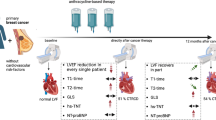
Abstract
Anthracyclines are active drugs against breast cancer, but can exert cardiotoxic effects. We analyzed the association between the kinetics of various biomarkers during chemotherapy, and the risk of subsequent cardiac toxicity. 50 patients (49 women) with early breast cancer surgically treated and eligible to anthracycline-based adjuvant chemotherapy were analyzed. The left ventricular ejection fraction (LVEF) together with the plasma concentration of several blood markers was measured at the beginning of anthracycline chemotherapy (t 0), 5 months (t 1), 16 months (t 2), 28 months (t 3), and 40 months later (t 4). A single measured LVEF value less than 50% or a clinically overt congestive heart failure (CHF) was considered cardiotoxic effects. We tested whether the kinetics of LVEF and blood biomarkers measured during chemotherapy was predictive of subsequent cardiotoxicity and overall cardiac fitness. The left ventricular ejection fraction measured at the end of treatment as well as the rate of change of hemoglobin concentration during anthracycline-based chemotherapy predicted cardiotoxicity in a 3-year follow-up period. When LVEF at the end of chemotherapy was lower than 53% or hemoglobin blood concentration declined more than 0.33 g/dL/month during chemotherapy, the odds ratio of subsequent cardiotoxicity was 37.3 and 18, respectively. The specificity of these two tests was 93.3% and 80%, whereas the sensitivity was 90.9 and 81.2%, respectively. Testing the rate of change of hemoglobin concentration during anthracycline-based chemotherapy, as well as the left ventricular ejection fraction at the end of treatment, seems a powerful method to assess the effects of anthracyclines on cardiac fitness and identify patients at high risk of CHF. Further validation of these tests on a large cohort of patients and cost-benefit analysis should be encouraged.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Anthracyclines alone or in combination with other drugs are among the most active therapy and the most widely used agents for the treatment of breast cancer in adjuvant and metastatic settings. In the adjuvant setting, the EBCTCG [1] suggested a definite survival advantages after using these drugs. Notwithstanding their advantages, there is a concern about the potential development of cardiotoxicity, which can culminate in congestive heart failure (CHF) [2]. The overall risk of conventional anthracycline-induced cardiotoxicity is related to cumulative dose [3–5]. Moreover, doxorubicin-related CHF risk is schedule dependent, with lower incidence with schedules based on one administration per week compared to every 3 weeks [3]. The development of an overt CHF is more common within the first year after the discontinuation of therapy, but sometimes a longer time elapses. Identification of patients more prone to develop chronic cardiac damage is the primary object of medical oncologists. The early diagnosis of cardiac damage, in fact, might permit a more aggressive control of cardiac function, a different choice of antitumor agents, and, finally, a cardiologic support therapy. The most common way to study anthracycline potential cardiotoxicity is the evaluation of the left ventricular ejection fraction (LVEF) using radionuclide ventriculography (MUGA) or echocardiography. Considering that the LVEF obtained by MUGA is more accurate and less dependent by the operator, serial radionuclide LVEF monitoring has been advised in order to monitor cardiotoxicity [6, 7]. We have previously reported that in breast cancer patients treated with anthracyclines, the plasma levels of troponin I and brain natriuretic peptide (BNP) rise during the course of chemotherapy and might be useful to identify patients at risk of cardiotoxicity [7]. Prompted by these observations, we decided to systematically analyze the kinetics of multiple blood markers together with LVEF in breast cancer patients receiving anthracycline-based adjuvant chemotherapy evaluating their predictive value.
Methods
Patients
Patients with early breast cancer and candidate to adjuvant chemotherapy were included into a prospective evaluation of cardiac function. All patients underwent radical surgery, i.e., mastectomy or breast-conserving treatment, had no evidence of distant metastases, as well as an adequate bone marrow, renal, and hepatic function. The presence of coronary artery disease, valvular disease, and pre-treatment left ventricular dysfunction (LVEF < 50%) was considered exclusion criteria. The chemotherapy regimen consisted of 6 cycles of cyclophosphamide 600 mg/m2, epirubicin 90 mg/m2, and fluorouracil 600 mg/m2 all given intravenously on day one every 21 days. Locoregional radiotherapy was delivered at the end of the chemotherapy plan, if indicated. Before starting chemotherapy (t 0), all patients underwent a cardiac assessment consisting of ECG, LVEF measurement at rest by radionuclide angiocardiography, hemocytometry, and measurement of the blood biomarkers listed below. All the above measurements were repeated at 5 months (t 1), 16 months (t 2), 28 months (t 3), and 40 months (t 4) after the start of the chemotherapy plan, together with a complete clinical examination performed by a cardiologist. A cardiotoxic effect was defined as the development of congestive heart failure (orthopnea or dyspnoea on exertion, paroxysmal dyspnoea, swelling of legs or abdomen, basilar pulmonary rales, and S3 gallop) or a decline of LVEF below 50% at any time point during the 3-year follow-up. An informed consent was obtained from each patient enrolled in the study. The study protocol was conformed to the ethical guidelines of the Declaration of Helsinki. This manuscript has been prepared according to the principles of Ethical Publishing in Medicine.
Radionuclide Angiocardiography
The scan was obtained as a routine MUGA study (24 frames/cycle, 64 × 64 matrix size, word mode) with a GE Starcam with a small field-of-view (30 cm) in the left anterior oblique position aiming at optimal separation of right and left ventricles, often with a slight caudal tilt, after the intravenous injection of 11–13 MBq/kg of Tc-labeled human serum albumin. The presence of cardiac arrhythmias was accepted when regarded less than 10% of total cardiac beats [8]. LVEF less than 50% was classified abnormal. The confidence interval of a single measurement of LVEF by MUGA is ±5% in our laboratory.
Blood Biomarkers
BNP
Whole blood samples were collected by venous puncture and immediately analyzed with the bedside Triage B-type natriuretic fluorescence immunoassay (Biosite Diagnostics, La Jolla, CA, USA). The Triage Meter is used to measure the BNP concentration by detecting a fluorescent emission that reproduces the amount of BNP in the blood. The assay results were complete in 20 min. Performance characteristics of the test are as follows: Assay range 5–5,000 pg/mL; Total CV 9.2–11.4%.
Troponin I
Heparinized plasma samples were collected by venous puncture and immediately analyzed with the Stratus CS cTnI fluorescence immunoassay (Dade Behring Limited, Milton Keynes, UK). The assay results were complete in 20 min. Performance characteristics of the test are as follows: Assay range 0.03–50 ng/mL; Total CV 3.4–8.2%.
Aldosterone
Serum samples were collected by venous puncture and stored at −20°C until measurement. Aldosterone was measured by a competitive radioimmunometric assay (Aldosterone—RIA, IMMUNOTECH SA—Beckman Coulter—Marseille, France). Performance characteristics of the test are as follows: Assay range 6.0–2,000 pg/mL; Total CV 9.5–9.9%.
Catecholamines
Serum samples were collected by venous puncture and stored at −20°C until measurement process. Catecholamines were measured by an HPLC method (Plasma catecholamines, Deutschland Bio-RadLaboratories GmbH, Muenchen, Germany; electrochemical detection). Performance characteristics of the test are as follows: Norepinephrine: Assay range 0.0–2,700 ng/L; Total CV 3.32–3.58%; Epinephrine: Assay range 0.0–3,000 ng/L; Total CV 3.24–5.75%; Dopamine: Assay range 0.0–1,100 ng/L; Total CV 8.62–10.79%.
Statistical Analyses
Change rates (〈ΔLVEF/Δt〉, ΔLVEF/(t 1–t 0), Δhgb/(t 1–t 0), and Δtropo/(t 1–t 0)) were calculated using Excel 2007. Areas under each LVEF curve in Suppl. Fig. 1 were calculated using GraphPad Prism 4. The means of continuous variable distributions were compared using the unpaired t test for either equal or unequal variances, after comparing variances. A P-value <0.05 was considered significant; t tests, as well as logistic regression and ROC curve analysis, were run in Stata/SE 11.1. All graphs were prepared using GraphPad Prism 4.
LVEF kinetics in the 41 cases in which LVEF was measured at each time point during follow-up. a Box plots of LVEF values at each time point during follow-up, separately for patients experiencing (left bars) or not (right bars) cardiotoxicity. For each plot, inter-quartile range, median, and extreme values are shown. P-values summarize the results of unpaired, non-parametric Mann–Whitney tests comparing the distributions indicated by horizontal square brackets. b Average fractional decay of LVEF from baseline (t 0) in patients without cardiotoxicity following anthracycline-based chemotherapy (upper curve) in comparison with patients for whom at least one LVEF value between t 1 and t 4 was lower than 50% (lower curve). For every case, the value of LVEF at each time point was normalized to the corresponding LVEF value at t 0
Results
Clinical Characteristics and LVEF Kinetics
50 patients (49 women and 1 man) were enrolled in the study and followed for 3 years after the end of chemotherapy. General patient characteristics are listed in Table 1. In 41 patients, LVEF could be measured at baseline as well as at every follow-up time point (t 1–t 4). Thus, all the following analyses were conducted on this group unless otherwise specified. In 11 patients (11/41 = 26.8%), the value of LVEF decreased below 50% at some point, a sign of overt cardiotoxicity. In this group, the average measured LVEF was significantly lower during the entire follow-up as compared to the group not experiencing cardiotoxicity (Fig. 1a). On average, in the group experiencing cardiotoxicity, LVEF quickly dropped down to approx. 85% of its baseline value during chemotherapy and never reached baseline again during the period of observation (Fig. 1b). In one case (patient 10), LVEF quickly dropped during chemotherapy, staying lower than 50% for the remaining time. In other cases, LVEF descended below 50% at t 1 or t 2 (patients 14, 33, 35, 40, and 48), but then climbed above 50% (Suppl. fig. 1). In 2 patients (2/41 = 4.9%), an overt congestive heart failure developed and was treated with the appropriate drugs.
In order to quantitatively describe the kinetics of LVEF, we computed the area under the LVEF curve (AUCLVEF), assuming that this is a good proxy for the left ventricular performance over the entire follow-up period. The average AUCLVEF value was significantly lower in patients experiencing cardiotoxicity (21.08 vs. 24.25, t test with equal variances: P-value < 0.0001). Similarly, the average LVEF value in the period t 1−t 4 was significantly lower in patients with cardiotoxicity (0.52 vs. 0.61, t test with equal variances: P-value <0.0001). Next, we calculated the average LVEF change rate (ΔLVEF/Δt) in the interval t 0–t 4 by averaging the change rate of LVEF in each subsequent time interval (t 0–t 1, t 1–t 2, t 2–t 3, and t 3–t 4). On average, LVEF tended to drop during the follow-up period, but the decline occurred almost five time faster in patients experiencing cardiotoxicity (−0.49% per month vs. −0.1% per month, t test with equal variances: P-value = 0.0007). We excluded that this was due to a difference in basal LVEF, as patients developing cardiotoxicity had basal LVEF values similar to unaffected individuals (60% vs. 63%, t test with equal variances: P-value = 0.25).
Kinetics of Blood Biomarkers
We then examined the trend of several blood markers during the course of the study. Average plasma hemoglobin levels significantly declined at t 1, renormalized at t 2, and stayed normal until the end of the study. Average white blood cells (WBC) and platelet counts also decreased at t 1, then WBC counts normalized by t 4, whereas platelets counts stayed low. In contrast, average plasma troponin I levels increased tenfold between t 0 and t 1, renormalized at t 2, and remained normal until the end of the study. Average plasma BNP levels significantly increased at t 1 and stayed high until t 4. Average plasma dopamine levels significantly increased at t 3 and further at t 4. Average levels of epinephrine decreased at t 1 remaining low until t 4. In contrast, levels of norepinephrine and aldosterone on average did not significantly change during the course of the entire study (Suppl. fig. 2).
Biomarkers Kinetics During Chemotherapy Associates with Future Cardiotoxicity
We next examined whether the kinetics of the above biomarkers during chemotherapy associates with cardiotoxicity. First, we studied the kinetics of LVEF during the course of chemotherapy [ΔLVEF/(t 1–t 0)]. On average, the rate of change of LVEF was negative (−0.81% per month), but the LVEF decline occurred 5.4 times faster (−2% per month vs. −0.37% per month, t test with equal variances: P-value = 0.001) in patients experiencing cardiotoxicity later on. Moreover, the value of LVEF at t 1 (LVEF1) was on average lower in patients experiencing cardiotoxicity at some point between t 1 and t 4 (50.2% vs. 60.8%, t test with equal variances: P-value <0.0001). This indicates that a rapid drop of LVEF during anthracycline-based chemotherapy might signal a high risk of subsequent cardiotoxicity.
We next tested whether also the kinetics of blood biomarkers that change during chemotherapy (hemoglobin, WBC, platelets, troponin I, BNP, and epinephrine) is associated with cardiotoxicity. During chemotherapy, hemoglobin levels declined at significantly higher speed in patients experiencing cardiotoxicity at some point during t 1–t 4 (Δhgb/(t 1–t 0) = −0.39 g/dL/month vs. −0.17 g/dL/month, t test with unequal variances: P-value = 0.0038)(Fig. 2a). The speed at which troponin I levels rose during chemotherapy was also higher in patients with cardiotoxicity (Δtropo/(t 1–t 0) = 16.77 ng/L/month vs. 9.63 ng/L/month, t test with equal variances: P-value = 0.038)(Fig. 2b). In contrast, the kinetics of WBC, platelets, and BNP levels during t 1–t 4 was the same irrespective of cardiotoxicity. The linear relationship between ΔLVEF/(t 1–t 0) and Δhgb/(t 1–t 0) or Δtropo/(t 1–t 0) is depicted in Fig. 2c, d.
Blood biomarkers kinetics and its association with LVEF kinetics. a Average fractional decay of hemoglobin from baseline (t 0), in patients without cardiotoxicity following anthracycline-based chemotherapy (upper curve) in comparison with patients for whom at least one LVEF value between t 1 and t 4 was lower than 50% (lower curve). For every case, the concentration of hemoglobin in the blood at each time point was normalized to the corresponding value at t 0. b Average troponin concentration in the blood of patients without cardiotoxicity following anthracycline-based chemotherapy (lower curve) in comparison with patients for whom at least one LVEF value between t 1 and t 4 was lower than 50% (upper curve). c, d Scatter plots of LVEF speed versus hemoglobin (c) or troponin I (d) speed during chemotherapy (t 1–t 0). The dashed line represents the best linear fit to the data. The slope of both fits is significantly different than 0 (c P-value = 0.013; d P-value = 0.02), indicating an association between each variable pair. r 2 squared Pearson’s correlation coefficient. Hgb hemoglobin. Tropo troponin I
To corroborate these findings, we performed logistic regression using cardiotoxicity as the binary outcome. The value of LVEF measured at the end of chemotherapy was the best predictor of future cardiotoxicity (P-value = 0.006), followed by the change rate of LVEF (P-value = 0.007), hemoglobin (P-value = 0.025), and troponin I (P-value = 0.054) during chemotherapy. LVEF1 and the kinetics of LVEF during chemotherapy remained the best predictors of cardiotoxicity (P-value = 0.002) even after including in the analysis the 9 patients in which LVEF could not be measured at each time point during follow-up. In addition, upon linear regression analysis, we found that the two latter parameters significantly co-varied with AUCLVEF and ΔLVEF/Δt, which can serve as proxy of left ventricular performance during the entire follow-up.
Specificity and Sensitivity of Biomarker Kinetics Testing During Chemotherapy
To evaluate the predictive power, we generated receiver operating characteristic (ROC) curves for each of the biomarker found to be significantly associated with cardiotoxicity. LVEF1 gave the highest area under the ROC curve (0.95), followed by Δhgb/(t 1–t 0) (0.81), ΔLVEF/(t 1–t 0) (0.8), and Δtropo/(t 1–t 0) (0.69) (Fig. 3). These values did not significantly change even after including in the analysis the 9 patients in which LVEF could not be measured at each time point during follow-up. The specificity and sensitivity of LVEF1 test were 93.3 and 90.9%, respectively, at the best cut-off value of 53%. For Δhgb/(t 1–t 0), specificity and sensitivity were 80 and 81.2%, respectively, at the best cut-off value of −0.33 g/dL/month. Using these cut-offs, the odds ratio of cardiotoxicity was 37.3 for LVEF1 and 18 for Δhgb/(t 1–t 0).
Discussion
In this study, we have measured the left ventricular ejection fraction (LVEF) before and after chemotherapy in breast cancer patients undergoing epirubicin treatment, demonstrating that LVEF tends to drop during the follow-up period, but the decline occurs almost five time faster in patients experiencing cardiotoxicity (−0.49% per month vs. −0.1% per month, t test with equal variances: P-value = 0.0007). Overall, our results indicate that the kinetics of the left ventricular ejection fraction after anthracycline-based chemotherapy is reflective of the risk of developing cardiac failure in the follow-up, and this observation might help in the decision to treat patients who undergo anthracycline chemotherapy with protective drugs. In the last 5 years, beta-blockers [9, 10], bosentan [11], and enalapril [12] have been used to prevent the cardiotoxicity induced by anthracycline. These efforts in understanding what drug will be protective for the anthracycline-induced cardiotoxicity might be helped by a better prediction of chronic cardiac damage. However, Cardinale et al. [13, 14] have recently demonstrated that treatment of heart failure (carvedilol and enalapril) induced by anthracycline chemotherapy should be started early after the detection of ventricular dysfunction because delaying treatment reduces the possibility of restoring left ventricular function. In these studies, LVEF measured by echocardiography or MUGA was poor in predicting the development of cardiac dysfunction. It was suggested that, when the left ventricle systolic function reduces, cardiac damage through myocytes necrosis has already occurred, so that therapy may only help to reduce the evolution of cardiac remodeling [14].
In our study, the analysis of plasma biomarkers demonstrated that the decline of hemoglobin during chemotherapy occurs significantly faster in patients experiencing cardiotoxicity at some point during t 1–t 4 as well as the rise in troponin I levels higher during t 1–t 0. The rise in plasma level of troponin (I or T) after chemotherapy was observed in previous experiences [15–17] in which the magnitude of elevation of the biomarkers predicted left ventricular dilatation and wall thinning [15] or left ventricular dysfunction at 3-year follow-up [16, 17]. The rise in troponin I early after anthracycline chemotherapy seemed to be very common, but the persistence of plasma elevation might be correlated to cardiac dysfunction in the follow-up [18]. Noteworthy, the absence of a significant plasma release of troponin I identified a favorable outcome. In our kinetics analysis, the speed at which troponin I levels rose during chemotherapy was higher in patients with cardiotoxicity (Δtropo/(t 1–t 0) = 16.77 ng/L/month vs. 9.63 ng/L/month, P-value = 0.038), and the faster was the elevation the worse was the left ventricular function. During chemotherapy, hemoglobin levels declined at significantly higher speed in patients experiencing cardiotoxicity (Δhgb/(t 1–t 0) = −0.39 g/dL/month vs. −0.17 g/dL/month, P-value = 0.0038). The decline of hemoglobin is more difficult to be explained. Anthracyclines have an intrinsic hemolyzing effect on red cells [19]. Furthermore, doxorubicin or its alcohol metabolite doxorubicinol may negatively affect red blood cell function and integrity [20]. Although aldosterone was dosed in patients with congestive heart failure as a prognostic marker [21], this experience failed to demonstrate a correlation with cardiotoxicity induced by anthracycline at 3-year follow-up. The reduction in average plasma epinephrine levels at t 1 that remained low until t 4 confirmed our previous experience [8] obtained at 2-year follow-up. Nevertheless, Suzuki et al. [22] did not observe significant modification in plasma level of epinephrine and norepinephrine in 27 patients treated with anthracycline. The persistent reduction in epinephrine is not easily explainable and might be related to a direct effect of the drugs.
Our experience indicates that measuring the left ventricular ejection fraction together with hemoglobin and troponin I at the beginning and end of anthracycline-based breast cancer chemotherapy and calculating their kinetics might be a powerful approach to predict subsequent cardiotoxicity (3-year follow-up). In particular, a patient profile including a decline of LVEF (<−2%/month), a reduction of hemoglobin (<−0.39 g/dL/month), and rising troponin I levels (>16.77 ng/L/month) during epirubicin chemotherapy identifies a high risk for developing cardiac dysfunction at 3-year follow-up. The prevention of the anthracycline-induced cardiotoxicity should be a joint goal for cardiologists and oncologists in the treatment of malignancy remains. The American College of Cardiology and the American Heart Association [23] recommend routine echocardiography in order to evaluate early cardiac abnormalities identifying a status of possible reversible cardiotoxic effect. Although some biochemical markers (above all plasma troponin and BNP) have been positively correlated with the development of cardiotoxicity, this study pointed out that the early kinetics of simple biological variables (LVEF evaluated with MUGA and hemoglobin) might be useful in the prediction of the myocardial damage after chemotherapy.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). (2005). Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet, 365, 1687–1717.
Singal, P. K., & Iliskovic, N. (1995). Doxorubicin-induced cardiomyopathy. New England Journal of Medicine, 332, 1738–1743.
Von Hoff, D., Layard, M., Basa, P., Davis, H. L., Von Hoff, H. L., Rozencweig, M., et al. (1979). Risk factors for doxorubicin-induced congestive heart failure. Annals of Internal Medicine, 91, 710–717.
Ryberg, M., Nielsen, D., Skovsgaard, T., Hansen, J., Jensen, B. V., Dombernowsky, P. (1998). Epirubicin cardiotoxicity: An analysis of 469 patients with metastatic breast cancer. Journal of Clinical Oncology, 16, 3502–3508.
Praga, C., Trave, F., & Petroccione, A. (1991). Anthracycline-induced cardiotoxicity and its relevance in cancer treatment. In W. S. Nimmo & G. T. Tucker (Eds.), Clinical measurement in drug evaluation (pp 131–142). Boca Raton, FL: CRC Press.
Ganz, W. I., Sridhar, K. S., Ganz, S. S., Gonzales, R., Chakko, S., Serafini, A. (1996). Review of tests for monitoring doxorubicin-induced cardiomyopathy. Oncology, 53, 461–470.
Mitani, I., Jain, D., Joska, T. M., Burtness, B., Zaret, B. L. (2003). Doxurubicin cardiotoxicity: Prevention of congestive heart failure with serial function monitoring with equilibrium radionuclide angiocardiography in the current era. Journal of Nuclear Cardiology, 10, 132–139.
Feola, M., Garrone, O., Occelli, M., Francini, A., Biggi, A., Visconti, G., et al. (2011). Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: Effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. International Journal of Cardiology, 148, 194–198.
Corbett, J. R., Akinboboye, O. O., Bacharach, S. L., Borer J. S., Botvinick, E. H., DePuey, E. G., et al. (2006). Equilibrium radionuclide angiocardiography. Journal of Nuclear Cardiology, 13, e56–e79.
De Nigris, F., Rienzo, M., Schiano, C., Fiorito, C., Casamassimi, A., Napoli, C. (2008). Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. European Journal of Cancer, 44, 334–340.
Kalay, N., Basar, E., Ozdogru, I., Er, O., Cetinkaya, Y., Dogan, A., et al. (2006). Protective effects of carvedilol against anthracycline-induced cardiomyopathy. Journal of the American College of Cardiology, 48, 2258–2262.
Bien, S., Riad, A., Ritter, C. A., Gratz, M., Olshausen, F., Westermann, D., et al. (2007). The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Research, 67, 10428–10435.
Cardinale, D., Colombo, A., Sandri, M. T., Lamantia, G., Colombo, N., Civelli, M., et al. (2006). Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation, 114, 2474–2481.
Cardinale, D., Colombo, A., Lamantia, G., Colombo, N., Civelli, M., De Gaicomi, G., et al. (2010). Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. Journal of the American College of Cardiology, 55, 213–220.
van Dalen, E. C., Caron, H. N., Dickinson, H. O., & Kremer, L. C. (2008). Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews (2), CD003917.
Lipshultz, S. E., Rifai, N., Sallan, S. E., Lipsitz, S. R., Dalton, V., Sacks, D. B., & Ottlinger, M. E. (1997). Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation, 96, 2641–2648.
Cardinale, D., Sandri, M. T., Martinoni, A., Borghini, E., Civelli, M., Lamantia, G., et al. (2002). Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Annals of Oncology, 13, 710–715.
Cardinale, D., Sandri, M. T., Colombo, A., Colombo, N., Boeri, M., Lamantia, G., et al. (2004). Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation, 109, 2749–2754.
Shinohara, K., & Tanaka, K. R. (1980). The effects of adriamycin (doxorubicin HCl) on human red blood cells. Hemoglobin, 4, 735–745.
Misiti, F., Giardina, B., Mordente, A., & Clementi, M. E. (2003). The secondary alcohol and aglycone metabolites of doxorubicin alter metabolism of human erythrocytes. Brazilian Journal of Medical and Biological Research, 36, 1643–1651.
Catena, C., Colussi, G., Marzano, L., & Sechi, L. A. (2011). Aldosterone and the heart: From basic research to clinical evidence. Hormone and Metabolic Research [Epub ahead of print].
Suzuki, T., Hayashi, D., Yamazaki, T., et al. (1998). Elevated B-type natriuretic peptide levels after anthracycline administration. American Heart Journal, 136, 362–363.
Cheitlin, M. D., Armstrong, W. F., Aurigemma, G. P., Beller, G. A., Biermon, F. Z., Davis, J. l., et al. (2003). ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: Summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 42, 954–970.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12012_2011_9149_MOESM1_ESM.tif
Supplementary figure 1: representative kinetic profiles of LVEF for 3 out of the 41 cases in which LVEF was measured at each time point during follow-up. Red lines indicate the 50% cut-off LVEF value. (TIFF 111 kb)
12012_2011_9149_MOESM2_ESM.tif
Supplementary figure 2: average kinetic profiles of several blood biomarkers. P-values summarize the results of unpaired t tests comparing the distributions indicated by horizontal square brackets. WBC: white blood cells count. (TIFF 182 kb)
Rights and permissions
About this article
Cite this article
Garrone, O., Crosetto, N., Lo Nigro, C. et al. Prediction of Anthracycline Cardiotoxicity after Chemotherapy by Biomarkers Kinetic Analysis. Cardiovasc Toxicol 12, 135–142 (2012). https://doi.org/10.1007/s12012-011-9149-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9149-4