Abstract
Alcohol exposure during pregnancy may cause congenital heart disease (CHD), but the underlying mechanisms are not clear. Recent evidence suggests that ethanol and its metabolites can selectively increase histone H3 acetylation at lysine 9 (H3AcK9) residue in rat hepatocytes. This may be a mechanism by which ethanol alters gene expression. The goal of current study is to investigate the effect of ethanol and its metabolites on H3AcK9 acetylation and the mRNA expression of heart development-related genes (GATA4, Mef2c, and Tbx5) in cardiac progenitor cells. We used mitochondrial activity (MTT) assay to assess the viability of cardiac progenitor cells. Western blotting and real-time PCR were employed to determine H3AcK9 acetylation and gene expression. Low levels of ethanol (50 mM), acetaldehyde (4 mM), and acetate (4 mM) had no effect on cell proliferation. However, high concentrations of ethanol (200 mM), acetaldehyde (12 mM), and acetate (16 mM) reduced cell viability by 30%, respectively (P < 0.05). Low levels of ethanol and acetate increased the acetylation of H3 lysine 9 by 2.4- and 2.2-fold, respectively (P < 0.05), but did not significantly change the expression of the heart development-related genes. High concentrations of ethanol and acetate increased H3 lysine 9 acetylation by 5.3- and 5.6-fold, respectively (P < 0.05). Moreover, high levels of ethanol and acetate significantly augmented the expression of GATA4 and Mef2c. Conversely, acetaldehyde (4 or 12 mM) had little effect on H3 lysine 9 acetylation or the expression of the heart development-related genes. Our studies demonstrate that high levels of ethanol or its metabolites induce H3AcK9 acetylation and impair cardiac progenitor cells. The altered histone H3 acetylation at lysine 9 has an important impact on the expression of the heart development-related genes, which may be one of the mechanisms underlying the alcohol-induced CHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CHD is one of the most common congenital malformations in children. However, the mechanisms responsible for it remain elusive. At present, the interactions between genes and environment are generally considered as the mechanism of CHD. However, the mode of gene–environment interactions is still unclear. A large number of studies have shown that epigenetic mechanisms, including DNA methylation and histone modification, are affected by the surrounding environmental factors [1, 2]. However, the epigenome consists of DNA methylation and histone modifications involved in controlling gene expression. Thus, epigenetic mechanisms provide a plausible link between environmental factors and gene expression. Environmental factors can cause adverse pattern of gene expression by establishing a wrong procedure of epigenetic which is called epimutation. Such mechanism would cause a lot of diseases such as cancer, senescence, and developmental disorder.
Alcohol has become widely accepted as a teratogen by the scientific community and the general public. Prenatal alcohol exposure is acknowledged as a cause of CHD. Burd et al. [3] analysis of all the published cases with fetal alcohol syndrome (FAS) before 2007 revealed that the proportion of CHD in patients with FAS accounted for 67%. Several studies showed that animal models of CHD could be produced by treatment of the experimental animals with alcohol [4, 5]. Generally speaking, researchers have approached the etiologies of alcohol teratogenesis from one or more of the following perspectives: genetic, biochemical, cellular, and morphological. For example, research has associated ethanol with reduced growth factor levels [6]; inhibition of such factors is likely to result in reduced cellular proliferation [7]. However, FAS cannot be understood as if it were a single localized insult on an otherwise normal organism. Instead, it must be approached as an emergent property of deregulated developmental pathways and interactions. The wide range of morphological and physiological abnormalities that have been associated with in utero alcohol exposure suggests that there is a high correlation between the primary insults at the molecular and cellular levels and the defects observed clinically. This, in turn, suggests that the etiology of FAS may be related to a potentially bewildering array of heterogeneity.
Cardiogenesis is a precise proceeding by sequential gene regulatory steps. At specific point during development, the heart development-related genes sequential activation and expression is the developmental biology basis for the formation of a perfect heart [8]. However, the accuracy of epigenetic in genome is necessary to regulate the transcription of heart development-related genes [9]. Many studies have shown that the balance of histone acetylation modification plays an extremely important role in the process of muscle formation and heart development by acting as a switch in regulating gene expression [10–12]. In our previous studies, we found that histone acetylation imbalance would affect the expression of heart development-related genes, leading to the obstruction of the differentiation of mesenchymal stem cells (MSCs) into cardiomyocytes [13, 14]. This prompts us to believe that imbalance of histone acetylation may be associated with CHD.
A growing body of evidence showed that ethanol and its metabolites can selectively increase acetylation of histone H3 at lysine 9, but have a negligible effect at lysine 14, 18, or 23 [15–18]. However, increased level of histone H3 acetylation at lysine 9 can be considered as a mechanism by which ethanol can alter gene expressions. Therefore, combined with our previous study, we hypothesized that histone acetylation imbalance and its potential to result in abnormal expression of heart development-related genes may associate with CHD caused by ethanol.
To resolve some of these issues, we select the cardiac progenitor cells as our study object. Next, we investigate the effect of ethanol and its metabolites on histone acetylation and measure heart development-related genes (GATA4, Mef2c, and Tbx5) mRNA levels in cardiac progenitor cells.
Materials and Methods
Culture and Treatment of Cardiac Progenitor Cells
The cardiac progenitor cells were favored by Molecular Oncology Laboratory at the University of Chicago Medical Center [19]. After recovery from nitrogen, the cardiac progenitor cells were cultured with Dulbecco’s modified Eagle medium (DMEM)/high glucose (Thermo, USA) containing 10% fetal bovine serum (FBS) (PAA, Austria). At 80% confluence, the cells were subcultured for 3 passages. We took the fourth generation of cardiac progenitor cells for our studies. According to our results of cytotoxicity test, we selected 50 mM ethanol, 4 mM acetaldehyde, 4 mM acetate (Kelong, China) which did not affect the viability of cells as low concentration groups and selected 200 mM ethanol, 12 mM acetaldehyde, 16 mM acetate which affected the viability about 30% as high concentration groups. The opening of flasks was covered with seal to avoid the evaporation of ethanol and its metabolites. The control group was cultured with DMEM/high glucose containing 10% FBS without adding drugs. At 24 h after the treatment mentioned earlier, the cells were prepared for subsequent experiments.
MTT Assay
Cell viability as well as mitochondrial activity was determined with a spectrophotometric assay using 3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) (Ameresco, USA). The dose of ethanol involved in our study was designed at 200, 100, and 50 mM, respectively. Meanwhile, the doses of acetaldehyde and acetate were both designed at 16, 12, 8, 4 mM. After 24-h treatment, MTT solution was added to the cells and incubated for 4 h at 37°C. This water-soluble tetrazolium salt is cleaved by the mitochondria of living cells to an insoluble purple formazan. Formazan was next dissolved with dimethylsulfoxide (DMSO), and optical density was measured at 490 nm with plate reader (Thermo, USA).
Western Blotting
Nuclear extracts were isolated from cells using nuclear extract kit (ActiveMotif, USA) according to manufacturer’s instructions. Protein concentrations were determined using a bicinchoninic acid (BCA) protein assay kit (Pierce, USA). Identical amounts (100 μg) of nuclear extracts were run on 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto polyvinylidene difluoride (PVDF) membrane. After blocking with 5% nonfat dried milk for 1.5 h, the membrane was incubated with primary antibody with the dilution of 1:1,000 for anti-H3AcK9, which was purchased from Abcam (ab61231, Britain). When incubating with specific acetylated lysine antibody, the same membrane was also incubated at the same time with β-actin at the dilution of 1:2,000 (Abcam, ab6276, Britain) overnight at 4°C. Membranes were incubated with horseradish-conjugated secondary antibody (both anti-mouse and anti-rabbit) (Zhong Shan-Golden Bridge, China) with the dilution of 1:2,000 for 1 h at room temperature. The horseradish peroxidase was detected by enhanced chemiluminescence (Pierce, USA). The bands were scanned and analyzed with Quantity One Version 4.4 software (Bio-Rad, USA). The western blotting experiments were repeated three times.
Quantitative Real-Time PCR Analysis
Quantitative RT-PCR assay was used to assess the transcriptional expression of heart development-related genes including GATA4, Mef2c, and Tbx5. Total RNAs were extracted from the cells using RNA extract kit (Bioteke, China) and reverse-transcribed (RT) with a kit from Takara (Takara, Japan) for quantitative RT-PCR assay using SYBR Green RealMasterMix kit (Tiangen, China) following the manufacturer’s protocols. β-Actin was used as the endogenous “house-keeping” gene to normalize the mRNA levels. The primer sequences were as follows: for GATA4: 5′-CCCTCCCGCACGATTTCT-3′ (upper) and 5′-AGAGGCCCAACTCGCTCAA-3′ (lower); for Mef2c:5′-GCGCAGGGAATGGATACGG-3′ (upper) and 5′-TGCCAGGTGGGATAAGACG-3′ (lower); for Tbx5 : 5′-CCAAAGACAGGTCTTGCGATTCG-3′ (upper) and 5′-TTCTCCTCCCTGCCTTGGTGAT-3′ (lower). The results were expressed as mean of 2−△△Ct ± standard deviation (SD).
Statistical Analysis
All data were expressed as mean ± SD and statistically analyzed by one-way ANOVA. Differences were considered statistically significant when P < 0.05.
Results
Cultured Cells Morphological Observations
The fourth generation of cardiac progenitor cells were adherent. The morphology showed different, such as short spindle, polygonal (Fig. 1).
Cytotoxicity Effect of Ethanol and Its Metabolites to Cardiac Progenitor Cells
We determined the cytotoxicity effect by MTT assay. The absorbance value indicated the cell viability. As shown in the Fig. 2a, the concentrations of ethanol at 200 mM and 100 mM inhibited cell viability (P < 0.01), which was about 31.5% in 200 mM. However, no change was observed when cells were treated with 50 mM (P > 0.05). In Fig. 2b, the concentrations of acetaldehyde at 16, 12, and 8 mM inhibited cell viability (P < 0.01), which was about 36% in 12 mM. No change was observed when cells were treated with 4 mM (P > 0.05). In Fig. 2c, the concentrations of acetate at 16 mM and 12 mM inhibited cell viability (P < 0.05) around 32.1% in 16 mM, but no change was observed when cells were treated with 8 and 4 mM of acetaldehyde (P > 0.05).
Effect of different concentrations of ethanol and its metabolites on cardiac progenitor cells mitochondrial activity. Cardiac progenitor cells were treated with 50, 100, 200 mM ethanol (a), 4, 8, 12, 16 mM acetaldehyde (b) and 4, 8, 12, 16 mM acetate (c) for 24 h. After treatment, mitochondrial activity was assessed using the MTT assay (see “Materials and Methods”). Data represent mean ± SD, n = 3 experiments. ** P < 0.01, * P < 0.05 when compared with control
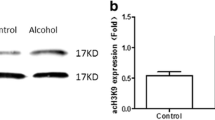
Effect of Ethanol and Its Metabolites on Histone H3 Acetylation at Lysine 9 in Cardiac Progenitor Cells
We analyzed the effect of ethanol and its metabolites on histone H3 acetylation at lysine 9 in cardiac progenitor cells by western blotting. The ratio of H3AcK9 to β-actin was determined in order to normalize sample recovery and loading. As shown in Fig. 3a, low concentration groups of 50 mM ethanol and 4 mM acetate, respectively, increased 2.4-, 2.2-fold acetylation of H3 at lysine 9 in cardiac progenitor cells when compared with control (P < 0.05). In Fig. 3b, the results showed that high concentration groups of 200 mM ethanol and 16 mM acetate, respectively, increased 5.3-, 5.6-fold acetylation of H3 at lysine 9 in cardiac progenitor cells (P < 0.05). However, whether the concentration of acetaldehyde was 4 or 12 mM, all had a negligible effect on the acetylation of H3 at lysine 9 in cardiac progenitor cells when compared with control (P > 0.05).
Effect of ethanol and its metabolites on histone H3 acetylation at lysine 9 by western blotting. Cardiac progenitor cells were treated with low concentration groups of 50 mM ethanol(e), 4 mM acetaldehyde(ald), 4 mM acetate(ate) (a) and high concentration groups of 200 mM ethanol, 4 mM acetaldehyde, 4 mM acetate (b) for 24 h. The control was treated without drugs. The experiments were performed as described in “Materials and Methods”. Quantitative analysis of acetylated histone H3 was performed by densitometric analysis and was presented as mean ± SD, n = 3 experiments. Values represent ratio of acetylated H3 lysine 9 and β-actin. * P < 0.05 when compared with control
Effect of Ethanol and Its Metabolites on mRNA Expression of Heart Development-Related Genes in Cardiac Progenitor Cells
The levels of transcriptional expression (mRNA) of heart development-related genes GATA4, Mef2c, and Tbx5 were examined by real-time PCR in cardiac progenitor cells. As shown in Fig. 4a, 50 mM ethanol had no significant effect on expression of heart development-related genes GATA4 (0.89 ± 0.06), Mef2c (1.14 ± 0.06), and Tbx5 (0.87 ± 0.16) when compared with control (P > 0.05), but 200 mM ethanol increased their expression levels of GATA4 (1.77 ± 0.17), Mef2c (3.30 ± 0.47), and Tbx5 (2.12 ± 0.50) compared to control or 50 mM ethanol (P < 0.01). In Fig. 4b, we found that whether at 4 or 12 mM concentrations, acetaldehyde had a negligible effect on the expression of GATA4, Mef2c, and Tbx5 (P > 0.05). In Fig. 4c, 4 mM acetate had no significant effect on the expression of GATA4 (0.85 ± 0.05) and Mef2c (0.78 ± 0.05) (P > 0.05), but 16 mM increased the expression levels of GATA4 (1.52 ± 0.18) and Mef2c (1.88 ± 0.59) compared to the controls and the cells given to 4 mM acetate (P < 0.05). However, 4 mM acetate repressed the expression of Tbx5 (0.58 ± 0.10) (P < 0.01), while at 16 mM concentration, the expression of Tbx5 (0.85 ± 0.18), no significant difference was observed compared to the controls (P > 0.05).
Effect of ethanol and its metabolites on mRNA expression of heart development-related genes GATA4, Mef2c, and Tbx5 by real-time PCR. Cardiac progenitor cells were treated with low concentration groups of 50 mM ethanol(e), 4 mM acetaldehyde(ald), 4 mM acetate(ate), and high concentration groups of 200 mM ethanol (a), 12 mM acetaldehyde (b), 16 mM acetate (c) for 24 h. The control was treated without drugs. The results were expressed as mean of 2−△△Ct ± SD. ** P < 0.01, * P < 0.05 when compared with control
Discussion
Cell viability is a basic property of cellular life processes. Their quantitative research can reflect the development status of groups [20]. To investigate the effect of foreign chemical substances on cell viability in vitro is helpful to understand the chemical toxicity and differentiation toxicity compensating for the lack of studies in vivo. So, we explored the MTT assay to investigate the effects of ethanol and its metabolites on the viability of cardiac progenitor cells. We found that high concentrations of ethanol and its metabolites all inhibited cellular viability. It makes known that ethanol and its metabolites have cytotoxicity to cardiac progenitor cells.
The normal development of embryo requires correct activation of specific genes. Inappropriate expression or repression of these genes can change trajectories and results in developmental malformation [21]. It is becoming increasingly clear that chromatin plays a fundamental role in the control of gene transcription in multicellular organisms. Epigenetic modifications are responsible for chromatin dependent regulatory mechanism and play a key role in the regulation of gene expression during embryonic development. Epigenetic mechanisms include DNA methylation and histone modification (acetylation, methylation, phosphorylation, etc.). Histone acetylation modification plays an important role in the regulation of chromatin state and then gene expression. Cell differentiates to a particular type by limiting a certain gene expression pattern. This limited role includes regulation of tissue-specific gene activation and repression. Histone acetylation modification participates in this regulation of gene activation and repression by acting on the chromatin. Studies [22] have shown that histone acetylation modification patterns have changed during the process of embryonic stem cell differentiation. The overall level of histone acetylation modification associated with gene activation temporarily declines. However, the level of histone deacetylation associated with gene repression relatively increases. Simultaneously, histone deacetylase (HDAC) inhibitor TSA (trichostatin) can prevent the differentiation of embryonic stem cells. This illustrated that the transcriptional repression caused by histone deacetylation was essential for early embryonic development. Another study showed that [23] there was genome-wide reduction in H3AcK9 during human embryonic stem cell differentiation. This prompted that the low acetylation of H3 lysine 9 is necessary for the normal development of embryos.
There are several particularly important periods during embryonic development. These periods of teratogenesis correlate with peak periods of epigenetic reprogramming. Ethanol-induced abnormalities may arise through disruption of these epigenetic reprogramming events [24]. We demonstrated for the first time that low concentration groups of 50 mM ethanol and 4 mM acetate, respectively, increased 2.4-, 2.2-fold acetylation of H3 at lysine 9 in cardiac progenitor cells, while high concentration groups of 200 mM ethanol and 16 mM acetate, respectively, increased it 5.3-, 5.6-fold. Whether 4 or 12 mM acetaldehyde all had a negligible effect on the acetylation of H3 lysine 9. This revealed that the increase in histone acetylation induced by ethanol and acetate was dose dependent. However, the other metabolite acetaldehyde had a negligible effect. Therefore, acetaldehyde itself may not be involved in histone acetylation, but its further metabolism (e.g., to acetate) is needed.
Many evidences suggest that acetylation of core histones plays an important role in transcriptional activation by altering chromatin structure [25]. Especially, acetylation of histone H3 at lysine 9 is considered to be a specific marker of active genes [26]. Low concentration of ethanol increased the histone acetylation, but did not affect the level of heart development-related genes such as GATA4, Mef2c, and Tbx5. When concentration of ethanol increased to 200 mM, the level of histone acetylation and as well as the mRNA level of GATA4, Mef2c, and Tbx5 increased. One of its metabolites acetate had similar changes, but another one, acetaldehyde, regardless of its concentration, had a negligible effect on the acetylation of histone H3 lysine 9 and the expression of heart development-related genes. Our results have shown that the effect of ethanol and its metabolites on histone acetylation was consistent with the changes of heart development-related genes in cardiac progenitor cells. So we speculated that the changes of heart development-related genes may be caused by the increase in histone acetylation.
However, why do lower doses of ethanol and acetate activate H3 acetylation, but fail to induce the mRNA expression of the heart-related genes? Although histones are associated with virtually all eukaryotic genomic DNA sequences, histone acetylases and deacetylases do not universally affect the transcription of all genes. As we have known, transcription initiation requires RNA-pol II identifying and combining to promoter sequences with the help of many transcription factors. However, there are two different promoters to acetylation: acetylation-sensitive and acetylation-insensitive promoters [27]. There are two putative and nonmutually exclusive differences between them. First, because of their inherent DNA sequence preferences, nucleosomes on the acetylation-sensitive promoter are more tightly packed and/or positioned to preclude the accessibility to the Pol II machinery. In contrast, nucleosomes on acetylation-insensitive promoters are not tightly packed and/or positioned to facilitate accessibility of the Pol II machinery to the promoter. Second, the acetylation-sensitive promoter responds to an activator protein whose binding is strongly inhibited by nucleosomes, whereas the acetylation-insensitive promoter responds to an activator that is largely unaffected by nucleosomes. For acetylation-sensitive promoter, we speculated that if the promoter is not enough acetylated, the nucleosomes on the acetylation-sensitive promoter may be also tightly packed, and the Pol II machinery or the protein activators are not easy to access to the promoter. When histone acetylation reaches certain levels, the nucleosomes become loosen so that they are easily accessed by the Pol II machinery or the protein activators. It may also have a dose-dependent relationship. Only when ethanol and its metabolites reach to a certain level, then they can induce the histone acetylation, resulting in an alteration of the expression of the heart development-related genes. However, further studies, in particular, some in vivo studies are needed to reveal the mechanisms underlying ethanol-induced alteration of the expression levels of the heart development-related genes in the heart and to demonstrate the relationship between ethanol-induced histone acetylation and the alteration of the expression levels of the heart development-related genes in the heart at different developmental stages.
Heart development is a complex process that involves the accurate expression of many genes in a sequential manner, in different time and space. Any alterations in the regulation of these genes may result in the occurrence of congenital heart disease. GATA4, Mef2c, and Tbx5 are the most important transcription factors in the heart development. Any qualitative or quantitative changes in these factors would cause heart malformations. In addition, there are complex interactions among them. Since these transcription factors are located on the upstream of many genes, they may control the expression regulation of these genes in the heart. If the expression of GATA4, Mef2c, and Tbx5 changes during the heart development, it may affect the expression patterns of their downstream genes. This may prevent an accurate sequential expression of the genes during heart development and eventually lead to congenital heart malformations. Wu et al. [28] reported that after the treatment of the pregnant mice with sodium valproate (NaVP), the transcriptional levels of the heart development-related genes, such as CHF1, Tbx5, and MEF2, were significantly increased in fetal mouse hearts accompanied by an increase of CHD. Our studies are consistent with Wu’s report and demonstrate that high levels of ethanol and its metabolites can induce histone lysine 9 acetylation and change the expression of several heart development-related genes in cardiac progenitor cells.
References
Jirtle, R. L., & Skinner, M. K. (2007). Environmental epigenomics and disease susceptibility. Nature Reviews Genetics, 8(4), 253–262.
Dolinoy, D. C., & Jirtle, R. L. (2008). Environmental epigenomics in human health and disease. Environmental and Molecular Mutagenesis, 49(1), 4–8.
Burd, L., Deal, E., Rios, R., et al. (2007). Congenital heart defects and fetal alcohol spectrum disorders. Congenital Heart Disease, 2(4), 250–255.
Webster, W. S., Germain, M. A., Lipson, A., et al. (1984). Alcohol and congenital heart defects: An experimental study in mice. Cardiovascular Research, 18(6), 335–338.
Cavieres, M. F., & Smith, S. M. (2000). Genetic and developmental modulation of cardiac deficits in prenatal alcohol exposure. Alcoholism, Clinical and Experimental Research, 24(1), 102–109.
Goodlett, C. R., & Horn, K. H. (2001). Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Research & Health, 25, 175–184.
Wozniak, D. F., Hartman, R. E., Boyle, M. P., Vogt, S. K., Brooks, A. R., Tenkova, T., et al. (2004). Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiology of Diseases, 17, 403–414.
Karamboulas, C., Swedani, A., Ward, C., et al. (2006). HDAC activity regulates entry of mesoderm cells into the cardiac muscle lineage. Journal of Cell Science, 119(20), 4305–4314.
Feinberg, A. P. (2007). Phenotypic plasticity and the epigenetics of human disease. Nature, 447(7143), 433–440.
Yang, X. J., & Seto, E. (2008). Lysine acetylation: codified crosstalk with other posttranslational modifications. Molecular Cell, 31(4), 449–461.
Ott, H. C., Matthiesen, T. S., Goh, S. K., et al. (2008). Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nature Medicine, 14(2), 213–221.
Backs, J., & Olson, E. N. (2006). Control of cardiac growth by histone acetylation/deacetylation. Circulation Research, 98(1), 15–24.
Jing, Z., Chuan, F., Li, L., Bing, D., & Jie, T. (2008). ShRNA on histone acetylation induces unusual cardiac-specific protein expression in MSCs differentiation into cardiomyocytes. Acta Academiae Medicinae Militaris Tertiae, 30(4), 303–306.
Jing, Z., Bing, D., Jingju, W., Jie, T., & Yingxiong, W. (2006). Detection of cardiac development gene after RNA interference for histone acetylation to MSCs induced by 5-azacytidine. Acta Academiae Medicinae Militaris Tertiae, 28(6), 535–538.
Park, P. H., Miller, R., & Shukla, S. D. (2003). Acetylation of histone H3 at lysine 9 by ethanol in rat hepatocytes. Biochemical and Biophysical Research Communications, 306, 501–504.
Park, P. H., Lim, R. W., & Shukla, S. D. (2005). Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: potential mechanism for gene expression. American Journal of Physiology, Gastrointestinal and Liver Physiology, 289(6), G1124–G1136.
Choudhury, M., & Shukla, S. D. (2008). Surrogate alcohols and their metabolites modify histone H3 acetylation: Involvement of histone acetyl transferase and histone deacetylase. Alcoholism, Clinical and Experimental Research, 32(5), 829–839.
Kim, J. S., & Shukla, S. D. (2005). Histone H3 modifications in rat hepatic stellate cells by ethanol. Alcohol and Alcoholism, 40(5), 367–372.
Zhu, G. H., Huang, J., Bi, Y., et al. (2009). Activation of RXR and RAR signaling promotes myogenic differentiation of myoblastic C2C12 cells. Differentiation, 78(4), 195–204.
Gong, Z., & Wenzeman, F. H. (2004). Inhibitory effect of alcohol on osteogenic differentiation in human bone marrow-derived mesenchymal stem cells. Alcoholism, Clinical and Experimental Research, 28(3), 468–479.
Horsthemke, B. (2006). Epimutations in human disease. Current Topics in Microbiology and Immunology, 310, 45–59.
Lee, J. H., Hart, S. R., & Skalnik, D. G. (2004). Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis, 38(1), 32–38.
Krejcí, J., Uhlírová, R., Galiová, G., et al. (2009). Genome-wide reduction in H3K9 acetylation during human embryonic stem cell differentiation. Journal of Cellular Physiology, 219(3), 677–687.
Haycock, P. C. (2009). Fetal alcohol spectrum disorders: the epigenetic perspective. Biology of Reproduction, 81(4), 607–617.
Grunstein, M. (1997). Histone acetylation in chromatin structure and transcription. Nature, 389(6649), 349–352.
Morinobu, A., Kanno, Y., & O’nd Shea, J. J. (2004). Discrete roles for histone acetylation in human T helper 1 cell-specific gene expression. Journal of Biological Chemistry, 279(39), 40640–40646.
Struhl, K. (1998). Histone acetylation and transcriptional regulatory mechanisms. Genes and Development, 12(5), 599–606.
Wu, G., Nan, C., Rollo, J. C., Huang, X., & Tian, J. (2010). Sodium valproate-induced congenital cardiac abnormalities in mice are associated with the inhibition of histone deacetylase. Journal of Biomedical Science, 17(1), 16.
Acknowledgments
This study was supported by The National Basic Research Program of China (973 Program) (2010CB529505), by the National Natural Science Foundation of China (Grant Number: 30672266) and the Research Fund for the Doctoral Program of Higher Education(20060631004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, L., Zhu, J., Lv, T. et al. Ethanol and Its Metabolites Induce Histone Lysine 9 Acetylation and an Alteration of the Expression of Heart Development-Related Genes in Cardiac Progenitor Cells. Cardiovasc Toxicol 10, 268–274 (2010). https://doi.org/10.1007/s12012-010-9081-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-010-9081-z