 at an atmosphere of 95% N2 and 5% CO2 in an anoxia chamber, while the control cells were maintained in complete DMEM/F12 under normal conditions.
CCK8 Assay
The viability of BMSCs was determined by CCK-8 assay. Briefly, BMSCs were seeded in 96-well plates at a density of 5 × 103 per well. After pre-treatment with catalpol for 24 h and OGD stimulation for 12 h, 10 µl of CCK-8 solution (KeyGEN BioTECH, Nanjing, China) was added to cells. The results were measured at 450 nm by a microplate reader (Bio Tek, USA) after incubation for 2 h at 37 °C.
Annexin V/PI Apoptosis Assay
The apoptosis of BMSCs was assessed using an Annexin V-FITC/PI apoptosis detection kit (Wanleibio, Shenyang, China). In short, BMSCs with different treatments were harvested, washed with cold PBS twice, and resuspended with 500 µl binding buffer. Then the BMSCs were stained with 5 µl of Annexin V-FITC and 10 µl of PI for 15 min in the dark. The stained BMSCs were immediately detected on a flow cytometer (BD, USA).
ELISA
The level of VEGF in the supernatant liquid of BMSCs or the heart tissues of rats was assessed by a commercial rat VEGF ELISA kit (Multi Sciences, Hangzhou, China), according to the manufacturer’s instructions.
Western Blot Analysis
The BMSCs or heart tissues were lysed at 4 °C by RIPA (Beyotime, Haimen, China) containing 1% phenylmethanesulfonyl fluoride (Beyotime). The protein concentration was determined by BCA Protein Assay Kit (Beyotime). The protein samples were loaded onto SDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fluoride membranes (Millipore, USA). After blocking with 5% not-fat milk, the membranes were blotted with corresponding primary antibodies against VEGF-A (1:500, Proteintech, 19003-1-AP, USA), Bcl-2 (1:1000, BOSTER, A00040-1, China), Bax (1:500, Proteintech, 50599-2-lg, USA), and cleaved caspase-3 (1:1000, Cell Signaling Technology, #9661, USA) and HIF-1α (1:400, Bioss, bs-0737R, China) at 4 °C overnight, respectively. Thereafter, the membranes were incubated with goat anti-rabbit secondary antibody (1:5000, Beyotime, A0208) at 37 °C for 45 min. The results were visualized by BeyoECL Plus (Beyotime). The density of the bands was analyzed by Gel-Pro-Analyzer software.
Animal Experiments
Healthy male Wistar rats weighing 200–220 g were obtained from Liaoning Changsheng Biotechnology Co. Ltd. (Benxi, China), and raised at 22 ± 1 °C under 12-h light–dark cycles with free access to food and water. All animal-related experimental procedures were in accordance with and approved by the Ethics Committee of Shengjing Hospital, China Medical University.
The rats were randomly divided into four groups (n = 12 per group): sham, MI, MI + BMSCs, MI + BMSCs + catalpol. Briefly, the rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) and connected to the respirator through a tracheotomy. The chest was opened via a left thoracic incision. Then MI was triggered by left anterior descending artery ligation via a 5–0 prolene suture. The rats in the sham group suffered the same operation without left anterior descending artery ligation. Immediately after the ligation, the rats in BMSCs transplantation groups were injected with 2 × 106 BMSCs (with or without pre-treatment with 49 µg/ml catalpol for 24 h) in the area of MI, while the rats in MI group were injected with equal volume vehicle. Then the chest was closed and the incision was sutured. After recovery of spontaneous breathing, the rats were extubated and placed in box on the warm pad for recovery. Four weeks after the transplantation, the rats were euthanatized and the heart tissues were collected for further experiments.
Survival Detection of the Transplanted BMSCs
To determine the in vivo survival of BMSCs, before transplantation, the BMSCs were stained with PKH26 (Sigma, USA), according to the manufacturer’s instruction. The collected heart tissues at 4 weeks after transplantation were cut into 5-µm sections and the nuclei were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI). Then the survived BMSCs that expressed red fluorescence were detected by a fluorescence microscopy.
Echocardiography
At 4 weeks after the transplantation, the rats were anesthetized and the heart function was determined by two-dimensional targeted M-mode echocardiography using an ultrasonic echocardiographic system. The following parameters including left ventricular end-systolic diameter (LVESD) and left ventricular end-diastolic diameter (LVEDD) were measured from three consecutive cardiac cycles. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were calculated as follows: LVEF% = [(LVEDD)3 − (LVESD)3]/(LVEDD)3 × 100; LVFS% = (LVEDD − LVESD)/LVEDD × 100.
Histological Examinations
The heart tissues were fixed in 10% formalin, then embedded in wax, and cut into 5-µm sections. The sections were subjected to routine hematoxylin–eosin (HE) and Masson staining to assess pathological changes and myocardial fibrosis. The images were photographed under a light microscope (Olympus, Japan).
TUNEL
To evaluate apoptosis of heart tissues, TUNEL staining was performed using In Situ Cell Death Detection Kit (Roche, Switzerland) according to the manufacturer’s protocols. The nuclei of the apoptotic cells were stained with green fluorescence. Under a fluorescence microscope, the images were obtained at a magnification of × 400.
Immunofluorescence Staining
The expression of CD31 in heart tissues was detected by immunofluorescence staining. Briefly, the 5-µm sections of heart tissues were blocked in goat serum (Solarbio, Beijing, China) and incubated with primary antibody against CD31 (1:200, Abcam, ab119339, UK) or Myosin (1:200, Proteintech, 10906-1-AP, USA) at 4 °C overnight. Then FITC-labeled Goad Anti-Mouse secondary antibody (Beyotime, A0568) or cy3-labeled Goad Anti-Rabbit secondary antibody (Beyotime, A0516) was added to the sections. After stained with DAPI, the sections were observed under a fluorescence microscope (Olympus, Japan).
Statistical Analysis
Statistical analysis was determined by one-way ANOVA followed by Bonferroni’s post hot test using GraphPad Prism 5 software. The results were presented as mean ± standard deviation (SD). Statistical difference was considered as P value < 0.05.
Results
Identification of BMSCs
As shown in Fig. 1a–d, the expressions of surface markers of BMSCs were assessed by flow cytometry. The results indicated that the percentages of CD44 and CD90 positive BMSCs were 92.1 and 75.7%, while the percentages of CD34 and CD45 positive cells were 1.7 and 2.3%. Thus, BMSCs were successfully isolated and identified.
Effect of Catalpol on VEGF Secretion in OGD-Treated BMSCs In Vitro
The secretion of VEGF in BMSCs was determined by ELISA and shown in Fig. 2a. The VEGF level in the BMSCs culture supernatant was slightly increased by OGD stimulation, although there was no statistical difference. Pre-treatment with catalpol could dose-dependently enhance the VEGF secretion in BMSCs under OGD condition. In addition, the protein levels of VEGF-A and HIF-1α in BMSCs were evaluated by western blot analysis. As illustrated in Fig. 2b–d, the levels of VEGF-A, nuclear and total HIF-1α appeared to be on the rise under OGD condition, which was significantly up-regulated by catalpol pre-treatment.
Catalpol facilitated the VEGF secretion in OGD-treated BMSCs. BMSCs were pre-treated with catalpol (L, 12.25 µg/ml; M, 24.5 µg/ml; H, 49 µg/ml) for 24 h, and then cultured under OGD condition for 12 h. a The level of VEGF in the supernatant of BMSCs that subjected to various treatments was determined by ELISA. b The protein level of VEGF-A, nuclear and total HIF-1α in BMSCs with different treatments was detected by western blot assay. β-Actin was used as a loading control. c, d The gray-scale values of the bands were analyzed. The experimental data were presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01 versus the BMSCs group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus the BMSCs + OGD group
Effect of Catalpol on Survival of BMSCs Under OGD Condition In Vitro
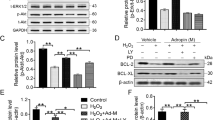
To assess the effect of catalpol on survival of OGD-treated BMSCs, CCK8 assay was performed. As presented in Fig. 3a, the viability of BMSCs was obviously decreased by stimulation of OGD, which could be effectively improved by pre-treatment with catalpol. Moreover, the results suggested that OGD-induced apoptosis of BMSCs was strikingly restrained by catalpol (Fig. 3b, c). To further evaluate the mechanisms through which catalpol regulated the apoptosis of BMSCs, the expressions of apoptosis-related proteins were assessed. As shown in Fig. 3d–g, the level of anti-apoptotic protein Bcl-2 was down-regulated, while the pro-apoptotic Bax and cleaved caspase-3 levels were up-regulated in BMSCs under OGD condition. As expected, pre-treatment with catalpol could remarkably reverse the above changes.
Catalpol promoted the survival of BMSCs under OGD condition in vitro. BMSCs were pre-treated with catalpol (L, 12.25 µg/ml; M, 24.5 µg/ml; H, 49 µg/ml) for 24 h, and then cultured under OGD condition for 12 h. a The viability of BMSCs was assessed by CCK8 assay. b The apoptosis of BMSCs was determined by Annexin V/PI staining on flow cytometry. c The apoptosis rate of BMSCs was shown. d The levels of apoptosis-related proteins Bcl-2, Bax, and cleaved caspase-3 were assessed by western blot assay. β-Actin was used as a loading control. e, g The gray-scale values of the bands were analyzed. The experimental data were presented as mean ± SD (n = 3). ***P < 0.001 versus the BMSCs group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus the BMSCs + OGD group
Effect of Catalpol on Survival of BMSCs After In Vivo Transplantation
The survival of BMSCs after 4 weeks of transplantation was determined. As shown in Fig. 4, the surviving transplanted BMSCs in heart tissues were labeled with PKH26, a red fluorescent dye. According to the result, catalpol pre-treatment obviously improved the survival of BMSCs after in vivo transplantation.
Catalpol enhanced the survival of BMSCs after in vivo transplantation. The BMSCs were stained with PKH26 before transplantation. After transplantation for 4 weeks, the surviving BMSCs (red) in heart tissue were observed under a fluorescent microscope (× 400). Scale bar is 50 µm. (Color figure online)
Effect of Catalpol on Curative Effect of BMSCs Transplantation on Myocardial Infarction in Rats
As illustrated in Fig. 5a, b, pathological changes and myocardial fibrosis was obvious in MI group as assayed by HE and Masson staining. BMSCs transplantation effectively alleviated MI-induced pathological changes and myocardial fibrosis, which could be enhanced by catalpol pre-treatment. Furthermore, TUNEL and myosin staining results indicated that MI caused increased apoptosis in myocardial cells in the border area. However, BMSCs transplantation inhibited MI-induced apoptosis and this inhibitory effect was more pronounced when BMSCs were pre-treated with catalpol (Fig. 5c). As presented in Fig. 5d–g, cardiac function was detected by echocardiography. LVEF and LVFS were remarkably decreased in MI group. However, BMSCs transplantation raised LVEF and LVFS, which could be further enhanced when BMSCs were pre-treated with catalpol. In addition, the increased LVESD and LVEDD induced by MI were restrained by BMSCs transplantation. As could be expected, catalpol pre-treated BMSCs could further decrease LVESD and LVEDD. These results suggested that catalpol pre-treatment further improved the efficacy of BMSCs in MI.
Catalpol improved the curative effect of BMSCs transplantation on myocardial infarction in rats. a The pathological changes of heart tissues were determined by HE staining (× 200). Scale bar is 100 µm. b The myocardial fibrosis was evaluated by Masson’s staining at 4 weeks after transplantation (× 100). Scale bar is 200 µm. The lines were added in the border of the infarct area and non-infarct area. c The apoptosis in the border area was determined by TUNEL and myosin staining at 4 weeks after transplantation (× 400). Scale bar is 50 µm. The representative apoptotic cardiomyocytes were pointed out by arrowheads. The LVEF (d), LVFS (e), LVESD (f), and LVEDD (g) of rats were assessed by echocardiographic measurements at 4 weeks after transplantation. The experimental data were presented as mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 versus the indicated group
Effect of Catalpol on VEGF Secretion in Ischemic Myocardium After BMSCs Transplantation
As VEGF is an important pro-angiogenesis factor, the angiogenesis in the ischemic myocardium was determined by CD31 immunofluorescence staining. As shown in Fig. 6a, the expression of CD31 was significantly enhanced in ischemic myocardium by BMSCs transplantation, which was strengthened by pre-treatment with catalpol. Moreover, the protein level of VEGF-A in heart tissues was evaluated by western blot. Catalpol pre-treatment promoted BMSCs-induced increase in VEGF-A level in heart tissues (Fig. 6b, c). As shown in Fig. 6d, the result of ELISA further demonstrated the enhancement of VEGF level by transplanting BMSCs that were pre-treated with catalpol.
Catalpol promoted the VEGF secretion in ischemic myocardium after BMSCs transplantation. a The expression of CD31, a endothelial cell marker, in ischemic myocardium was determined by immunofluorescent staining at 4 weeks after transplantation (× 400). Scale bar is 50 µm. b The protein level of VEGF-A in heart tissue was assessed by western blot assay. β-Actin was used as a loading control. c The gray-scale values of the bands were analyzed. d The level of VEGF in heart tissue was evaluated by ELISA. The experimental data were presented as mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 versus the indicated group
Discussion
This is the first report that investigates the effect of catalpol on survival and VEGF secretion of BMSCs, and the efficacy of BMSCs transplantation in a rat model of MI. We found that catalpol promoted the survival and VEGF secretion of BMSCs under OGD condition. Moreover, in vivo experiments indicated that pre-treatment with catalpol significantly facilitated the survival of transplanted BMSCs, inhibited apoptosis, increased VEGF level in the ischemic myocardium, and improved cardiac function.
The recent progress in stem cell therapy has shown new hope for treating MI. BMSCs are easy to obtain, and can not induce remarkable reject reaction in allograft [20]. Due to such characteristics, BMSCs have a bright clinical prospect in MI. A large of research data showed that intramyocardial transplantation of BMSCs in animal models of MI facilitated myocardial regeneration and angiogenesis, decreased infarct size, alleviated cardiac remodeling, and improved left heart function [21, 22]. However, after MI the myocardial ischemic and anoxic injury, oxidative stress, and inflammatory response are aggravated, which are all not beneficial to the survival of transplanted BMSCs and compromise their therapeutic efficacy. To solve this problem, previous studies have provided various strategies, such as pre-treatment with protective substances [23], genetic modification [24], and ischemic preconditioning [25]. In the present study, we focused on the role of catalpol pre-treatment in enhancing the therapeutic efficacy of transplanted BMSCs.
Catalpol has been demonstrated to resist apoptosis induced by various factors, including OGD [26], hydrogen peroxide [27], ionizing radiation [28], and chronic cerebral hypoperfusion [29], and thus promotes cell survival. According to our results, catalpol significantly facilitated the survival and restrained apoptosis of BMSCs under OGD condition in vitro and transplantation in vivo. Apoptosis is a programmed process regulated by various genes. Therefore, we next evaluated the mechanisms involved in the regulation of apoptosis by catalpol in OGD-treated BMSCs. Bcl-2 is a well-known anti-apoptotic protein, while Bax is a pro-apoptotic protein of the Bcl-2 family [30]. Caspase-3 is an important mediator of apoptosis, which is dependent on or independent of mitochondrial apoptosis [31]. Our results showed that catalpol increased Bcl-2 level, whereas decreased Bax and cleaved caspase-3 levels in BMSCs under OGD condition. These results suggested that catalpol restrained OGD-induced apoptosis via up-regulating Bcl-2/Bax ratio and inactivation of caspase-3 pathway.
Cardiac fibrosis is an important characteristic of MI, and can cause a decrease in left ventricular function or more severe heart failure [32]. So cardiac fibrosis plays crucial roles in the development of MI. Myocardial apoptosis is present in the infarct area after MI, which further exacerbates the unfavorable left ventricular remodeling [33]. Thus, effective suppression of myocardial apoptosis can reduce the infarct size and improve cardiac function. In this study, cardiac fibrosis and apoptosis after MI were further repressed by catalpol-treated BMSCs transplantation. In addition, catalpol-treated BMSCs transplantation has much better effects on improving cardiac function as evidenced by increase in LVEF and LVFS, while decrease in LVESD and LVEDD.
Angiogenesis plays pivotal roles in delivering nutrients and oxygen to the ischemic myocardium after MI. It has been indicated that BMSCs transplantation may improve blood infusion of the heart muscle via promoting angiogenesis [34]. Paracrine mechanism is involved in the beneficial role of BMSCs transplantation in MI. Many angiogenic cytokines, such as VEGF, can be secreted by BMSCs. VEGF has been recognized to promote angiogenesis. Beyond that, pro-survival potential of VEGF has also been verified [35]. In stroke model, catalpol could improve infarcted-brain angiogenesis and stimulate VEGF expression [16, 36]. According to the present study, the secretion of VEGF was enhanced by catalpol in OGD-treated BMSCs in vitro. Moreover, catalpol-pretreated BMSCs transplantation facilitated angiogenesis and VEGF expression in the infracted myocardium. From the above results, catalpol contributed to the efficacy of BMSCs transplantation via promoting VEGF-mediated angiogenesis in a paracrine fashion.
In conclusion, our results suggest that catalpol contributes to the survival and VEGF secretion of BMSCs and therefore enhances the therapeutic effect of BMSCs transplantation in MI. Although further experiments are needed to elucidate the detailed mechanisms, our study provides evidence for better efficacy of BMSCs pre-treated with catalpol to protect against MI.
References
Wu, Z., Chen, G., Zhang, J., Hua, Y., Li, J., Liu, B., Huang, A., Li, H., Chen, M., & Ou, C. (2017). Treatment of myocardial infarction with gene-modified mesenchymal stem cells in a small molecular hydrogel. Scientific Reports, 7, 15826.
Deluyker, D., Ferferieva, V., Driesen, R. B., Verboven, M., Lambrichts, I., & Bito, V. (2017). Pyridoxamine improves survival and limits cardiac dysfunction after MI. Scientific Reports, 7, 16010.
Elmadbouh, I., & Ashraf, M. (2017). Tadalafil, a long acting phosphodiesterase inhibitor, promotes bone marrow stem cell survival and their homing into ischemic myocardium for cardiac repair. Physiological Reports 5, e13480.
Amado, L. C., Saliaris, A. P., Schuleri, K. H., St John, M., Xie, J. S., Cattaneo, S., Durand, D. J., Fitton, T., Kuang, J. Q., Stewart, G., Lehrke, S., Baumgartner, W. W., Martin, B. J., Heldman, A. W., & Hare, J. M. (2005). Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America, 102, 11474–11479.
Zhang, G. W., Gu, T. X., Guan, X. Y., Sun, X. J., Qi, X., Li, X. Y., Wang, X. B., Lv, F., Yu, L., Jiang, D. Q., & Tang, R. (2015). HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Proliferation, 48, 661–670.
Gao, L. R., Pei, X. T., Ding, Q. A., Chen, Y., Zhang, N. K., Chen, H. Y., Wang, Z. G., Wang, Y. F., Zhu, Z. M., Li, T. C., Liu, H. L., Tong, Z. C., Yang, Y., Nan, X., Guo, F., Shen, J. L., Shen, Y. H., Zhang, J. J., Fei, Y. X., Xu, H. T., Wang, L. H., Tian, H. T., & Liu, D. Q. (2013). A critical challenge: Dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. International Journal of Cardiology, 168, 3191–3199.
Zhang, H., Hou, J. F., Shen, Y., Wang, W., Wei, Y. J., & Hu, S. (2010). Low level laser irradiation precondition to create friendly milieu of infarcted myocardium and enhance early survival of transplanted bone marrow cells. Journal of Cellular and Molecular Medicine, 14, 1975–1987.
Gao, X. R., Xu, H. J., Wang, L. F., Liu, C. B., & Yu, F. (2017). Mesenchymal stem cell transplantation carried in SVVYGLR modified self-assembling peptide promoted cardiac repair and angiogenesis after myocardial infarction. Biochemical and Biophysical Research Communications, 491, 112–118.
Carmeliet, P. (2005). Angiogenesis in life, disease and medicine. Nature, 438, 932–936.
Xia, J. B., Wu, H. Y., Lai, B. L., Zheng, L., Zhou, D. C., Chang, Z. S., Mao, C. Z., Liu, G. H., Park, K. S., Zhao, H., Kim, S. K., Song, G. H., Cai, D. Q., & Qi, X. F. (2017). Gene delivery of hypoxia-inducible VEGF targeting collagen effectively improves cardiac function after myocardial infarction. Scientific Reports, 7, 13273.
Gao, F., He, T., Wang, H., Yu, S., Yi, D., Liu, W., & Cai, Z. (2007). A promising strategy for the treatment of ischemic heart disease: Mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Canadian Journal of Cardiology, 23, 891–898.
Shieh, J. P., Cheng, K. C., Chung, H. H., Kerh, Y. F., Yeh, C. H., & Cheng, J. T. (2011). Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry, 59, 3747–3753.
Zhang, X., Zhang, A., Jiang, B., Bao, Y., Wang, J., & An, L. (2008). Further pharmacological evidence of the neuroprotective effect of catalpol from Rehmannia glutinosa. Phytomedicine, 15, 484–490.
Shen, C. Y., Jiang, J. G., Yang, L., Wang, D. W., & Zhu, W. (2017). Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. British Journal of Pharmacology, 174, 1395–1425.
Wang, L., & Xue, G. B. (2018). Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochemical and Biophysical Research Communications, 495, 27–34.
Dong, W., Xian, Y., Yuan, W., Huifeng, Z., Tao, W., Zhiqiang, L., Shan, F., Ya, F., Hongli, W., Jinghuan, W., Lei, Q., Li, Z., & Hongyi, Q. (2016). Catalpol stimulates VEGF production via the JAK2/STAT3 pathway to improve angiogenesis in rats’ stroke model. Journal of Ethnopharmacology, 191, 169–179.
Hu, L. A., Sun, Y. K., Zhang, H. S., Zhang, J. G., & Hu, J. (2016). Catalpol inhibits apoptosis in hydrogen peroxide-induced cardiac myocytes through a mitochondrial-dependent caspase pathway. Bioscience Reports 36, e00348.
Chen, W., Li, X., Jia, L. Q., Wang, J., Zhang, L., Hou, D., & Ren, L. (2013). Neuroprotective activities of catalpol against CaMKII-dependent apoptosis induced by LPS in PC12 cells. British Journal of Pharmacology, 169, 1140–1152.
Li, L., Guan, Q., Dai, S., Wei, W., & Zhang, Y. (2017). Integrin beta1 increases stem cell survival and cardiac function after myocardial infarction. Frontiers in Pharmacology, 8, 135.
Minguell, J. J., & Erices, A. (2006). Mesenchymal stem cells and the treatment of cardiac disease. Experimental Biology and Medicine, 231, 39–49.
Schuleri, K. H., Feigenbaum, G. S., Centola, M., Weiss, E. S., Zimmet, J. M., Turney, J., Kellner, J., Zviman, M. M., Hatzistergos, K. E., Detrick, B., Conte, J. V., McNiece, I., Steenbergen, C., Lardo, A. C., & Hare, J. M. (2009). Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. European Heart Journal, 30, 2722–2732.
Liu, X., Hou, J., Shi, L., Chen, J., Sang, J., Hu, S., Cong, X., & Chen, X. (2009). Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells and Development, 18, 947–954.
Khan, I., Ali, A., Akhter, M. A., Naeem, N., Chotani, M. A., Mustafa, T., & Salim, A. (2016). Preconditioning of mesenchymal stem cells with 2,4-dinitrophenol improves cardiac function in infarcted rats. Life Sciences, 162, 60–69.
Meng, X., Li, J., Yu, M., Yang, J., Zheng, M., Zhang, J., Sun, C., Liang, H., & Liu, L. (2018). Transplantation of mesenchymal stem cells overexpressing IL10 attenuates cardiac impairments in rats with myocardial infarction. Journal of Cellular Physiology, 233, 587–595.
Kim, H. W., Haider, H. K., Jiang, S., & Ashraf, M. (2009). Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. Journal of Biological Chemistry, 284, 33161–33168.
Wang, Z., An, L. J., Duan, Y. L., Li, Y. C., & Jiang, B. (2008). Catalpol protects rat pheochromocytoma cells against oxygen and glucose deprivation-induced injury. Neurological Research, 30, 106–112.
Hu, L., Sun, Y., & Hu, J. (2010). Catalpol inhibits apoptosis in hydrogen peroxide-induced endothelium by activating the PI3K/Akt signaling pathway and modulating expression of Bcl-2 and Bax. European Journal of Pharmacology, 628, 155–163.
Chen, C., Chen, Z., Xu, F., Zhu, C., Fang, F., Shu, S., Li, M., & Ling, C. (2013). Radio-protective effect of catalpol in cultured cells and mice. Journal of Radiation Research, 54, 76–82.
Cai, Q., Yao, Z., & Li, H. (2014). Catalpol promotes oligodendrocyte survival and oligodendrocyte progenitor differentiation via the Akt signaling pathway in rats with chronic cerebral hypoperfusion. Brain Research, 1560, 27–35.
Labi, V., Grespi, F., Baumgartner, F., & Villunger, A. (2008). Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: A breakthrough in anticancer therapy? Cell Death & Differentiation, 15, 977–987.
Porter, A. G., & Janicke, R. U. (1999). Emerging roles of caspase-3 in apoptosis. Cell Death & Differentiation, 6, 99–104.
Cohn, J. N., Ferrari, R., & Sharpe, N. (2000). Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. Journal of the American College of Cardiology, 35, 569–582.
Abbate, A., Biondi-Zoccai, G. G., Bussani, R., Dobrina, A., Camilot, D., Feroce, F., Rossiello, R., Baldi, F., Silvestri, F., Biasucci, L. M., & Baldi, A. (2003). Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. Journal of the American College of Cardiology, 41, 753–760.
Rahbarghazi, R., Nassiri, S. M., Ahmadi, S. H., Mohammadi, E., Rabbani, S., Araghi, A., & Hosseinkhani, H. (2014). Dynamic induction of pro-angiogenic milieu after transplantation of marrow-derived mesenchymal stem cells in experimental myocardial infarction. International Journal of Cardiology, 173, 453–466.
Nor, J. E., Christensen, J., Mooney, D. J., & Polverini, P. J. (1999). Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. The American Journal of Pathology, 154, 375–384.
Zhu, H. F., Wan, D., Luo, Y., Zhou, J. L., Chen, L., & Xu, X. Y. (2010). Catalpol increases brain angiogenesis and up-regulates VEGF and EPO in the rat after permanent middle cerebral artery occlusion. International Journal of Biological Sciences, 6, 443–453.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no competing financial interest for this work.
Additional information
Handling Editor: Gen Suzuki
Rights and permissions
About this article
Cite this article
Ju, X., Xue, D., Wang, T. et al. Catalpol Promotes the Survival and VEGF Secretion of Bone Marrow-Derived Stem Cells and Their Role in Myocardial Repair After Myocardial Infarction in Rats. Cardiovasc Toxicol 18, 471–481 (2018). https://doi.org/10.1007/s12012-018-9460-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-018-9460-4