Abstract
Certain metals play a role in the pathogenesis of diabetes. This study aimed to investigate the potential association of plasma magnesium (Mg) and dietary intake of Mg with glycaemic markers. A cross-sectional study was conducted on 2373 subjects in Guangxi, China. Dietary Mg was obtained through a food frequency questionnaire containing 109 common foods. Plasma Mg concentration was determined by inductively coupled plasma mass spectrometry. Multiple linear regression combined with multivariable restricted cubic spline (RCS) functions was applied to evaluate the association of plasma Mg and dietary Mg with haemoglobin A1c (HbA1c) and fasting plasma glucose (FPG). In linear regression, dietary Mg was significantly associated with FPG in the overall population (β = − 0.087, P < 0.05) and in women (β = − 0.098, P < 0.05). Plasma Mg was significantly associated with FPG in the overall population (β = − 0.096, P < 0.05) and in men (β = − 0.110, P < 0.05) and women (β = − 0.088, P < 0.05). In the RCS model, no non-linear association was found between dietary and plasma Mg and HbA1c levels. Dietary and plasma Mg are significantly negatively associated with fasting glucose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of diabetes and pre-diabetes is increasing extensively with the ageing population and the obesity epidemic [1]. Type 2 diabetes mellitus (T2DM) has become a global health crisis, and methods to prevent it are urgently needed. The central aspects of T2DM are believed to be insulin resistance and b-cell dysfunction, and an imbalance of metal ions in the body may be associated with insulin resistance [2, 3].
Mg is an essential mineral that acts as a cofactor in any ATP-driven reaction and thus has a significant effect on metabolism [4]. Mg regulates insulin sensitivity and influences insulin-mediated glucose uptake [5]. Epidemiological investigations have identified low Mg levels as an independent risk factor for prediabetes and T2DM [1, 6]. Mg deficiency may lead to impaired tyrosine kinase and glucose transport across cell membranes, and it is associated with reduced insulin secretion from pancreatic B cells, post-receptor insulin resistance, and reduced cellular glucose utilisation [5, 7].
This process may be altered by dietary Mg intake, which reduces the risk of impaired glucose and insulin metabolism and the progression from prediabetes to diabetes [8,9,10]. Mg is obtained from diet, and it is mainly found in various unprocessed foods, including nuts, legumes, and vegetables; by contrast, meat, fish, and dairy products are relatively low in Mg [11]. The US Food and Nutrition Board recommends a daily Mg intake of 320 mg for women and 420 mg for men [12]. Despite the clear recommendations, low Mg intake is common worldwide [13], and Mg deficiency remains a global public health problem. Dietary Mg in the normal Western diet is generally insufficient to meet the body’s needs, and even in developed countries, such as the USA, many adults do not meet the recommended values [14].
Results regarding the relationship between serum or dietary Mg and the risk of diabetes in Asian populations are currently inconsistent [15,16,17,18,19]. In addition, racial differences in Mg intake exist, which could lead to certain health differences [14]. Therefore, the present study aimed to understand the relationship between dietary Mg and serum Mg and that between fasting blood glucose and glycated haemoglobin in rural Southern China. This study contributes to clear dietary guidelines and guides public awareness, thereby reducing the risk of Mg deficiency-related diseases.
Materials and Methods
Study Population
A cross-sectional survey was conducted from 2018 to 2019 on the general population in the rural areas of Gongcheng Yao Autonomous County, which is located in Guangxi in the southwest region of China. Study subjects who met the following criteria were selected: (a) resided in the study area and (b) 30 years old and older. A total of 2373 participants were included in this study after excluding the following: (a) participants who did not complete the questionnaire or the questionnaire contained missing data on covariates; (b) participants who had missing data on height, weight, systolic or diastolic blood pressure, plasma triglycerides or total cholesterol, fasting plasma glucose (FPG), and haemoglobin A1c (HbA1c); (c) participants who were treated with antidiabetic medication; (d) participants who had abnormal metal values (defined as three times of the 99th percentile); (e) participants with FPG and HbA1c greater or less than three standard deviations; and (f) participants who had invalid FFQs, defined as those with estimate daily caloric intake of 600 kcal/day or 4,000 kcal/day for women or 4,200 kcal/day for men [8]. Finally, 2373 participants were included for analysis (Fig. 1). This study was approved by the Medical Ethics Committee of Guilin Medical University (No. 20180702–3). Sample collection and questionnaire survey were conducted on participants who provided a written informed consent.
Determination of HbA1c Levels
Fasting blood samples were collected from the elbow vein in the morning after at least 12 h of fasting. HbA1c was measured using an MQ2000PT high-performance liquid chromatography analyser (Nanning Jinyu Medical Technology Co., Ltd). The laboratory reference range for HbA1c is 4.0–6.0%.
Determination of FPG Levels
Blood samples were sent to Gongcheng County People’s Hospital through cold-chain transportation on the same day for the determination of blood biochemical indicators, including FPG, plasma triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels. All blood biochemical indices were detected with a Chemistry Automatic Analyzer (Hitachi 7600–020, Kyoto, Japan) in accordance with the manufacturer’s instructions. The above indicators were set as parallel samples during testing to reduce errors. The laboratory reference range for FPG is 3.90–6.10 mmol/L.
Plasma Mg assessment
Professional nurses collected 16 mL of fasting venous blood samples from all subjects in the morning after fasting for at least 12 h and dispensed them into four blood collection tubes. After refrigeration, the samples were sent to the laboratory department of Gongcheng County People’s Hospital in time. After centrifugation at 4 °C for 10 min, the plasma samples were stored in a 1.5-mL centrifuge tube (Eppendorf, Germany) in an ultralow temperature refrigerator at − 80 °C until testing. Before the test, the sample was transferred from a − 80 °C refrigerator gradient to room temperature. Then, 100 µL of the plasma samples was diluted to 1:20 with 1.9 mL 1% HNO3. The glass instruments for inductively coupled plasma sampling system (atomisation chamber and atomiser) were immersed in 10% HNO3 for more than 24 h, and the sampling and intercepting cones were cleaned with 1% HNO3. They were rinsed with ultrapure water and dried for later use. Inductively coupled plasma mass spectrometry (Thermo Fisher Scientific iCAPRQ01408, USA) was used to determine Mg. The experiment was performed in accordance with the instruction manual. The standard solution consisted of a mixed standard solution of 43 and six elements; a single standard solution of Cu, Zn, Fe, Mg, and Ca; and 1% HNO3. Based on the results of the pre-experiment, we developed a standard magnesium curve ranging from 5.05 to 1010 ppb, requiring a correlation coefficient ≥ 0.999, and then the plasma samples were determined; we ensured that 80% of the samples were within the standard curve concentration range. ClinChek (level 1, No. 8883; level 2, No. 8884; Recipe Chemicals, Germany) was used for quality control. The quality control solution was measured every 25 samples; we also checked whether the magnesium content of the standard reference material was within the recommended range. The recovery rate of the standard addition of plasma Mg was in the range of 80.16‒114.65%. If the Mg concentration was lower than the limit of detection (LOD), it was replaced for (LOD)/√2. The LOD of Mg is 0.006 μg/L, and the concentration of Mg in the samples was above the LOD.
Dietary Mg Assessment
Diet was assessed using a food frequency questionnaire (FFQ) in reference to the questionnaire designed by Liu et al. [20]. Several previous studies have shown that FFQ is a reasonable, valid, and reliable method for assessing long-term average dietary intake [21, 22]. The FFQ included 15 groups and 109 items as follows: cereals (six items) and tuber crops (three items), processed vegetables (one item), fresh vegetables (33 items), fruits (26 items), processed meat (two items), fresh meat (13 items), fresh eggs (three items), processed eggs (two items), bean and products (eight items), nuts and seeds (five items), milk and dairy products (two items), alcohol (three items), and oil and tea intake. The subjects were asked whether they ever consumed a certain food. If the response was positive, a question was asked to indicate how often the consumption was and how much was consumed each time. A food album was attached to the FFQ, including dietary items, measurement tools, and weight of each portion size. The weight of each portion size was weighed by a standardised portable electronic kitchen scale, which can measure food in g and drinks in mL. The frequency of consumption of food/drink item was categorised into eight subgroups, ranging from never to three times per day. The intake amount of each food was obtained by multiplying the portion size by the weight of portion size. The contents of energy and nutrients in food were referred to the Chinese Food Composition Table (2009) [23]. Daily energy intake, dietary fibre intake, and dietary Mg were calculated.
Covariate Definition
A standardised and structured questionnaire was used to obtain information, particularly age, ethnicity (Han, Yao or other ethnic groups), educational level (≤ 6 years or > 6 years), smoking history (yes or no), and alcohol consumption (yes or no). Smoking was defined as currently smoking at least one cigarette per day. Alcohol consumption was defined as drinking at least 50 g of alcohol or more than once a month. Body mass index (BMI) was calculated by dividing the weight by height squared (kg/m2). Being overweight was defined as 23.0 kg/m2 ≤ BMI < 27.5 kg/m2, whereas obesity was defined as BMI ≥ 27.5 kg/m2 [24]. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. Hyperlipidaemia was defined as total cholesterol of > 5.72 mmol/L, triglycerides of > 1.70 mmol/L [25], or intake of lipid-lowering medication.
Statistical Analysis
Descriptive statistics was calculated for all demographic and clinical characteristics of the study subjects. Continuous variables were expressed as mean (SD) or median (IQR). Categorical variables were presented as numbers and percentages. The levels of metals were subjected to log10 (lg) transformation to reduce their skewness. Spearman correlation was used to explore the correlation between dietary Mg, plasma Mg, blood glucose level, and glycosylated haemoglobin. Multiple linear regression combined with multivariable RCS functions was used to investigate the dose–response relationship amongst dietary Mg, plasma Mg, FPG, and HbA1c, with adjustment for sex, age, ethnicity, education, smoking, alcohol consumption, BMI, hypertension, hyperlipidaemia, energy, and fibre. Dietary fibre was adjusted because it represents foods high in Mg, such as whole grain, which is associated with a reduced risk of T2DM. Five sections of RCS were located at 5%, 27.5%, 50%, 72.5%, and 95% of the element concentration range.
Patients with diabetes may have reduced blood Mg concentration due to increased renal Mg excretion [26]. Subjects with HbA1c level of ≥ 7.0 and FPG ≥ 7.0 were excluded for sensitivity analysis to rule out the potential effect of diabetes on plasma Mg [27, 28]. All statistical analyses were performed in SPSS 17.0 and R 4.0.3, and P values < 0.05 were considered as significant statistical difference.
Results
Characteristics of Participants
Table 1 shows the clinical and demographic characteristics of the 2373 study participants, comprising 890 men and 1483 women. Yao people were the most numerous, accounting for 75.4% of the total number of people, and 65.4% did not attend junior high school. The mean concentrations of HbA1c and FPG in the total study population were 5.72% ± 0.57% and 4.77 ± 0.67 mmol/L, respectively.
Spearman Correlation Analysis
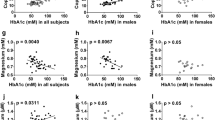
Spearman correlation analysis showed that plasma Mg was significantly negatively correlated with HbA1c (r = − 0.05, P < 0.05), whilst dietary Mg (r = − 0.059, P < 0.01) and plasma Mg (r = − 0.19, P < 0.01) were significantly negatively correlated with FPG in the total population. In men, plasma Mg was negatively associated with FPG (r = − 0.20, P < 0.01). In women, dietary Mg was significantly negatively associated with HbA1c (r = − 0.082, P < 0.01), and dietary Mg (r = − 0.12, P < 0.01) and plasma Mg (r = − 0.18, P < 0.01) were significantly negatively associated with FPG, as shown in Fig. 2.
Spearman correlation analysis was conducted to analyse the correlation between plasma Mg, dietary Mg, FPG and HbA1c. A Overall B Men. C Women. The distribution of each variable is shown on the diagonal. The bottom of the diagonal shows a binary scatter plot with fitted lines, and the top of the diagonal is the correlation value plus significance level (indicated by asterisks). Each significance level is associated with a sign: p value (0.001, 0.01, 0.05) assigns as (“***,” “**,” “*”)
Linear Regression Analysis
The results of linear regression analysis are shown in Table 2. In the overall participants and in men, dietary Mg and plasma Mg were not associated with HbA1c. Dietary Mg was significantly associated with HbA1c in women, but this relationship disappeared after adjustment for covariates. Plasma Mg was significantly associated with FPG in the whole population (β = − 0.096) and in men (β = − 0.110) and women (β = − 0.088) stratification (all P values < 0.05). In addition, significant differences were found in dietary Mg and FPG observed in the general population (β = − 0.087, P < 0.05) and in women (β = − 0.098, P < 0.05) but not in men (P > 0.05).
RCS Analysis
RCS was used to determine whether a non-linear association exists between dietary and plasma Mg and HbA1c levels. The results showed no non-linear association between dietary and plasma Mg and HbA1c levels, as shown in Fig. 3.
Dose–response relationship between dietary and plasma Mg and HbA1c levels. The solid and dotted line shadows represent the fitting curve and 95% confidence interval, respectively. Adjusted for sex, age, ethnicity, education, smoking and alcohol consumption, BMI, hypertension, hyperlipidaemia, energy, and fibre. A Overall. B Men. C Women
Sensitivity Analysis
After 53 study participants with HbA1c ≥ 7 and 24 with FPG ≥ 7 were excluded, a negative correlation between plasma Mg and FPG was still observed in the general population (β = − 0.096) and in men (β = − 0.109) and women (− 0.090), with P < 0.05. No linear relationship was found between plasma Mg and HbA1c, as shown in Table 3.
Discussion
The daily Mg intakes for men and women in this study were lower than the 2007–2014 Chinese Health and Nutrition Examination Survey intakes [29] and the US Food and Nutrition Board recommended intakes [12]. Meanwhile, the plasma Mg concentration of the study population was lower than that of the population in China reported by Zhang et al. [30]. These findings suggested an inadequate intake of Mg in the local population.
Dietary Mg was negatively associated with FPG, and our findings were consistent with the results of the 2007–2014 National Health and Nutrition Examination Survey [29]. However, a pilot cross-sectional study in women of childbearing age in Ghana [31] and a cross-sectional study in an occupational population in Japan [32] did not find a significant association between dietary Mg and fasting glucose. Controversial results were also found in randomised controlled trials, with one randomised controlled trial of 116 people aged 30–65 years with hypomagnesemia and newly diagnosed pre-diabetes finding that Mg supplementation significantly reduced fasting blood glucose levels [33]. By contrast, a randomised double-blind crossover trial of 98 patients with T2DM showed no significant improvement in fasting blood glucose with Mg supplementation in patients with diabetes [34]. The reasons for the discrepancy in the results of these studies may be related to the different durations of Mg salts and/or Mg supplementation and the small sample size and short follow-up period [33, 34]. Previous meta-analyses of prospective cohort studies have consistently concluded that dietary Mg intake reduces the prevalence of T2DM. For example, Fang et al. [13] conducted a meta-analysis of 25 prospective cohort studies involving 637,922 subjects and found a 17% reduction in the risk of T2DM compared with those with the lowest Mg intake in the population. Similarly, Tian et al. [35] included 539,735 participants and found a significantly lower risk of T2DM in those with the highest Mg intake. In a sex-stratified analysis, a significant negative association was found between dietary Mg intake and blood glucose in women, whereas no significant association was observed in men. A prospective cohort study from Japan also found that Mg intake was significantly associated with a reduced risk of diabetes only in women and the gender difference may be explained by the fact that the role of dietary Mg in insulin regulation is more important for women than for men [17].
This study provides additional evidence that plasma Mg may indeed affect FPG and the results are consistent with those of cross-sectional studies of occupational populations in China and Japan [32, 36]. In Rotterdam, Kieboom et al. reported that reduced plasma Mg concentrations were associated with an increased risk of diabetes and prediabetes [37]. As sensitivity analyses excluding patients with diabetes yielded consistent results, this finding suggested that the association between plasma Mg and fasting glucose is unlikely to be due to the opposite cause. The beneficial effects of Mg on diabetes have been demonstrated in cellular models and animal studies. Cellular experiments have demonstrated that long-term exposure to low Mg concentrations of adipocytes is associated with the development of insulin resistance [38]. In animal experiments, the activity of muscle insulin receptor tyrosine kinase was impaired in rats with hypomagnesemia [39] and Mg supplementation prevented fructose-induced insulin resistance [40]. Increased Mg intake may prevent deterioration of glucose tolerance and thus delay the development of idiopathic non-insulin-dependent diabetes mellitus [41]. However, the current study failed to find a significant association between dietary and plasma Mg and glycated haemoglobin. HbA1c is an indicator of long-term glucose metabolism due to its low variability in the body; it is independent of food intake and minimally disturbed by short-term glucose levels [42]. The effect of Mg supplementation on HbA1c was not found to be significant in a meta-analysis of randomised double-blind controlled trials [43, 44]. Saeed et al. [45] also found no significant association between plasma Mg and HbA1c in patients with T2DM in a cross-sectional study. These findings are consistent with the present results and suggested that Mg intake may not affect long-term glycaemic control. However, Galli-Tsinopoulou et al. found a significant negative correlation between plasma Mg and HbA1c in patients with type 1 diabetes [11]. The association between Mg and HbA1c remains controversial, and more epidemiological and experimental evidence is needed to clarify the association.
The strength of this study is reflected in the inclusion of dietary and plasma Mg, the different markers of glucose metabolism, and the analysis of the interaction between dietary and serum Mg and gender.
This study has limitations. Firstly, this cross-sectional study was unable to assess the causal relationship between Mg intake and plasma Mg and glucose markers. Secondly, although this study adjusted for some potential confounders, information regarding family history and the genetic characteristics of diabetes was not obtained, and residual confounders remained unavoidable. Further research is needed to determine the relationship amongst Mg intake, plasma Mg, and glycaemic markers and the mechanisms involved in this relationship.
Conclusion
In conclusion, the results suggested that Mg intake and plasma Mg are significantly negatively associated with fasting glucose, with gender differences in this association.
References
Fang C, Wang X, Wu W, Gu X, Ye T, Deng H, Wang X, Shen F (2016) Association of serum magnesium level with odds of prediabetes and diabetes in a southern Chinese population: a prospective nested case-control study. Biol Trace Elem Res 172(2):307–314. https://doi.org/10.1007/s12011-015-0594-y
Lv Y, Xie L, Dong C, Yang R, Long T, Yang H, Chen L, Zhang L, Chen X, Luo X, Huang S, Yang X, Lin R, Zhang H (2021) Co-exposure of serum calcium, selenium and vanadium is nonlinearly associated with increased risk of type 2 diabetes mellitus in a Chinese population. Chemosphere 263:128021. https://doi.org/10.1016/j.chemosphere.2020.128021
Bjørklund G, Dadar M, Pivina L, Doşa MD, Semenova Y, Aaseth J (2020) The role of zinc and copper in insulin resistance and diabetes mellitus. Curr Med Chem 27(39):6643–6657. https://doi.org/10.2174/0929867326666190902122155
Topf JM, Murray PT (2003) Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord 4(2):195–206. https://doi.org/10.1023/a:1022950321817
Barbagallo M, Dominguez LJ (2007) Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome and insulin resistance. Arch Biochem Biophys 458(1):40–47. https://doi.org/10.1016/j.abb.2006.05.007
Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL (1999) Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the atherosclerosis risk in communities study. Arch Intern Med 159(18):2151–2159. https://doi.org/10.1001/archinte.159.18.2151
Noor MM, Nazir Q, Khan TM, Gillani S, Abbasi MA, Rauf A, Qureshi ZU (2019) Association between low serum magnesium level and type 2 diabetes mellitus in Abbottabad. J Ayub Med Coll Abbottabad : JAMC 31(2):226–229
Hruby A, Meigs JB, O’Donnell CJ, Jacques PF, McKeown NM (2014) Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care 37(2):419–427. https://doi.org/10.2337/dc13-1397
Larsson SC, Wolk A (2007) Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med 262(2):208–214. https://doi.org/10.1111/j.1365-2796.2007.01840.x
Dong JY, Xun P, He K, Qin LQ (2011) Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 34(9):2116–2122. https://doi.org/10.2337/dc11-0518
Galli-Tsinopoulou A, Maggana I, Kyrgios I, Mouzaki K, Grammatikopoulou MG, Stylianou C, Karavanaki K (2014) Association between magnesium concentration and HbA1c in children and adolescents with type 1 diabetes mellitus. J Diabetes 6(4):369–377. https://doi.org/10.1111/1753-0407.12118
Food and Nutrition Board IoM (1997) Magnesium. dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academies Press, Washington, pp 190–249
Fang X, Han H, Li M, Liang C, Fan Z, Aaseth J, He J, Montgomery S, Cao Y (2016) Dose-response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: a systematic review and meta-regression analysis of prospective cohort studies. Nutrients 8(11). https://doi.org/10.3390/nu8110739
Ford ES, Mokdad AH (2003) Dietary magnesium intake in a national sample of US adults. J Nutr 133(9):2879–2882. https://doi.org/10.1093/jn/133.9.2879
Weng LC, Lee NJ, Yeh WT, Ho LT, Pan WH (2012) Lower intake of magnesium and dietary fiber increases the incidence of type 2 diabetes in Taiwanese. J Formosan Med Assoc Taiwan Yi Zhi 111(11):651–659. https://doi.org/10.1016/j.jfma.2012.07.038
Villegas R, Gao YT, Dai Q, Yang G, Cai H, Li H, Zheng W, Shu XO (2009) Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai Women’s Health Study. Am J Clin Nutr 89(4):1059–1067. https://doi.org/10.3945/ajcn.2008.27182
Konishi K, Wada K, Tamura T, Tsuji M, Kawachi T, Nagata C (2017) Dietary magnesium intake and the risk of diabetes in the Japanese community: results from the Takayama study. Eur J Nutr 56(2):767–774. https://doi.org/10.1007/s00394-015-1122-8
Nanri A, Mizoue T, Noda M, Takahashi Y, Kirii K, Inoue M, Tsugane S (2010) Magnesium intake and type II diabetes in Japanese men and women: the Japan public health center-based prospective study. Eur J Clin Nutr 64(10):1244–1247. https://doi.org/10.1038/ejcn.2010.138
Shi ZM, Hu XS, Yuan BJ, Gibson R, Dai Y, Garg M (2008) Association between magnesium : iron intake ratio and diabetes in Chinese adults in Jiangsu Province. Diabetic Med J Br Diabetic Assoc 25(10):1164–1170. https://doi.org/10.1111/j.1464-5491.2008.02558.x
Liu X, Wang X, Lin S, Song Q, Lao X, Yu IT (2015) Reproducibility and Validity of a food frequency questionnaire for assessing dietary consumption via the dietary pattern method in a chinese rural population. PLoS ONE 10(7):e0134627. https://doi.org/10.1371/journal.pone.0134627
Barak F, Falahi E, Keshteli AH, Yazdannik A, Saneei P, Esmaillzadeh A (2015) Red meat intake, insulin resistance, and markers of endothelial function among Iranian women. Mol Nutr Food Res 59(2):315–322. https://doi.org/10.1002/mnfr.201400333
Esfahani FH, Asghari G, Mirmiran P, Azizi F (2010) Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol 20(2):150–158. https://doi.org/10.2188/jea.je20090083
Y YX (2009) Ingredient list of Chinese food. Peking University Medical Press, Beijing
(2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England) 363(9403):157–163. https://doi.org/10.1016/s0140-6736(03)15268-3
Li Z, Xu Y, Huang Z, Wei Y, Hou J, Long T, Wang F, Hu H, Duan Y, Guo H, Zhang X, Chen X, Yuan H, Wu T, Shen M, He M (2019) Association between exposure to arsenic, nickel, cadmium, selenium, and zinc and fasting blood glucose levels. Environ Pollut (Barking, Essex : 1987) 255(Pt 2):113325. https://doi.org/10.1016/j.envpol.2019.113325
Pham PC, Pham PM, Pham SV, Miller JM, Pham PT (2007) Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol 2(2):366–373. https://doi.org/10.2215/cjn.02960906
(2021) 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes care 44(Suppl 1):S73–s84. https://doi.org/10.2337/dc21-S006
(2021) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes care 44(Suppl 1):S15–s33. https://doi.org/10.2337/dc21-S002
Yan S, Li M, Ma X, Jiang S, Sun M, Wang C, Pan Y, Sun C, Yao Y, Jin L, Li B (2019) Association of multiple mineral and vitamin B group intake with blood glucose using quantile regression analysis: NHANES 2007–2014. Food Nutr Res 63. https://doi.org/10.29219/fnr.v63.3560
Zhang Z, Zhao S, Wu H, Qin W, Zhang T, Wang Y, Tang Y, Qi S, Cao Y, Gao X (2022) Cross-sectional study: Relationship between serum trace elements and hypertension. J Trace Elem Med Biol 69:126893. https://doi.org/10.1016/j.jtemb.2021.126893
Bentil HJ, Abreu AM, Adu-Afarwuah S, Rossi JS, Tovar A, Oaks BM (2021) Association between dietary magnesium intake and glycemic markers in Ghanaian women of reproductive age: a pilot cross-sectional study. Nutrients 13(11). https://doi.org/10.3390/nu13114141
Akter S, Eguchi M, Nanri A, Kochi T, Kashino I, Kuwahara K, Hu H, Miki T, Kabe I, Mizoue T (2018) Association of dietary and serum magnesium with glucose metabolism markers: the Furukawa nutrition and health study. Clin Nutr ESPEN 24:71–77. https://doi.org/10.1016/j.clnesp.2018.01.011
Guerrero-Romero F, Simental-Mendía LE, Hernández-Ronquillo G, Rodriguez-Morán M (2015) Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: a double-blind placebo-controlled randomized trial. Diabetes Metab 41(3):202–207. https://doi.org/10.1016/j.diabet.2015.03.010
Navarrete-Cortes A, Ble-Castillo JL, Guerrero-Romero F, Cordova-Uscanga R, Juárez-Rojop IE, Aguilar-Mariscal H, Tovilla-Zarate CA, Lopez-Guevara Mdel R (2014) No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes Res 27(2):48–56. https://doi.org/10.1684/mrh.2014.0361
Xu T, Chen GC, Zhai L, Ke KF (2015) Nonlinear reduction in risk for type 2 diabetes by magnesium intake: an updated meta-analysis of prospective cohort studies. Biomed Environ Sci 28(7):527–534. https://doi.org/10.3967/bes2015.075
Ge X, Yang A, Huang S, Luo X, Hou Q, Huang L, Zhou Y, Li D, Lv Y, Li L, Cheng H, Chen X, Zan G, Tan Y, Liu C, Xiao L, Zou Y, Yang X (2021) Sex-specific associations of plasma metals and metal mixtures with glucose metabolism: an occupational population-based study in China. Sci Total Environ 760:143906. https://doi.org/10.1016/j.scitotenv.2020.143906
Kieboom BCT, Ligthart S, Dehghan A, Kurstjens S, de Baaij JHF, Franco OH, Hofman A, Zietse R, Stricker BH, Hoorn EJ (2017) Serum magnesium and the risk of prediabetes: a population-based cohort study. Diabetologia 60(5):843–853. https://doi.org/10.1007/s00125-017-4224-4
Kandeel FR, Balon E, Scott S, Nadler JL (1996) Magnesium deficiency and glucose metabolism in rat adipocytes. Metabolism Clin Exp 45(7):838–843. https://doi.org/10.1016/s0026-0495(96)90156-0
Suárez A, Pulido N, Casla A, Casanova B, Arrieta FJ, Rovira A (1995) Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia 38(11):1262–1270. https://doi.org/10.1007/bf00401757
Balon TW, Jasman A, Scott S, Meehan WP, Rude RK, Nadler JL (1994) Dietary magnesium prevents fructose-induced insulin insensitivity in rats. Hypertension (Dallas, Tex : 1979) 23(6 Pt 2):1036–1039. https://doi.org/10.1161/01.hyp.23.6.1036
Balon TW, Gu JL, Tokuyama Y, Jasman AP, Nadler JL (1995) Magnesium supplementation reduces development of diabetes in a rat model of spontaneous NIDDM. Am J Physiol 269(4 Pt 1):E745-752. https://doi.org/10.1152/ajpendo.1995.269.4.E745
Kehl KG, Findeisen HM, Fardo DW, Bruemmer D, Mannino DM, Sanderson WT (2011) Race-ethnicity as an effect modifier of the association between HbAlc and mortality in US adults without diagnosed diabetes. Eur J Endocrinol 165(2):275–281. https://doi.org/10.1530/eje-11-0171
Song Y, He K, Levitan EB, Manson JE, Liu S (2006) Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabetic Med 23(10):1050–1056. https://doi.org/10.1111/j.1464-5491.2006.01852.x
Simental-Mendía LE, Sahebkar A, Rodríguez-Morán M, Guerrero-Romero F (2016) A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol Res 111:272–282. https://doi.org/10.1016/j.phrs.2016.06.019
Saeed H, Haj S, Qasim B (2019) Estimation of magnesium level in type 2 diabetes mellitus and its correlation with HbA1c level. Endocrinol Diabetes Metab 2(1):e00048. https://doi.org/10.1002/edm2.48
Acknowledgements
The authors want to express their gratitude to all the participants and cooperating units who helped in completing this study.
Funding
The study was supported by the National Natural Science Foundation of China (Grant Nos. 81760577, 81960583, and 81560523), the Guangxi Science and Technology Development Project (Grant Nos. AD 17129003 and 18050005), the Guangxi Natural Science Foundation for Innovation Research Team (2019GXNSFGA245002), and Guangxi Scholarship Fund of Guangxi Education, Department of China.
Author information
Authors and Affiliations
Contributions
All authors designed the study and collected samples. Min Xu, Jiansheng Cai, Xiaoting Mo, Junling Zhang, Qiumei Liu, Yanfei Wei, Yinxia Lin, Shenxiang Huang, and Shuzhen Liu measured trace element. Min Xu and Jiansheng Cai drafted the manuscript. Professor Jian Qin and Zhiyong Zhang supervised this study.
Corresponding authors
Ethics declarations
Ethics Approval
Approval was obtained from the ethics committee of Guilin Medical University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate
Written informed consent files were received from all participants before the study.
Consent for Publication
The participant has consented to the submission of the case report to the journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, M., Cai, J., Mo, X. et al. Association of Dietary and Plasma Magnesium with Glycaemic Markers in a Chinese Population. Biol Trace Elem Res 201, 549–558 (2023). https://doi.org/10.1007/s12011-022-03172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03172-z