Abstract
Heavy metal accumulation in medicinal plants has increased dramatically in recent years due to agricultural and industrial activities leading to pollution of natural sources. This study is focused on the concentration of trace elements and heavy metals in aboveground parts of 33 medicinal plants from the Eastern Mediterranean of Turkey. Results showed that the Al concentrations varied between 4.368 and 1104.627, the B level varied between 47.850 and 271.479, Ca values ranged between 1971.213 and 22,642.895, Cd concentrations ranged between 0.011 and 0.651, Cr contents varied between 1.371 and 41.692, Cu values varied between 13.278 and 42.586, Fe concentrations varied between 20.705 and 1276.783, K levels ranged between 652.143 and 14,440.946, Mg concentrations varied from 336.871 to 1869.486, Mn contents varied between 46.383 and 849.492, Na concentrations varied between 167.144 and 3401.252, Ni values varied between 0.065 and 9.968, Pb levels ranged between 1.311 and 16.238, and Zn concentrations ranged between 67.250 and 281.954 mg kg−1, respectively. Furthermore, Recommended Dietary Allowance (RDA) values for trace elements and estimated daily intake (EDI), target hazard quotient (THQ), and hazard index (HI) for heavy metals were calculated. The concentrations of heavy metals in some studied plants distributed in industrial and mining regions were found as slightly higher than the acceptable limits determined by WHO. Consequently, in order to prevent this heavy metal accumulation, when collecting medicinal aromatic plants, rural areas, close to clean rivers, or mountainous areas should be preferred, away from highway, mining, and industrial areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The earliest archeological evidence on the relationship between humans and plants dates back 1.2 million years. Recently, chemical analysis of human dental calculus belonging to ancient times has demonstrated that 70–80% of daily calorie was provided from plant products [1]. In recent years, the demand for medicinal plants has been increasing especially due to the chemical side effects of drugs although the use of plants for medicinal purposes in traditional treatment methods in history has lost its old value due to technological developments. It is known that 80% of the world’s population benefits from medicinal plants against diseases and more than 80,000 plant species are used for medicinal purposes [2]. Like other plants, medicinal plants also need macroelements (Ca, Cl, N, Na, P, K, Mg, etc.) and trace elements (B, Fe, Cu, Mn, Zn, etc.) for their structure, growth, and metabolic activities in maintaining a proper life. Their uptake from the environment is crucial since these elements cannot be synthesized by the organism itself [3].
These trace elements have important roles in metabolic activities such as seed and fruit formation, pollen health, fertilization, protein synthesis, formation and transport of carbohydrates, transport of calcium, and formation of hormones, development of the cell tissues, the formation of roots and flowers, the structural and physiological stability of plant tissue, cell division, cell wall formation, cell expansion, and activation of enzymes in plant metabolism [4, 5]. Essential nutrient deficiency causes necrosis in young leaves, vegetative and reproductive growth, blossom-end rot of fruits, the significant losses in crop yield and quality, cell breakdown, reduction in plant fertility, loss of membrane integrity, and inhibition of the cell expansion in plant functions [6,7,8].
However, numerous anthropogenic activities such as industry, fossil fuel burning, mining, smelting, forest fires, traffic, municipal wastes, sewage disposal, chemical fertilizers, and pesticides have caused dramatically increased pollution in the period since the Industrial Revolution [2, 9, 10]. Consequently, many natural aquatic and terrestrial ecosystems have been contaminated by heavy metals like aluminum (Al), arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), iron (Fe), lead (Pb), nickel (Ni), silver (Ag), and zinc (Zn) elements [11, 12]. Numerous medicinal plants may accumulate heavy metals while growing in their natural habitats. Transition of heavy metals and trace elements from environments to plants is greatly affected by soil pH. At different pH levels, some heavy metals can be accumulated less, while others can reach extreme levels that cause toxicity [13]. Salinity is another factor affecting both uptake and accumulation of heavy metals and trace elements [14]. It is also dependent on the competition rates between the plant species growing in the same lands [15]. In addition, it is known that there is a decrease in the concentrations of trace elements in plants grown in densely populated and polluted environments compared to those grown in unpolluted ones [16]. Moreover, some plants are also capable of accumulating extraordinarily high amounts of heavy metals, well above the levels found in most other plant species, without showing any phytotoxic effects. Among these plants, which are called as hyperaccumulators, some could be used for medicinal purposes and can cause toxic effects when consumed [2, 17].
It was reported that heavy metal pollution can be deadly harmful to living species including humans. Instead of treating various diseases, toxic heavy metals can pass directly or indirectly to humans, causing cardiovascular and stomachic diseases; excretory, nervous, and respiratory system diseases; cancer; and even death [18,19,20]. In addition, relevant data show that pollution causes at least 9 million early deaths, and this number is many times higher than the deaths caused by the COVID-19 pandemic [21, 22]. Therefore, it is very important to analyze the accumulation of heavy metals in medicinal plants traditionally used by humans, to monitor pollution, and to understand the effect on plant and animal species in their natural habitats.
The aim of the present work was to determine the concentrations of the nutritional trace elements and heavy metals in medicinal plants traditionally used in the East Mediterranean Turkey in terms of human health risk. It is an attempt to increase the knowledge available and provide protection for natural and cultural ethnobotanical legacy.
Material and Methods
Plant Material Sampling and Study Area
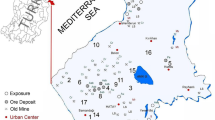
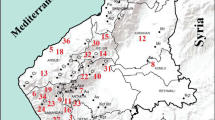
Aboveground parts of a total of 33 medical plant samples were collected from mining, farming, industrial, and rural sites of the East Mediterranean of Turkey during their flowering time in 2020 and 2021 (Fig. 1). Plant taxa were identified using the “Flora of Turkey” [23,24,25]. Detailed information on their collected localities and scientific and vernacular names is also given together with their ethnobotanical uses and pharmacological activities for each plant taxa (20 perennial, and the others annual or biannual) belonging to 23 plant families and 30 genera (Table 1). Phytogeographical origins of the studied medicinal species were determined as Irano-Turanian (9 taxa), Mediterranean (9 taxa), Euro-Siberian elements (4 taxa), East Mediterranean, and cosmopolitan elements (1 taxon each), while the phytogeographical origins of the remaining taxa were unknown. Erodium amanum of the studied taxa is endemic to Turkey, while the others are commonly distributed. Life forms of the studied medicinal species were determined as Hemicryptophyte (14 taxa), Therophyte (8 taxa), Chamaephyte (6 taxa), Geophyte (4 taxa), and Phanerophyte (1 taxon) according to the Raunkiaer method [26].
Localities of the studied medicinal plants and mineral maps of the study area modified from MTA [27]
The study area falls into the 35° 48′–37° 00′ N, 35° 46′–36° 41′ E in the east Mediterranean part of Turkey. The climate is clearly Mediterranean type with 18.1 °C annual temperature and 1.078 mm annual rainfall. The study area has about 2000 plant taxa, and a rich ethnobotanical heritage transferred from generation to generation since this region is known to be the cradle of many civilizations throughout history [2].
Determination of Trace Element Level and Statistical Analysis
Plant samples were isolated by transferring into sterile bags and dried in glass petri dishes at 80 °C for 48 h. Plant samples were grounded in porcelain mortar, powdered, and weighed. Samples weighed in the range of 0.200–0.250 g were transferred to Teflon Wessel. For plant samples, 8 ml of 65% HNO3 (Merck) solution was added to Teflon Wessels. Prepared samples were digested by using a Berghof-MSW2 brand model microwave device. After the process, the samples were transferred to 50-ml sterile falcon tubes using ultrapure water by filtering with a blue band Whatman filter, then they were filled up to a total volume of 50 ml with ultrapure water. The quantities of Al, B, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Pb, and Zn elements (mg kg−1 dry weight) were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES/PerkinElmer-Optima 7000DV) [2].
Quality Control and Quality Assurance
All chemicals used in this study were of analytical grade. Ultrapure water (Human-Zener Power I) was used as a solvent in dilution processes and in all experimental steps. Elemental values of medicinal plant samples were determined in triplicate (for linearity). All elemental concentrations were determined with a very low margin of error of ± 0.72–1.95% (RSD) using the prepared calibration standards (Table 2). The EPA 3051A Analytical Method for ICP-OES was applied using Mars Microwave to dissolve medicinal plant samples. Al, B, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, and Zn contents of the samples were determined using ICP-OES (PerkinElmer Optima 7000DV).
The advantages of ICP are long working hours, providing little or no organic molecules, a few number of ionization interferences, and the ability to sequentially analyze a large number of elements [102]. Calibration standards were prepared by diluting 1000 mg L−1 of ICP multi-element standard solution (Merck) in order to calculate the concentration values of each element examined for medicinal plant samples. Calibration curves using calibration standards prepared in 8 different concentrations were obtained for each element and found to be R2 > 0.999 (Table 2). After the initial calibration, the calibration standards were reanalyzed after every ten samples during the experimental process, and their margins of error were checked to prove that the initial calibration parameters remained constant throughout the analysis process.
The accuracy and consistency of the elemental analyzes were also proved by repeated analysis of multi-element calibration solutions with known concentrations. LoD (limit of detection) and LoQ (limit of quantification) values were determined by analyzing blank solutions and calculated for each element (Table 2):
In the given equations, the term SD denotes the standard deviation of ten replicates of the blank solution, while the values of \({\varnothing }_{n}\) and \({\varnothing }_{q}\) explain multiplying factors of 3 and 10, respectively [103, 104]. To determine the elemental compositions of the medicinal plant samples, the spectral lines were selected as listed in Table 2 according to the related literature [104,105,106].
Risk Assessment for Toxic Metals (Cd–Cr–Ni–Pb)
Exposures of Cd, Cr, Ni, and Pb leading up to the health risks to consumers through consumption of plant leaves were assessed according to the estimated daily intake (EDI), target hazard quotient (THQ), and hazard index (HI) [107].
In this equation, Cn is the toxic metal concentration in the plant samples (mg kg-1); n is the toxic metals (CdCr-Ni-Pb); IR is the uptake rate of the plant for an adult person (11.4 g/person/day); TRn stands for the transference rate of the toxic metal; and BW represents body weight (70 kg of adult person) [107, 108]. The TR values used in this study are 6.6% for Cd, 42% for Cr, 30% for Ni, and 19.8% for Pb [107, 109, 110].
THQ was used for the quantitative evaluation of the potential non-carcinogenic effects of each individual toxic metal [107]. A THQ value less than 1 indicates no significant risk of carcinogenicity to the exposed population. RfDn (mg/kg/day) for each metal (n) represents the reference dose determined by FAO/WHO. EDIn is the daily average exposure dose (mg/kg/day), and THQn is the target hazard quotient of the toxic metal. The combined risk of multiple toxic metals on general human health through the consumption of medicinal plants may arise from exposure to multiple pollutants. Therefore, the hazard index (HI) was used to estimate the total health hazards that are carcinogenic caused by exposure to multiple toxic metals.
In this equation, HI represents the total health risk due to exposure to toxic metals. An HI value less than 1 indicates that the negative effects of toxic metals on human health are less likely to occur. On the other hand, if the HI value is greater than 1, it is very likely that toxic metals cause adverse effects on human health.
Evaluation of Recommended Dietary Allowance Values
In order to reveal the Dietary Reference Intake potentials of E. cicutarium, M. campanuloides, and P. vulgaris used as daily food among the plants examined in this study, Recommended Dietary Allowance (RDA) values were calculated for Ca, Cu, Fe, K, Mg, Mn, Na, and Zn elements [10, 17].
In this equation: RDA (%) is Recommended Dietary Allowance percentages in 100 g/dw;
MV is trace element values in the studied plant samples (mg/kg; ppm);
RDAst is international standards (mg per 100 g/dw).
Results and Discussion
The mean concentrations of micronutrients and toxic metals (mg/kg) in the most commonly used whole plants are shown in Table 3. Our experimental results showed that heavy metal and trace element in the plant samples vary between 4.368 (E. hirsutum) and 1104.627 (E. amanum) for Al, 47.850 (P. lanceolata) and 271.479 (E. italicum) for B, 1971.213 (C. erythraea) and 22,642.895 (R. chalepensis) for Ca, 0.011 (P. vulgaris) and 0.651 (D. sericea) for Cd, 1.371 (P. vulgaris) and 41.692 (E. amanum) for Cr, 13.278 (E. hirsutum) and 42.586 (A. pyramidalis) for Cu, 20.705 (C. creticum) and 1276.783 (E. amanum) for Fe, 652.143 (D. maritima) and 14,440.946 (W. somnifera) for K, 336.871 (D. maritima) and 1869.486 (E. amanum) for Mg, 46.383 (C. maritima) and 849.492 (E. amanum) for Mn, 167.144 (S. nigrum) and 3401.252 (C. maritima) for Na, 0.065 (D. maritima) and 9.968 (A. apterocarpa) for Ni, 1.311 (R. suaveolens) and 16.238 (S. nigrum) for Pb, 67.250 (R. chalepensis) and 281.954 (D. maritima) for Zn (mg/ kg), respectively (Table 3).
The results obtained from our study were also compared with the permissible limits set for medicinal plants by FAO/WHO and the American Herbal Products Association (AHPA). It was detected that the heavy metal concentrations determined in the study materials were mostly within the permissible limits [111,112,113,114,115,116,117,118,119,120,121,122].
Numerous countries such as Brazil, Canada, China, Germany, India, Malaysia, Republic of Korea, Poland, Singapore, Union of Europe, UK, Thailand, and Turkey have implemented their own programs to ensure safe plant-based production regarding the heavy metal content (Table 4).
When comparing our results according to the acceptable limits, it was shown that Al levels of A. pyramidalis, E. amanum, and S. aegyptiaca were higher than the permissible limits, and they were within normal limits in other taxa; the content of Cd was slightly higher than the permissible limits in D. sericea, while it was within the permissible limits in all others; the levels of Cr were higher than the acceptable limits in A. pyramidalis, E. amanum, and S. aegyptiaca, while they were within the acceptable limits in others; the value of Cu was slightly higher than the acceptable limits in A. pyramidalis, D. strictus, S. nigrum, and W. somnifera, while it was within the acceptable limits in others; the values of Fe were found as higher than the permissible limits in A. apterocarpa, A. pyramidalis, E. amanum, E. cicutarium, G. kotschyanus, R. chalepensis, S. columbaria, S. aegyptiaca, and D. maritima, while they were within the acceptable limits in others; the concentrations of Mn were slightly higher than the permissible limits in E. amanum and E. cicutarium, while they were normal in others; the concentrations of Ni were slightly higher than the permissible values in A. apterocarpa, A. pyramidalis, C. creticum, G. kotschyanus, P. vulgaris, S. columbaria, S. aegyptiaca, T. orientale, and W. somnifera, while they were within normal limits in others; the contents of Pb were slightly higher than the permissible limits in A. apterocarpa, A. visnaga, C. erythraea, D. sericea, E. amanum, M. campanuloides, S. nigrum, and T. orientale; and Zn concentrations were found as slightly higher than the permissible limits in A. apterocarpa, C. creticus, C. creticum, D. sericea, D. orientalis, D. strictus, G. kotschyanus, M. campanuloides, D. maritima, V. hispanica, V. officinalis, and W. somnifera taxa, while they were within normal limits in other taxa (Table 3).
Normally, plants need and tend to uptake some heavy metals (Cu, Fe, Mn, Ni, and Zn) in low concentrations as trace elements for plant biochemistry and physiology, but their higher concentrations have toxic effects for both plant metabolism and its consumer people [17]. Long-term heavy metal exposure can lead to (ROS) resulting in lipid peroxidation, enzyme inactivation and DNA damage, inhibited respiration and gas exchange, reduced photosynthesis, diminished water balance, and disturbed carbohydrate and nutrient uptake metabolisms resulting in visible symptoms such as root blackening, necrosis, chlorosis, wilting, stunted plant growth, senescence, general reductions in biomass production limited seed numbers, and even death in plant metabolism [18, 123,124,125].
When the damages of heavy metal toxicity on human health are evaluated, higher levels of Al in the human body can cause Alzheimer, dialysis dementia, neurotoxicity, dermatitis, lung and bone disease, kidney disease, microcytic anemia, and lung and bladder cancer [126, 127]. The toxicity of Cd has harmful multiple human functions including pulmonary emphysema, gastrointestinal disorder, lung cancer, renal dysfunction, kidney stones, high blood pressure, skeletal defects, and bronchitis [18, 128, 129]. Cu overload has been associated with anemia, gastrointestinal, cardiovascular, liver and kidney dysfunction, intestinal irritation, abdominal pain, cramps, and nausea [128, 130, 131]. An excessive level of Cr can lead to nervous system damage, lung cancer, liver and kidney problems, fatigue, and irritability [128, 130]. Fe toxicity can result in diarrhea, vomiting, gastrointestinal bleeding, metabolic acidosis, shock, hypotension, tachycardia, cardiovascular and central nervous system problems, and possibly death [130,131,132]. A high concentration of Ni may lead to nausea, headache, cough, epigastric, chest pain, gastrointestinal problems, and weakness [127]. Mn toxicity causes damage to cardiovascular and central nervous systems. Pb toxicity is excessively harmful due to causing infant encephalopathy, developmental delay, and mental retardation in children, congenital paralysis, nervous system damage, gastrointestinal damage, and liver and kidney diseases [128]. A high amount of Zn can lead to skin diseases, vomiting, chest tightness, nausea, excitement, coldness, unconsciousness, liver damage, and pulmonary edema [131].
On the map of Hatay mines of MTA, it is seen that there is a Cr mine in the Kızıldağ, Arsuz, and Belen regions that have soils rich in Cr, and there are iron and steel, fertilizer, cement, and paint factories in the Payas-İskenderun regions [27]. According to our evaluation using the results of the total heavy metal profile, high Cr concentrations in A. pyramidalis, E. amanum, and S. aegyptiaca show that these samples were collected from soils rich in Cr (Samandağ, Arsuz, and Belen), while Al, Fe, Cu, Cr, Ni, Pb, and Zn accumulation of M. campanuloides, S. aegyptiaca, D. maritime, and R. chalepensis taxa indicates that these samples were collected from industrial areas and highways with heavy traffic (Iskenderun and Payas). In addition, contamination was observed in the A. visnaga and S. columbaria samples collected from agricultural soils (Kırıkhan and Dörtyol) as high levels of Ni were detected. Finally, lower levels of heavy metal contamination were observed in plant samples collected from relatively uncontaminated areas (Fig. 1; Table 3).
Our results revealed that the highest heavy metal accumulations were determined in A. apterocarpa, A. visnaga, A. pyramidalis, G. kotschyanus, M. campanuloides, S. aegyptiaca, S. nigrum, and W. somnifera. These species can be utilized in green phytoremediation technology for cleaning heavy metal–polluted agricultural soil and water resources. Similarly, previous studies were in agreement with our results, reporting that the species mentioned could be considered as natural hyperaccumulators [92, 133, 134].
Hitherto, numerous previous studies have been carried out on trace element and heavy metal accumulation in medicinal plants in many different parts of the world. In previous studies, concentrations of Cd and Cr in edible plants from rural areas of Eastern Iran were investigated and it was found that Cd and Cr values were 0.45 and 0.648 in A. capillus-veneris and 0.016 and 0.704 mg kg−1 in P. lanceolata [135]. The toxic heavy metal levels of P. lanceolata at the smelter area near Meza valley (Serbia) were measured, and they were found to be 0.5–16.0 for Cd, 59.4–338.7 mg kg−1 for Fe, 1.4–195.9 mg kg−1 for Pb, and 33.3–799.5 mg kg−1 for Zn, clearly higher than the permissible limits. Also, the content of various heavy metals in P. lanceolata growing in metal-contaminated and non-contaminated soil in southern parts of Poland has been measured for monitoring soil pollution [136]. The results of the study demonstrated a wide range between 1 and 13.8 for Cd, 175.7 and 1065.6 for Fe, 6.03 and 121.3 for Pb, and 101.3 and 420.1 mg kg−1 for Zn in the samples from non-metalliferous and metalliferous sites, which shows that plants in the polluted area have high heavy metal content as compared to those in non-polluted regions. Similarly, these elements were found within normal limits in the related taxa according to our results. In another study, accumulation of heavy metals (Cu, Ni, Pb, and Zn) in natural plants collected from the Sarcheshmeh copper mining site (Iran) was measured. Concentrations of Cu and Pb were found to be clearly higher than the permissible limit in E. hirsutum (1300–1581 and 20 mg kg−1), P. lanceolata (128 and 11 mg kg−1), and S. nigrum (119 and 12 mg kg−1) expectedly, while the Ni and Zn values were within the normal limit in these taxa [137]. However, it was found that Cu, Ni, and Zn accumulations in S. nigrum collected from the nearby agricultural area (Karacay) were above the permissible limits, while other values were found to be within normal levels in E. hirsutum and P. lanceolata. Trace element (Ca, Mg, Na) and heavy metal (Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) levels of the S. nigrum and W. somnifera samples in industrial areas in Islamabad (Pakistan) and also sold in Indian herbalist markets were investigated, and it was reported that the content of Cd (0.65 and 0.97 mg kg−1), Cu (311.88 mg kg−1), and Pb (37.8, 36.47, and 19.87 mg kg−1) was clearly above the permissible limits set by the WHO, while the others were within normal limits [90, 92, 138]. In another study, W. somnifera samples were collected from the field of the PCSIR Lab. complex in Karachi (Pakistan) and it was determined that the concentrations of Cu (245.7 and 135.8 mg kg−1), Fe (9417.7 and 3750.3 mg kg−1), Mn (833.5 and 452.6 mg kg−1), Ni (16.2 and 10.3 mg kg−1), Pb (60.6 and 23.3 mg kg−1), and Zn (422.2 and 375 mg kg−1) in shoot and leaves were above the permissible limits [101]. Similarly, they were found that Cu and Pb in S. nigrum and Cu, Ni, and Zn in W. somnifera collected from the nearby agricultural area (Samandag-Karacay) were above the permissible limits, while the other metals were within normal limits. For R. chalepensis, the concentrations of Cd and Pb metals were measured from different locations in Ethiopia and Jordan, and the studies suggested that the mean values of Cd (0.58–0.71) and Pb (22.8) mg kg−1 were above normal limits in the samples from industrial and agricultural areas [139, 140]. However, it was found that only Fe accumulations in R. chalepensis collected from the nearby industrial area (Belen) were above the permissible limits, while the other metals were within the permissible limits in our study. The results of all obtained from previous studies and our results show that plant samples in the polluted area have high heavy metal content as compared to non-polluted regions.
Herein, the dendrogram constructed by using the results of average linkage cluster analysis revealed 2 main groups as groups 1 and 2 (Fig. 2). Group 1 containing R. chalepensis, V. hispanica, and E. italicum showed a general distribution in close regions (Payas-İskenderun) having similar habitat characteristics, and the members of this subgroup were probably affected by the similar environmental conditions. They are also Mediterranean element, therophyte, and annual taxa. Group 2 included A and B subgroups. Subgroup A comprised P. lanceolata, S. aegyptiaca, S. nigrum, T. terrestris, and W. somnifera which are generally annual taxa and showed distribution generally in close ranges (Payas-Samandağ/Kırıkhan-Kumlu). The other 25 taxa were placed in subgroup B.
The human body needs at least 22 mineral elements in order to maintain its metabolic activities in a healthy way. Plants are the most important natural sources for these essential nutrients [141]. Therefore, the Recommended Dietary Allowance (RDA) values for the elements Ca, Cu, Fe, K, Mg, Mn, Na, and Zn were calculated in E. cicutarium, M. campanuloides, and P. vulgaris taxa used as daily food (Table 5). These RDA values are as follows: the RDA value of Ca was measured in E. cicutarium > P. vulgaris > M. campanuloides; the RDA values of Cu and Zn were found in M. campanuloides > E. cicutarium > P. vulgaris; the RDA values for Fe and Mn were found in E. cicutarium > P. vulgaris > M. campanuloides; the RDA values for K, Mg, and Na were found in E. cicutarium > M. campanuloides > P. vulgaris. As it is known, these plants have edible properties. This indicates their possible usability as dietary supplements.
When the functions and importance of trace elements studied on human health are evaluated, Ca affects many intracellular and extracellular processes, and is essential for the development, growth,, and maintenance of teeth and bone, and the stability of the cytoskeleton [142]. It also plays an important role in vitamin D absorption, regulation of the activity of intracellular enzymes, and neuronal transmission [143]. Ca deficiency can lead to congestive heart failure, cardiac arrhythmias, scurvy, sarcopenia, rickets, and osteoporosis in the human body [144, 145]. Dairy products contain high levels of calcium, and the RDA value for Ca is reported as 1000 mg day−1. According to our results, the lowest and highest RDA values were found to be 323.71 (in M. campanuloides) and 702.01 (in E. cicutarium) for Ca. In other words, the daily consumption of 100 g of M. campanuloides meets ~ 32.37% of daily Ca need and this rate is ~ 70.20% for E. cicutarium (Table 5).
Cu plays a key role in metabolic activities such as bone mineralization, brain development, immune system, energy metabolism, maintenance of hematopoietic activity, growth, maturation of red and white blood cells, and Fe transport and metabolism [146]. Deficiency of Cu can lead to many health problems such as retarded growth and bone abnormalities, anemia, leukopenia, and gray hair [147]. Some foods such as grain products, seafood, meat and offal, fruits, and vegetables are rich in Cu element, and the RDA for Cu is reported as 0.9 mg per day [148]. The lowest and highest RDA values were found to be 1.68 (in P. vulgaris) and 3.22 (in M. campanuloides). Daily consumption of 100 g of P. vulgaris meets ~ 187.01% of daily Cu need, while M. campanuloides is ~ 357.36% in adults (Table 5).
Fe plays an essential role in biological processes, including DNA biosynthesis, transport of hemoglobin, the formation of oxygen transport proteins and enzymes in electron transfer and oxidation reductions, electron transfer in the redox cycling, and cellular energy generation [149,150,151]. Fe can be obtained from food sources such as eggs, pulses, cereals and black mulberry, legumes, vegetables, and fruits. The RDA value for Fe is reported as 8 mg day−1 for men and 18 mg day−1 for women [152]. However, Fe deficiency has been associated with many different diseases including chronic anemia in adults, weakness, fatigue, weakened immune function, reduced cognitive function in children, delays in childhood development, sepsis, miscarriage, maternal and perinatal death, and low birth weight during pregnancy [132, 151,152,153,154]. In our study, the lowest and highest RDA values were found to be 11.77 in M. campanuloides and 92.59 in E. cicutarium, respectively. In other words, the daily consumption of 100 g M. campanuloides meets ~ 147.07% of daily Fe need in men and ~ 65.37% in women, while this ratio is ~ 1157.32% and ~ 514.36% in E. cicutarium, respectively (Table 5).
K is critical for nerve, skeletal, and cardiac muscle activity [155]. Deficiency of K can lead to high cardiac arrhythmias, blood pressure, kidney disease, muscle cramps and weakness, bone demineralization, constipation, fatigue, nausea, and vomiting [145]. It can be obtained from vegetables, fruits, and foods, including tomato, potato, blueberries, beet greens, yogurt, and milk [156, 157]. The RDA for K has been reported as 4700 mg day−1 for men [158]. Our study revealed that the RDA values for K were between 495.46 in P. vulgaris and 780.61 in E. cicutarium, which shows that daily consumption of 100 g P. vulgaris provides ~ 10.54% and E. cicutarium ~ 16.61% of the required daily amounts of K, respectively (Table 5).
Mg is necessary for energy production, transport of electrolytes across cell membranes, permeability of cell membranes, preservation of the anatomical and functional integrity of various cellular organelles, muscle contraction, development of bone structure, neuron excitability, synthesis of DNA and RNA, and release of hormones and neurotransmitters [142, 159]. Mg deficiency causes atrial fibrillation, appetite, weakness, hypertension, atherosclerosis, cerebrovascular disease, congestive heart failure, migraines, asthma, Alzheimer’s, nausea, hypocalcemia, fatigue and hypophosphatemia, premenstrual syndrome, dermatological allergy, rapid heartbeat, and mental disorders [159, 160]. The RDA for Mg is listed as 420 mg day−1 for men and 320 mg day−1 for women [158]. Our results show that the RDA values of Mg were found between 42.09 in P. vulgaris and 113.12 in E. cicutarium, which means that the daily consumption of 100 g P. vulgaris provides ~ 10.02% and 13.15% and E. cicutarium ~ 26.93% and 35.35% of the daily requirements of Mg for men and women, respectively (Table 5).
Mn is an essential nutrient for several vital biological processes including synthesis and activation of many enzymes, antioxidant defense, glucose and lipid metabolism, bone development, catalysis of hematopoiesis, immune response, and neural functions [161, 162]. Common dietary sources of Mn include cereals, rice, tea, and nuts. However, Mn deficiency results in problems such as birth defects, impaired growth and fertility, poorer bone formation, abnormal glycosylation patterns, carbohydrate and lipid metabolism, skeletal problems, and general psychomotor disability [162,163,164]. The RDA values for Mn are stated as 2.3 mg day−1 for men and 1.8 mg day−1 for women [152]. In our results, minimum and maximum RDA values for Mn were found to be 6.69 in M. campanuloides and 45.03 in E. cicutarium. As a result, daily consumption of 100 g of M. campanuloides provides ~ 290.83% and 371.62%, while E. cicutarium provides ~ 1957.98% and 2501.87% of the daily requirements of Mn for men and women, respectively (Table 5).
Na plays a critical role in healthy muscle, nerve activity, acid–base balance, and plasma volume [155]. Na deficiency can cause severe health problems like weakness, headache, lethargy, anorexia, nausea, vomiting, muscle cramps, confusion, seizures, coma, and altered consciousness [145]. Our analyses demonstrated that our studied medicinal plants are rich in trace element such as Ca, K, Mg, and Na. The RDA for Na is stated as 1500 mg day−1 for men [165]. The RDA values of Na were found between 31.45 in P. vulgaris and 146.14 in E. cicutarium in our results. Additionally, daily consumption of 100 g of P. vulgaris provides ~ 2.10% of daily required Na and ~ 9.74% of E. cicutarium (Table 5).
Zn is a critical element for several vital biological functions and processes, such as bone structure, protein synthesis, immune system functions, DNA synthesis, cell division, and development of pregnancy, for growth during childhood and adolescence. It is also involved in more than 300 metalloenzymes, as well as Zn matrix metalloproteinases. Common dietary sources of Zn include oysters, whole grains, crab, red meat, and beans [166,167,168].
Thus, Zn deficiency results in a number of detrimental effects, such as immune systems disorders, weight loss, infertility and hypogonadism in males, mental disorders, anorexia, depression, mental lethargy, decreased wound healing, skin lesions, rough skin, acrodermatitis, alopecia, diarrhea, loss of appetite, and retarded growth in children [166]. The RDA value of Zn is presented as 11 mg day−1 for men and 8 mg day−1 for women [169, 170]. According to our results, the lowest RDA values of Zn were found in P. vulgaris (8.27), while it was the highest in M. campanuloides (18.88). Moreover, a daily intake of 100 g of P. vulgaris provides ~ 75.21% and 103.41% of the daily requirement for men and women, while M. campanuloides provides ~ 171.68% and 236.06% of the daily requirement (Table 5). However, it is estimated that more than 60% of the world’s 6 billion people are iron (Fe) deficient and more than 30% are zinc (Zn) deficient. In addition, high calcium deficiencies of Ca, Mg, and Cu are common in many developed and developing countries [141]. Therefore, our RDA results clearly demonstrate that the studied plant species have a high potential for essential elements.
As a result of our study, it was seen that the EDI, THQ, and HI values determined for studied medicinal plants were within the acceptable limits. Considering the determined THQ values and cumulative HI values for Cd, Cr, Ni, and Pb, the risk of adverse effects in adults was within the permissible limits after exposure to the four toxic heavy metals tested through the consumption of medicinal plants. The lowest and highest HI values were found to be 0.012 for P. lanceolata and 0.154 for E. amanum, respectively (Table 6). Furthermore, risk assessment of toxic metals in these plants is important not only for assessing their adverse effects on human health, but also for obtaining realistic estimates of their uptake through this traditional use.
Additionally, the Joint FAO/WHO Expert Committee on Food Additives [112] has allocated the maximum daily intake of toxic elements for a 70-kg adult person (TDI: tolerable daily intake). TDI values are considered to be within permissible limits for toxic heavy metals such as As, Br, Cd, Pb, and Sb, which have hazardous effects for human body and metabolism [10]. Our results suggested that the Cd and Pb values of all the medicinal plants investigated when compared with the TDI values were beyond the TDI limits (for Cd 0.014 and Pb 0.021 mg/day/70 kg adult person). These results demonstrate to us that the use of medicinal plants is a phenomenon that should be evaluated scientifically.
Conclusion
Long-term environmental damages caused by pollutants such as mining, agricultural activities, burning of fossil fuels, and wastewater, which have increased due to rapid population growth in recent years, affect many plant species living in terrestrial and aquatic ecosystems. In addition, highly toxic metals in soils can threaten wildlife and human health through the consumption of contaminated plants. In order to prevent this heavy metal accumulation, when collecting medicinal aromatic plants, rural areas, close to clean rivers, or mountainous areas should be preferred, away from highways and mining and industrial areas. In addition, this accumulation should be controlled by similar heavy metal analyses carried out periodically in widely used medicinal plants, and the results should be shared with local users and managers. It is clearly seen from our results that in most of the analyzed plant samples, the selected heavy metals are within the permissible limits set by international authorities or in some plants slightly above these limits. Briefly, it was observed that the heavy metal levels in the collected plants were generally at consumable levels, although they were slightly higher in the samples collected from the areas close to the industrial zones and mining and agricultural areas.
Data Availability
The author can confirm that all data generated or analyzed are included in the article.
References
Hardy K, Radini A, Buckley S et al (2017) Diet and environment 1.2 million years ago revealed through analysis of dental calculus from Europe’s oldest hominin at Sima del Elefante. Spain Sci Nat 104:7–11. https://doi.org/10.1007/s00114-016-1420-x
Karahan F, Ozyigit II, Saracoglu IA et al (2020) Heavy metal levels and mineral nutrient status in different parts of various medicinal plants collected from eastern Mediterranean region of Turkey. Biol Trace Elem Res 197:316–329. https://doi.org/10.1007/s12011-019-01974-2
Zeiner M, Cindrić IJ (2017) Review–trace determination of potentially toxic elements in (medicinal) plant materials. Anal Methods 9:1550–1574. https://doi.org/10.1039/C7AY00016B
Jones C, Jacobsen J (2001) Plant nutrition and soil fertility. Nutrient management module 2. Montana State University Extension Service. Publication 4449–2
Aydın A (2017) Turunçgillerde Bitki Besleme TÜRKTOB Dergisi 22:49–54
Olle M, Bender I (2009) Causes and control of calcium deficiency disorders in vegetables: a review. J Hortic Sci Biotechnol 84:577–584. https://doi.org/10.1080/14620316.2009.11512568
Herrera-Rodríguez MB, González-Fontes A et al (2010) Role of boron in vascular plants and response mechanisms to boron stresses. Plant Stress 4:115–122
Vatansever R, Ozyigit II, Filiz E (2017) Essential and beneficial trace elements in plants, and their transport in roots: a review. Appl Biochem Biotechnol 181:464–482. https://doi.org/10.1007/s12010-016-2224-3
Erdogan A, Seker ME, Kahraman SD (2022) Evaluation of environmental and nutritional aspects of bee pollen samples collected from East Black Sea region, Turkey, via elemental analysis by ICP-MS. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03217-3
Ozyigit II, Karahan F, Yalcin IE et al (2022) Heavy metals and trace elements detected in the leaves of medicinal plants collected in the southeast part of Turkey. Arab J Geosci 15:27. https://doi.org/10.1007/s12517-021-09264-9
Turan O, Özdemir H, Demir G (2020) Deposition of heavy metals on coniferous tree leaves and soils near heavy urban traffic. Front Life Sci Relat Technol 1:35–41
Turksoy R, Terzioglu G, Yalcin IE, Turksoy O, Demir G (2021) Removal of heavy metals from textile industry wastewater. Front Life Sci Relat Technol 2:44–50
Eskin B, Ozyigit I, Doğan I, Demir G, Yarci C, Serin M (2019) Ecophysiological properties of Turkish endemic Centaurea consanguinea DC. Fresenius Environ Bull 28:1082–1092
Ozyigit II, Eskin B et al (2018) Some heavy metals and mineral nutrients of narrow endemic Cirsium byzantinum Steud. from Istanbul, Turkey: Plant-Soil interactions. Fresenius Environ Bull 27:668–674
Ozyigit II, Dogan I et al (2021) Mineral nutrient compositions of field-grown weed and maize (Zea mays L.) plants in terms of competition. Pak J Agric Sci 58:115–123
Yalcin IE, Ozyigit II, Dogan I, Demir G, Yarci C (2020) Determining element accumulations in Turkish red pine used as a bioindicator for estimating of existing pollution on both sides of Bosphorus in Istanbul. Fresenius Environ Bull 29:4963–4972
Ozyigit II, Yalcin B, Turan S et al (2018) Investigation of heavy metal level and mineral nutrient status in widely used medicinal plants’ leaves in Turkey: insights into health implications. Biol Trace Elem Res 182:387–406. https://doi.org/10.1007/s12011-017-1070-7
Hocaoglu-Ozyigit A, Genc BN (2020) Cadmium in plants, humans and the environment. Front Life Sci Relat Technol 1:12–21
Nzediegwu C, Prasher S, Elsayed E et al (2020) Impact of soil biochar incorporation on the uptake of heavy metals present in wastewater by Spinach plants. Water Air Soil Pollut 231:123. https://doi.org/10.1007/s11270-020-04512-2
Wang P, Yuan Y, Xu K et al (2021) Biological applications of copper-containing materials. Bioact Mater 6:916–927. https://doi.org/10.1016/j.bioactmat.2020.09.017
Moelling K, Broecker F (2020) Air microbiome and pollution: composition and potential effects on human health, including SARS coronavirus infection. J Environ Public Health 2020:1646943. https://doi.org/10.1155/2020/1646943
Uras ME (2021) In silico comparative analysis of SARS-CoV-2 nucleocapsid (N) protein using bioinformatics tools. Front Life Sci Relat Technol 2:1–9
Davis PH (1965–1988) Flora of Turkey and the East Aegean Islands, Vol. 1–9. Edinburgh University Press, Edinburgh
Davis PH, Mill RR, Tan K (1988) Flora of Turkey and the East Aegean Islands (Supplement), vol 10. Edinburgh University Press, Edinburgh
Güner A, Aslan S, Ekim T, Vural M, Babaç MT (eds) (2012) Türkiye Bitkileri Listesi (Damarlı Bitkiler). Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği, İstanbul
Raunkiaer C (1934) Life forms of plants. Oxford, University Press, Tehran
Uzer M, Paker S (2019) Mineral map of Antakya. Available online: https://www.mta.gov.tr/v3.0/sayfalar/hizmetler/maden-haritalari/Antakya.pdf. Accessed 16 Apr 2022
Baytop T (1999) Therapy with medicinal plants in Turkey (Past and Present), 2. ed. 2nd ed. Nobel Medical Publishing, Istanbul
Benítez G, González-Tejero MR, Molero-Mesa J (2010) Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): ethnopharmacological synthesis. J Ethnopharmacol 129:87–105. https://doi.org/10.1016/j.jep.2010.02.016
Owfi RE (2020) An overview of important endemic plants and their products in Iran. In: Cooper R, Deakin JJ (eds) Natural products of silk road plants, 1st edn. CRC Press, Boca Raton, pp 171–200
Ezer N, Arisan OM (2006) Folk medicines in Merzifon (Amasya, Turkey). Turk J Bot 30:223–230
Yeşil Y, Akalın E (2009) Folk medicinal plants in Kürecik area (Akçadağ/Malatya Turkey). Turk J Pharm Sci 6:207–220
Altundag E, Ozturk M (2011) Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia - Soc Behav Sci 19:756–777. https://doi.org/10.1016/j.sbspro.2011.05.195
Aksoy A, Celik J, Tunay H (2016) Gazipaşa (Antalya) ilçe pazarında satılan ve halk tarafından kullanılan bazı bitkiler ve kullanım amaçları. Biy Bil Araş Derg 9:55–60
Keskin C (2018) Mardinde geleneksel halk hekimliğinde kullanılan tıbbi bitkiler ve geleneksel kullanımları. In: Congress Book. p. 1
Mart S, Türkmen N (2018) A survey on wild plants with ethnobotanical use in the Bahçe and Hasanbeyli districts of Osmaniye, Turkey. GSC Bio Pharm Sci 5: 28–35. https://doi.org/10.30574/gscbps.2018.5.3.0133
Al-Snafi AE (2013) Chemical constituents and pharmacological activities of Ammi majus and Ammi visnaga. A review. Int J Pharm Indust Res 3:257–265
Terzioğlu S, Coşkunçelebi K (2021) Medicinal plants of Northeast Anatolia. In Ozturk M, Altay V, Efe R (eds) Biodiversity, conservation and sustainability in Asia, Springer, Cham, pp 275–337. https://doi.org/10.1007/978-3-030-59928-7_11
Sargın SA, Selvi S, Büyükcengiz M (2015) Ethnomedicinal plants of Aydıncık district of Mersin, Turkey. J Ethnopharmacol 174:200–216. https://doi.org/10.1016/j.jep.2015.08.008
Sargın SA, Büyükcengiz M (2019) Plants used in ethnomedicinal practices in Gülnar district of Mersin. Turkey. J Herb Med 15:100224. https://doi.org/10.1016/j.hermed.2018.06.003
Bozyel ME, Merdamert-Bozyel E (2020) Ethnomedicinal uses of Orchidaceae taxa in Turkish traditional medicine. Int Res J Bio Sci 9:52–63
Ksouri R et al (2007) Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem 45:244–249. https://doi.org/10.1016/j.plaphy.2007.02.001
Stevanovic Z, Stankovic, MS, Stankovic J, Janackovic P, Stankovic M (2019) Use of halophytes as medicinal plants: Phytochemical diversity and biological activity. In: Hasanuzzaman M, Shabala S, Fujita M (eds) Halophytes and climate change: Adaptive mechanisms and potential uses, 1st edn. CABI, Wallingford, pp 343–358
Güzel Y, Güzelşemme M, Miski M (2015) Ethnobotany of medicinal plants used in Antakya: A multicultural district in Hatay province of Turkey. J Ethnopharmacol 174:118–152. https://doi.org/10.1016/j.jep.2015.07.04
Al-Khalil S (1995) A survey of plants used in Jordanian traditional medicine. Int J Pharmacogn 33:317–323
Atalan E, Bulbul AS, Ceylan Y (2020) Cephalaria syriaca (L.): Investigation of antimicrobial, antibiofilm, antioxidant potential and seed morphology. Fresenius Environ Bull 29:3641–3649.
Kavak C, Baştürk A (2020) Antioxidant activity, volatile compounds and fatty acid compositions of Cephalaria syriaca seeds obtained from different regions in Turkey. Grasas Aceites 71:e379. https://doi.org/10.3989/gya.0913192
González-Tejero MR, Casares-Porcel M, Sánchez-Rojas CP et al (2008) Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. J Ethnopharmacol 116:341–357. https://doi.org/10.1016/j.jep.2007.11.045
Polat R, Satıl F (2012) An ethnobotanical survey of medicinal plants in Edremit Gulf (Balıkesir–Turkey). J Ethnopharmacol 139:626–641. https://doi.org/10.1016/j.jep.2011.12.004
Emre G, Doğan A et al (2021) An ethnobotanical study of medicinal plants in Mersin (Turkey). Front Pharmacol 12:664500. https://doi.org/10.3389/fphar.2021.664500
El-Shazly A, Sarg T, Witte L, Wink M (1996) Pyrrolizidine alkaloids from Cynoglossum creticum. Phytochem 42:1217–1221
Sargin SA, Akcicek E, Selvi S (2013) An ethnobotanical study of medicinal plants used by the local people of Alaşehir (Manisa) in Turkey. J Ethnopharmacol 150:860–874. https://doi.org/10.1016/j.jep.2013.09.040
Tosun A (2006) Daphne L. türlerinin kimyasal içeriği ve biyolojik aktiviteleri. Ankara Ecz Fak Derg 35:43–68
Güneş S, Savran A, Paksoy MY, Çakılcıoğlu U (2018) Survey of wild food plants for human consumption in Karaisalı (Adana-Turkey). Indian J Tradit Knowl 17:290–298
Nacakçı FM, Dutkuner I (2018) A study of ethnobotany in Kumluca (Antalya). Turk J Forestry 19: 113–119. https://doi.org/10.18182/tjf.421970
Ghasemi Pirbalouti A, Momeni M, Bahmani M (2013) Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan districts, Ilam Province. Iran Afr J Tradit Complement Altern Med 10:368–385. https://doi.org/10.4314/ajtcam.v10i2.24
Ghamari S, Mohammadrezaei-Khorramabadi R, Mardani M, Shahsavari S (2017) An overview of the most important medicinal plants with anti-toothache property based on ethnobotanical sources in Iran. J Pharm Sci Res 9:796–799
Al-Shamma A, Lester AM (1979) Comprehensive survey of indigenous Iraqi plants for potential economic value. 1. Screening results of 327 species for alkaloids and antimicrobial agents. J Nat Prod 42:633–642
Baydoun S, Chalak L, Dalleh H, Arnold N (2015) Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J Ethnopharmacol 173:139–156. https://doi.org/10.1016/j.jep.2015.06.052
Passalacqua NG, Guarrera PM, De Fine G (2007) Contribution to the knowledge of the folk plant medicine in Calabria region (Southern Italy). Fitoterapia 78:52–68. https://doi.org/10.1016/j.fitote.2006.07.005
Yücel E (2008) Türkiye’de yetişen tıbbi bitkiler 1 (A-L). Cetemenler Dijital, Eskişehir
Yücel E (2014) Türkiye'de yetişen tıbbi bitkiler tanıma klavuzu. Türmatsan, İstanbul
Bulut Y (2006) Manavgat (Antalya) yöresinin faydalı bitkileri. Dissertation, Süleyman Demirel Üniversitesi
Egamberdieva D, Jabborova D (2018) Medicinal plants of Uzbekistan and their traditional uses. In: Egamberdieva D, Ozturk M (eds) Vegetation of Central Asia and environs. Springer, Cham, pp 211–237
Altay V, Karahan F (2012) Tayfur Sökmen Kampüsü (Antakya-Hatay) ve çevresinde bulunan bitkiler üzerine etnobotanik bir araştırma. Karadeniz Fen Bilimleri Dergisi 3:13–28
Altay V, Karahan F, Sarcan YB, Ilcim A (2015) An ethnobotanical research on wild plants sold in Kırıkhan district (Hatay/Turkey) herbalists and local markets. Biol Divers Conserv 8:81–91
Özturk M, Altay V, Gönenç TM (2017) Herbal from high mountains in the East Mediterranean. In: Bhojraj S et al (eds) Drug Discovery from Herbs. DAYA Publishing House, New Delhi, pp 327–367. https://doi.org/10.1007/978-3-319-99728-5_8
İlçim A (2014) Hatay’ın Sessiz Güzelleri 900 Yabani Çiçek, Hatay Bitki Envanteri. Hatay Valiliği, Hatay
Al-Quran S (2008) Taxonomical and pharmacological survey of therapeutic plants in Jordan. J Nat Prod 1:10–26.
Cakilcioglu U, Turkoglu I (2010) An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey). J Ethnopharmacol 132:165–175. https://doi.org/10.1016/j.jep.2010.08.017
Khatib C, Nattouf A, Hasan Agha MI (2021) Ethnobotanical survey of medicinal herbs in the Western region in Syria (Latakia and Tartus). Res Sq 1–75. https://doi.org/10.21203/rs.3.rs-355008/v1
Guvenc A, Akkol EK et al (2012) Wound healing and anti-inflammatory activities of the Michauxia L’Hérit (Campanulaceae) species native to Turkey. J Ethnopharmacol 139:401–408. https://doi.org/10.1016/j.jep.2011.11.024
Altay V, Keskin M, Karahan F (2015) An assessment of the plant biodiversity of Mustafa Kemal University Tayfur Sokmen Campus (Hatay-Turkey) for the view of human health. Int J Sci Technol Res 1:83–103.
Mükemre M, Behçet L, Çakılcıoğlu U (2015) Ethnobotanical study on medicinal plants in villages of Çatak (Van-Turkey). J Ethnopharmacol 166:361–374. https://doi.org/10.1016/j.jep.2015.03.040
Öztürk M, Altay V et al (2018) Herbals in Iğdır (Turkey), Nakhchivan (Azerbaijan), and Tabriz (Iran). In: Öztürk M, Hakeem KR (eds) Plant and human health, Vol 1, Springer, Cham, pp 197–266. https://doi.org/10.1007/978-3-319-93997-1_6
Cornara L, La Rocca A, Marsili S, Mariotti MG (2009) Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J Ethnopharmacol 125:16–30. https://doi.org/10.1016/j.jep.2009.06.021
Polat R, Çakılcıoğlu U et al (2015) An ethnobotanical study on medicinal plants in Espiye and its surrounding (Giresun-Turkey). J Ethnopharmacol 163:1–11. https://doi.org/10.1016/j.jep.2015.01.008
Mattalia G, Sõukand R, Corvo P, Pieroni A (2019) Scholarly vs. traditional knowledge: effects of sacred natural sites on ethnobotanical practices in Tuscany, Central Italy. Human Ecol 47:653–667. https://doi.org/10.1007/s10745-019-00102-x
Bonet MÀ, Parada M, Selga A, Valles J (1999) Studies on pharmaceutical ethnobotany in the regions of L’Alt Empordà and Les Guilleries (Catalonia, Iberian Peninsula). J Ethnopharmacol 68:145–168. https://doi.org/10.1016/S0378-8741(99)00083-5
Said O, Khalil K, Fulder S, Azaizeh H (2002) Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J Ethnopharmacol 83:251–265. https://doi.org/10.1016/S0378-8741(02)00253-2
Macía MJ, García E, Vidaurre PJ (2005) An ethnobotanical survey of medicinal plants commercialized in the markets of La Paz and El Alto, Bolivia. J Ethnopharmacol 97:337–350. https://doi.org/10.1016/j.jep.2004.11.022
Del Carmen J-Vázquez M, Carranza-Álvarez C et al (2013) Ethnobotany of medicinal plants used in Xalpatlahuac, Guerrero, México. J Ethnopharmacol 148:521–527. https://doi.org/10.1016/j.jep.2013.04.048
Ivanova A, Kostova I, Tsvetkova I, Najdenski H (2001) GC-MS investigation of Haplophyllum suaveolens extracts. Comptes Rendus de l’Academie Bulgare des Sci 54:6–35.
Ulubelen A, Ozturk M (2008) Alkaloids, coumarins and lignans from Haplophyllum species. Records Nat Prod 2:54–69
Rigat M, Bonet MÀ, Garcia S, Garnatje T, Valles J (2007) Studies on pharmaceutical ethnobotany in the high river Ter valley (Pyrenees, Catalonia, Iberian Peninsula). J Ethnopharmacol 113:267–277. https://doi.org/10.1016/j.jep.2007.06.004
Lucchetti L, Zitti S, Taffetani F (2019) Ethnobotanical uses in the Ancona district (Marche region, Central Italy). J Ethnobio Ethnomed 15:9. https://doi.org/10.1186/s13002-019-0288-1
Otang-Mbeng W, Idowu JS (2020) Anti-melanogenesis, antioxidant and anti-tyrosinase activities of Scabiosa columbaria L. Processes 8:236. https://doi.org/10.3390/pr8020236
Fakir H, Korkmaz M, Guller B (2009) Medicinal plant diversity of western Mediterranean region in Turkey. J App Bio Sci 3:33–43.
Mosaddegh M, Naghibi F, Moazzeni H, Pirani A, Esmaeili S (2012) Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J Ethnopharmacol 141:80–95. https://doi.org/10.1016/j.jep.2012.02.004
Subramanian R, Gayathri S, Rathnavel C, Raj V (2012) Analysis of mineral and heavy metals in some medicinal plants collected from local market. Asian Pacific J Trop Biomed 2:74–78. https://doi.org/10.1016/S2221-1691(12)60133-6
Eissa TA, Palomino OM, Carretero ME, Gómez-Serranillos MP (2014) Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula. Egypt J Ethnopharmacol 151:317–332. https://doi.org/10.1016/j.jep.2013.10.041
Kumar N, Kulsoom M, Shukla V et al (2018) Profiling of heavy metal and pesticide residues in medicinal plants. Environ Sci Pollut Res 25:29505–29510. https://doi.org/10.1007/s11356-018-2993-z
Erdemgil FZ, Yilmaz M, Kivanc M (2004) Antimicrobial activity of Thalictrum orientale’s extracts. J Marmara Pure App Sci 19:23–33
Bhat R, Kiran K, Arun AB, Karim AA (2010) Determination of mineral composition and heavy metal content of some nutraceutically valued plant products. Food Anal Methods 3:181–187. https://doi.org/10.1007/s12161-009-9107-y
Arituluk ZC, Nurten EZ (2012) Halk arasında diyabete karşı kullanılan bitkiler Türkiye-II. Hacettepe Uni J Fac Pharm 32:179–208
Zaurov DE, Belolipov IV, Kurmukov AG, Sodombekov IS, Akimaliev AA, Eisenman SW (2013) The medicinal plants of Uzbekistan and Kyrgyzstan. In: Eisenman S, Zaurov D, Struwe L (eds) Medicinal plants of Central Asia: Uzbekistan and Kyrgyzstan. Springer, New York, pp 15–273. https://doi.org/10.1007/978-1-4614-3912-7_5
Corlett JL, Clegg MS, Keen CL, Grivetti LE (2002) Mineral content of culinary and medicinal plants cultivated by Hmong refugees living in Sacramento, California. Int J Food Sci Nutrit 53:117–128. https://doi.org/10.1080/09637480220132139
Batugal, PA, Kanniah J, Young LS, Oliver JT (eds). 2004. Medicinal plants research in Asia, Vol 1: The framework and project workplans. International Plant Genetic Resources Institute-Regional Office for Asia, the Pacific and Oceania (IPGRI-APO). Serdang, Selangor DE, Malaysia
Attard E, Pacioni P (2012) The phytochemical and in vitro pharmacological testing of Maltese medicinal plants. In: Rasooli I (ed) Bioactive compounds in phytomedicine. InTech, Rijeka (Croatia), pp 93–112
Kultur S, Gurdal B, Sari A, Melikoglu G (2021) Traditional herbal remedies used in kidney diseases in Turkey: an overview. Turk J Bot 45:269–287. https://doi.org/10.3906/bot-2011-32
Shirin K, Imad S, Shafiq S, Fatima K (2010) Determination of major and trace elements in the indigenous medicinal plant Withania somnifera and their possible correlation with therapeutic activity. J Saudi Chem Soc 14:97–100. https://doi.org/10.1016/j.jscs.2009.12.015
Sharma I (2020) ICP-OES: An advance tool in biological research. Open J Environ Biol 5:27–33. https://doi.org/10.17352/ojeb.000018
Cao L, Zheng J, Tsukada H et al (2016) Simultaneous determination of radiocesium (135Cs, 137Cs) and plutonium (239Pu, 240Pu) isotopes in river suspended particles by ICP-MS/MS and SF-ICP-MS. Talanta 159:55–63. https://doi.org/10.1016/j.talanta.2016.06.008
Goncalves DA, de Souza ID, Rosa ACG et al (2019) Multi-wavelength calibration: determination of trace toxic elements in medicine plants by ICP OES. Microchem J 146:381–386. https://doi.org/10.1016/j.microc.2019.01.021
Barin JS, Pereira JSF, Mello PA et al (2012) Focused microwave-induced combustion for digestion of botanical samples and metals determination by ICP OES and ICP-MS. Talanta 94:308–314. https://doi.org/10.1016/j.talanta.2012.03.048
Lee J, hee, Kim JY, Park SG, et al (2017) A study on the hazardous metal content of herbal medicines in the Daegu Area. Korean J Environ Heal Sci 43:257–266. https://doi.org/10.5668/jehs.2017.43.4.257
Zhang J, Yang R, Chen R, Peng Y, Wen X, Gao L (2018) Accumulation of heavy metals in tea leaves and potential health risk assessment: A case study from Puan County, Guizhou Province, China. Int J Environ Res Public Heal 15:133. https://doi.org/10.3390/ijerph15010133
Peng CY, Zhu XH, Hou RY et al (2018) Aluminum and heavy metal accumulation in tea leaves: an ınterplay of environmental and plant factors and an assessment of exposure risks to consumers. J Food Sci 83:1165–1172. https://doi.org/10.1111/1750-3841.14093
Li L, Fu QL, Achal V, Liu Y (2015) A comparison of the potential health risk of aluminum and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi, China. Environ Monit Assess 187:1–12. https://doi.org/10.1007/s10661-015-4445-2
Gruszecka-Kosowska A, Mazur-Kajta K (2016) Potential health risk of selected metals for Polish consumers of oolong tea from the Fujian Province, China. Hum Ecol Risk Assess 22:1147–1165. https://doi.org/10.1080/10807039.2016.1146572
FAO, WHO, (1984) Contaminants, vol XVII. FAO/WHO, Rome
FAO/WHO (1999) Joint FAO/WHO Expert committee on food additives. Summary and conclusions. In: 53rd meeting, Rome, 1–10 June
FAO/WHO (2007) Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 13th Session. Report of the thirty eight session ofthe codex committee on food hygiene, Houston, United States of America, ALINORM 07/30/13. Available from: ftp://ftp.fao.org/codex/R
FAO/WHO (2011) Joint FAO/WHO food standards programme codex committee on contaminants in foods, fifth session. 52pp. March 2011, CL 2011/6-C
Joint FAO/WHO Expert Committee on Food Additives (2003): Rome I (2004) World Health Organization & Food and Agriculture Organization of the United Nations. Evaluation of certain food additives and contaminants: Sixty-first report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization
American Herbal Products Association (AHPA) (2012) About Us > AHPA’s Policies > Guidance Policies. In: AHPA
Tripathy V, Basak BB, Varghese TS, Saha A (2015) Residues and contaminants in medicinal herbs-a review. Phytochem Lett 14:67–78. https://doi.org/10.1016/j.phytol.2015.09.003
De Oliveira LM, Das S, da Silva EB et al (2018) Metal concentrations in traditional and herbal teas and their potential risks to human health. Sci Total Environ 633:649–657. https://doi.org/10.1016/j.scitotenv.2018.03.215
Liu C, Qin J, Dou X et al (2018) Extrinsic harmful residues in Chinese herbal medicines: types, detection, and safety evaluation. Chinese Herb Med 10:117–136. https://doi.org/10.1016/j.chmed.2018.02.002
Zolfaghari G, Akhgari Sang Atash Z, Sazgar A (2018) Baseline heavy metals in plant species from some industrial and rural areas: carcinogenic and non-carcinogenic risk assessment. MethodsX 5:43–60. https://doi.org/10.1016/j.mex.2018.01.003
Keshvari M, Nedaeinia R, Nedaeinia M et al (2021) Assessment of heavy metal contamination in herbal medicinal products consumed in the Iranian market. Environ Sci Pollut Res 28:33208–33218. https://doi.org/10.1007/s11356-021-13020-7
Soomro AH, Jatoi WB, Maitlo AA et al (2021) Assessment of heavy metal content of commonly consumed herbal medicines in Sindh, Pakistan. Environ Sci Pollut Res 28:32744–32753. https://doi.org/10.1007/s11356-021-13019-0
Ozyigit II, Yilmaz S, Dogan I, Sakcali MS, Tombuloglu G, Demir G (2016) Detection of physiological and genotoxic damages reflecting toxicity in kalanchoe clones. Glob Nest J 18:223–232. https://doi.org/10.30955/gnj.001349
Kumar V, Singh J, Kumar P (2019) Heavy metals accumulation in crop plants: sources, response mechanisms, stress tolerance and their effects. In: Kumar V, Kumar R, Singh J, Kumar P (eds) Contaminants in agriculture and environment: health risks and remediation. Agriculture and Environmental Science Academy, India, pp 38–57
Houri T, Khairallah Y, Zahab AA et al (2020) Heavy metals accumulation effects on the photosynthetic performance of geophytes in Mediterranean reserve. J King Saud Univ Sci 32:874–880. https://doi.org/10.1016/j.jksus.2019.04.005
Jaishankar M, Tseten T, Anbalagan N et al (2014) Toxicity, mechanism and health effects of some heavy metals. Interd Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
Fakhar A, Gul B et al (2022) Heavy metal remediation and resistance mechanism of Aeromonas, Bacillus, and Pseudomonas: A review. Crit Rev Environ Sci Technol 52:1868–1914. https://doi.org/10.1080/10643389.2020.1863112
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43:246–253. https://doi.org/10.4103/0253-7613.81505
Haider FU, Liqun C, Coulter JA et al (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887. https://doi.org/10.1016/j.ecoenv.2020.111887
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharmacol 38:232–242. https://doi.org/10.1016/S0273-2300(02)00020-X
Maurya PK, Malik DS, Yadav KK et al (2019) Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: Possible human health risks evaluation. Toxicol Rep 6:472–481. https://doi.org/10.1016/j.toxrep.2019.05.012
Yasar U, Ozyigit II, Demir G, Yilmaz YZ (2012) Determination of hair iron levels of healthy female high school students with AAS in the Pendik District, Istanbul-Turkey. Fresenius Environ Bull 21:2644–2264
Padmavathiamma PK, Li LY (2007) Phytoremediation technology: hyperaccumulation metals in plants. Water Air Soil Pollut 184:105–126. https://doi.org/10.1007/s11270-007-9401-5
Luo SL, Chen L et al (2011) Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 85:1130–1138. https://doi.org/10.1016/j.chemosphere.2011.07.053
Sayadi MH, Kharkan J et al (2020) Cadmium and chromium levels in water and edible herbs in a risk assessment study of rural residents living in Eastern Iran. Environ Sci Pollut Res 27:9901–9909. https://doi.org/10.1007/s11356-020-07600-2
Nadgórska-Socha A, Ptasiński B, Kita A (2013) Heavy metal bioaccumulation and antioxidative responses in Cardaminopsis arenosa and Plantago lanceolata leaves from metalliferous and non-metalliferous sites: A field study. Ecotoxicol 22:1422–1434. https://doi.org/10.1007/s10646-013-1129-y
Ghaderian SM, Ravandi AAG (2012) Accumulation of copper and other heavy metals by plants growing on Sarcheshmeh copper mining area. Iran J Geochem Explor 123:25–32. https://doi.org/10.1016/j.gexplo.2012.06.022
Parveen R, Abbasi AM, Shaheen N, Shah MH (2020) Accumulation of selected metals in the fruits of medicinal plants grown in urban environment of Islamabad, Pakistan. Arab J Chemis 13:308–317. https://doi.org/10.1016/j.arabjc.2017.04.010
Baye H, Hymete A (2010) Lead and cadmium accumulation in medicinal plants collected from environmentally different sites. Bull Environ Contam Toxicol 84:197–201. https://doi.org/10.1007/s00128-009-9916-0
Alomary A, Jamal E, Al-Momani I et al (2013) Pb in medicinal plants from Jordan. Environ Chem Lett 11:55–63. https://doi.org/10.1007/s10311-012-0378-y
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84. https://doi.org/10.1111/j.1469-8137.2008.02738.x
Ciosek Ż, Kot K, Kosik-Bogacka D et al (2021) The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules 11:506. https://doi.org/10.3390/biom11040506
Wrzosek M, Wo J, Kozioł-kaczorek D, Włodarek D (2019) The assessment of the supply of calcium and vitamin D in the diet of women regularly practicing sport. J Osteoporos 2019:9214926. https://doi.org/10.1155/2019/9214926
Du Y, Oh C, No J (2019) Advantage of dairy for improving aging muscle. J Obes Metab Syndr 28:167–174. https://doi.org/10.7570/jomes.2019.28.3.167
Baj J, Flieger W, Teresiński G et al (2020) Magnesium, calcium, potassium, sodium, phosphorus, selenium, zinc, and chromium levels in alcohol use disorder: a review. J Clin Med 9:1–24. https://doi.org/10.3390/jcm9061901
Toro-román V, Siquier-coll J, Bartolomé I et al (2021) Copper concentration in erythrocytes, platelets, plasma, serum and urine: Influence of physical training. J Int Soc Sports Nutr 18:28. https://doi.org/10.1186/s12970-021-00426-4
Hofmann P, Vidovic M, Debrunner J (2021) Copper deficiency J Clin Nutr Diet 7:1–4
Bost M, Houdart S, Oberli M, Kalonji E (2016) Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol 35:107–115. https://doi.org/10.1016/j.jtemb.2016.02.006
Dhanda A, Atkinson S, Vergis N et al (2020) Trace element deficiency is highly prevalent and associated with infection and mortality in patients with alcoholic hepatitis. Aliment Pharmacol Ther 52:537–544. https://doi.org/10.1111/apt.15880
Talebi S, Ghaedi E, Sadeghi E et al (2020) Trace element status and hypothyroidism: a systematic review and meta-analysis. Biol Trace Elem Res 197:1–14. https://doi.org/10.1007/s12011-019-01963-5
Roemhild K, von Maltzahn F et al (2021) Iron metabolism: pathophysiology and pharmacology. Trends Pharmacol Sci 42:640–656. https://doi.org/10.1016/j.tips.2021.05.001
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101:294–301
Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19:164–174
Hoes MF, Beverborg NG, Kijlstra JD et al (2018) Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 20:910–919. https://doi.org/10.1002/ejhf.1154
Van DC, Van VA, Abdelrazek M et al (2018) Minerals and sarcopenia; the role of calcium, ıron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc 19:6-11.e3. https://doi.org/10.1016/j.jamda.2017.05.026
He FJ, MacGregor GA (2008) Beneficial effects of potassium on human health. Physiol Plant 133:725–735. https://doi.org/10.1111/j.1399-3054.2007.01033.x
Udensi UK, Tchounwou PB (2017) Potassium homeostasis, oxidative stress, and human disease. Int J Clin Exp Physiol 4:111–122. https://doi.org/10.4103/ijcep.ijcep
National Institutes of Health (NIH) (2021) Dietary supplement fact sheet. https://ods.od.nih.gov/factsheets/list-all/. Accessed 6 Mar 2022
Al-Fartusie FS, Mohssan SN (2017) Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci 5:127–136. https://doi.org/10.22607/IJACS.2017.503003
Reddy P, Edwards LR (2019) Magnesium supplementation in vitamin D deficiency. Am J Ther 26:e124–e132. https://doi.org/10.1097/MJT.0000000000000538
Chen P, Bornhorst J, Aschner MA (2018) Manganese metabolism in humans. Front Biosci Landmark 23:1655–1679. https://doi.org/10.25932/publishup-42743
Li L, Yang X (2018) The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev 2018:7580707. https://doi.org/10.1155/2018/7580707
Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25:191–203. https://doi.org/10.1016/j.jtemb.2011.08.144
Liu Q, Barker S, Knutson MD (2021) Molecular cell research iron and manganese transport in mammalian systems. BBA - Mol Cell Res 1868:118890. https://doi.org/10.1016/j.bbamcr.2020.118890
Eckel RH, Jakicic JM, Ard JD et al (2014) 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardio 63:2960–2984. https://doi.org/10.1161/01.cir.0000437740.48606.d1
Sousa C, Moutinho C, Vinha AF, Matos C (2019) Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. Int J Sci Res Methodol 13:57–80
Kandasamy S, Narayanan V, Shanmugam S (2020) International Journal of Biological Macromolecules Zinc and manganese substituted hydroxyapatite/CMC/PVP electrospun composite for bone repair applications. Int J Biol Macromol 145:1018–1030. https://doi.org/10.1016/j.ijbiomac.2019.09.193
Shayganfard M (2021) Are essential trace elements effective in modulation of mental disorders? Update and perspectives. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02733-y
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365. https://doi.org/10.3390/ijerph7041342
Moon N, Figgins B, Altshuler E et al (2022) Concurrent zinc and vitamin B6 deficiencies in acutely exacerbated inflammatory bowel disease: case reports. Nutr Clin Pract 37:203–208. https://doi.org/10.1002/ncp.10665
Acknowledgements
The author would like to thank Dr. Ahmet Ilcim for the valuable contributions to the study.
Author information
Authors and Affiliations
Contributions
F. Karahan: conceived and designed the present study, collected and analyzed the plant samples, and did the writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
This work is a systematic and meta-analysis review that did not need ethical approval and did not receive any technical or financial support from any institution and was carried out by the authors at their own personal expense.
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karahan, F. Evaluation of Trace Element and Heavy Metal Levels of Some Ethnobotanically Important Medicinal Plants Used as Remedies in Southern Turkey in Terms of Human Health Risk. Biol Trace Elem Res 201, 493–513 (2023). https://doi.org/10.1007/s12011-022-03299-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03299-z