Abstract
Aluminum (Al) is linked to the development of many neurological disorders such as Alzheimer’s disease (AD), Parkinson’s disease, and autism. Centella asiatica (CA) is a regenerating herb traditionally used to stimulate memory. This study was designed to assess the neuroprotective role of ethanolic extract of CA (CAE) in AlCl3-induced neurological conditions in rats. Adult rats were chronically treated with AlCl3 (100 mg/kg b.w./day) for 60 days to establish the dementia model, and co-administration of CAE was evaluated for its ability to attenuate the toxic effect of AlCl3. CAE was given orally at a dose of 150 and 300 mg/kg b.w./day, for 60 days. The behavioral performances of rats were tested through Y-maze and open field tests. Lipid peroxidation, superoxide dismutase, and catalase activity were evaluated to measure oxidative stress; and acetylcholinesterase (AChE) activity was assessed to evaluate cholinergic dysfunction in the rat brain. H&E staining was used to assess structural abnormalities in the cortex and hippocampus. The result showed that AlCl3 induces cognitive dysfunction (impaired learning and memory, anxiety, diminished locomotor activity), oxidative stress, cholinergic impairment, and histopathological alteration in the rat brain. Co-administration of CAE with AlCl3 markedly protects the brain from AlCl3-induced cognitive dysfunction, oxidative stress, AChE activity, and cytoarchitectural alterations. Furthermore, 15 days CAE treatment after 45 days AlCl3 administration markedly ameliorates the AlCl3-induced neurotoxicity indicating its potential for therapeutic use.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) is the largest and most common element in the earth’s crust. The burden of biologically available Al is continuously increasing with global industrialization [1]. As humans are constantly being exposed to Al through diet, utensils, drinking water, cosmetics (antiperspirants), and medicines (antacids, buffered aspirin, adjuvant, antigen and allergen), there is an urgent need to understand the effect of Al exposure on humans [2]. AlCl3 enters the body through different routes and chronic exposure causes Al to accumulate in different regions of the rat brain, such as the hippocampus, cerebral cortex, and cerebellum, areas associated with learning and memory [3,4,5]. Increased Al concentration in the brain creates an alarming situation for several neurological disorders, e.g., encephalopathy, Alzheimer’s disease (AD), and Parkinson’s disease (PD) [6]. Furthermore, a study on the rodent model demonstrated that upon entering the brain, Al induces the generation of free radicals leading to oxidative stress in the cellular environment [7]. These free radicals alter the activity of superoxide dismutase (SOD) and catalase enzyme and also induce lipid peroxidation leading to neurodegeneration in the cerebral cortex and hippocampus [8, 9]. Metal-induced oxidative stress interferes with the cholinergic system [10] and eventually causes cognitive impairment, as cognitive functions are mainly dependent on the cholinergic neurotransmission [11]. Al exposure in rodents also is responsible for an increase in AChE activity that causes a reduction in acetylcholine concentration and loss of memory function [9]. Thus, Al-induced cognitive deficit has been widely used for translational research on oxidative stress-induced neurological conditions [8, 12, 13].
Extensive research is ongoing to explore the pharmacological activities of medicinal plants, primarily due to their antioxidant, and nootropic activities [14]. Centella asiatica (CA) (L) urban (family Apiaceae), also known as Indian pennywort or Gotu kola is a perennial herbaceous creeper [15]. It is considered a regenerating herb and has been used traditionally in the Indian subcontinent as a memory enhancer. The medicinal values of the plant are associated with the active constituents of CA, namely, asiatic acid, asiaticoside, madecassic acid, madecassoside, brahmoside, brahminoside, and flavonoids [16]. Asiatic acid is a powerful neuroprotective agent in the mouse model of cerebral ischemia [17]. It also is neuroprotective against AlCl3-induced amyloid pathology, oxidative stress, and apoptosis in rats [18]. Studies show that CA extract has antioxidant [19] and nootropic activity [20] and increases dendritic arborization in neuronal cells [21].
Only a limited number of studies have documented a neuroprotective role of Centella asiatica against AlCl3-induced neurotoxicity [22,23,24,25]. Most of them used AlCl3 in combination with D-galactose and also differ from the present study in terms of methodology, doses selection, and experimental measures. To the best of our knowledge, this is the first study to evaluate the neuroprotective role of ethanolic extract of Centella asiatica against AlCl3-induced neurotoxicity on different brain domains using behavioral, biochemical, and histological experiments. Furthermore, this study also assessed the therapeutic role of CAE to treat AlCl3-induced cognitive deficits and memory loss in animals.
Material and Methods
Preparation of Plant Extract
Green plant was collected from the university campus and identified, and a specimen was deposited in the botany department (voucher specimen number—Apia. 2019/1). The exact botanical name of the plant is Centella asiatica (L.) urban.
Plant material was shade dried, crushed to coarse material, and extracted with 70% ethanol (1 kg of the dried plant was extracted with 4.5 L of ethanol) for 3 days using Soxhlet apparatus exactly as described previously [26]. Finally, the thick green extract was obtained with a yield of 16.3% w/w.
Animals
Rats of Charles Foster strain (200–260 gm) were acquired from the central animal house of the university. Only male rats were used to avoid possible effects of gender variance. Animals were housed in standard conditions with a 12-h lighting regime and allowed to acclimatize for a week as described previously [26]. Standard animal diet (Krishna Valley Agrotech Private Ltd, India) and drinking water were provided ad libitum. The CPCSEA guidelines accepted by the Central Animal Ethical Committee of the University (letter-number—Dean/2017/CAEC/719, 30.03.2017) were followed to maintain the animals in this study.
Experimental Procedure
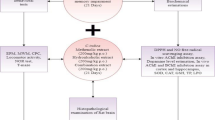
Animals were divided into five groups of five animals each, and all the treatments were given orally for a 60-day regime, as shown in Fig. 1.
-
1.
Control animals were given normal saline only.
-
2.
AlCl3-treated animals were given AlCl3 (100 mg/kg b.w./day) prepared in normal saline.
-
3.
CAE150-treated animals were given AlCl3 (100 mg/kg b.w./day) and aqueous solution of CAE (150 mg/kg b.w./day).
-
4.
CAE300-treated animals were given AlCl3 (100 mg/kg b.w./day) and aqueous solution of CAE (300 mg/kg b.w./day).
-
5.
CAE300, 15 days-treated animals were given AlCl3 (100 mg/kg b.w./day) for 45 days, and then, AlCl3 (100 mg/kg b.w./day) and aqueous solution of CAE (300 mg/kg b.w./day) for 15 days.
Doses of AlCl3 and CAE were based on previous reports [27, 28]. The animals were weighed weekly, and doses were adjusted accordingly. After this 60-day regime, the animals were subjected to behavioral tests.
Behavioral Study
Y-maze Test
The Y-maze test was used to evaluate spatial working memory and anxiety in animals. The experimental protocols were identical to those described earlier [26]. The Y-maze apparatus comprised of three arms namely, A, B, and C at 120° angle to each other. Briefly, one arm (novel arm, C) of the Y-maze was blocked and the animal was allowed to move between the other two arms (A and B) for 15 min (trial phase). After 4 h, the blocked arm was opened, and the rat was allowed to explore all three arms (A, B, and C) for 5 min (test phase). The movement of animals was traced using an attached camera. Alternate arm entries (ABC, CAB, BAC) are a measure of spatial working memory [29], which can be expressed as a spontaneous alternation score (SAS):
Furthermore, anxiety was measured as a tendency for animals to avoid visiting and staying in the novel arm and was expressed in terms of a reduction in coping behavior [30]:
Open Field Test (OFT)
To evaluate the exploratory nature and locomotor activity of the animals, the open field test was conducted [31] as described previously [26]. The animal was placed gently on one corner of the open field apparatus (divided into 16 equal squares) and allowed to explore the arena for 5 min. The animal’s movement was recorded, and the ambulation number was calculated by counting the number of squares crossed by the rat. The surface was cleaned with 70% alcohol before testing the next animal.
Biochemical Study
After the behavioral tests, rats were sacrificed with cervical dislocation. The brain was immediately isolated from the skull; the cerebrum and cerebellum were separated, and the cerebrum was divided by mid-sagittal cut into two halves. One-half of the cerebrums was washed with normal saline (cold) and kept at − 80 °C for histological study. The cerebellum and the other half of the cerebrum were washed with normal saline, weighed, and used to prepare homogenate for biochemical studies. The homogenate (10%, w/v) was prepared in PBS (pH 7.4) and centrifuged at 1000 g for 15 min at 4 °C. The pellet was discarded and the supernatant (S1) was collected and recentrifuged at 6000 g for 20 min at 4 °C in a cooling centrifuge (C-24 BL, Remi Instrumentation Ltd, India). The resulting supernatant (S2) was collected and used for all biochemical studies, namely, lipid peroxidation, SOD, catalase, and AChE assay.
Lipid Peroxidation Assay
To determine lipid peroxidation, malondialdehyde (MDA) content was measured using the Wills method [32]. Briefly, 0.5 ml of S2 supernatant was mixed with 0.5 ml of 20% trichloroacetic acid (TCA) and centrifuged at 2000 g for 10 min, and the supernatant was collected. A total of 0.5 ml of this supernatant was mixed with 0.5 ml glacial acetic acid (50%) and 1 ml thiobarbituric acid (TBA) (0.67%) in a tube and kept in the boiling water bath for 15 min. After cooling, the absorbance was measured at 532 nm. MDA content was calculated using an extinction coefficient of 1.56 × 105 M−1 cm−1 and reported as nmol/mg protein [33].
Catalase Assay
Aebi’s method was used to measure the catalase activity [34] by monitoring the rate of H2O2 breakdown at 240 nm. In brief, 990 µl of catalase buffer (0.036% H2O2 prepared in 50 mM phosphate buffer, pH 7.0) was taken in a cuvette, and the reaction was initiated by adding 10 µl S2 supernatant. Enzyme activity was measured immediately at 240 nm for 3 min and expressed as µmol/min/mg protein.
Superoxide Dismutase (SOD) Assay
The SOD assay was carried out by the method of Fridovich and Beauchamp [35] as described previously [26]. In brief, the sample tube had 130 mM L-methionine (300 µl), 250 µl S2 supernatant, 750 µM NBT (150 µl), 0.5 mM EDTA (75 µl), 60 µM riboflavin (100 µl), and SOD buffer (pH 7.8) in a total volume of 1500 µl. The Control and blank tubes had all the above chemicals except supernatant and riboflavin, respectively. Then, all the tubes were placed in front of the fluorescent light for 10 min and the color produced was read at 560 nm. Enzyme activity was calculated by measuring the percentage inhibition and expressed as µmol/min/mg protein.
Acetylcholinesterase (AChE) Activity
AChE assay was carried out by Ellman’s method [36] as described previously [26]. Briefly, the reaction mixture contains 0.1 M phosphate buffer (260 µl, pH 8.0), S2 supernatant (40 µl), and 10 mM DTNB (10 µl) in an ELISA plate. The reaction was initiated by adding 2 µl of acetylthiocholine (ATCI, 75 mM) and kept in the dark for 20 min. AChE hydrolyses ATCI and produces thiocholine which reacted with DTNB to give a yellow color product (read at 412 nm). AChE activity was expressed as µmoles of substrate hydrolyzed/min/mg protein.
Protein Estimation
Protein estimation was done by the Bradford method using BSA as standard [37].
Histological Study
Half cerebrum was taken out from − 80 °C, placed into the cryostat (Leica, Germany) at − 20 °C, and then mounted with the help of a tissue freezing medium. Sagittal Sects. (20 µm) were collected on gelatin pre-coated glass slides, and stored at − 20 °C.
H&E staining was done as described previously [26]. Briefly, slides were passed through xylene and a graded series of ethanol (100, 90, 70, and 50%) for 5 min each to hydrate the tissue sections. Then, slides were dipped in hematoxylin for 2 min, washed under running tap water, and counterstained with eosin (1 min). Slides were passed through a graded series of ethanol (50, 70, 90, and 100%, 5 min each) to dehydrate, and then placed in xylene before being coverslipped. Sections then were photographed with a camera (Magcam DC 5MP, Sony) attached to the light microscope (CX21iLED, Olympus).
Quantitative Analysis of Neurodegeneration
A quantitative analysis of the number of healthy cells was conducted in the cerebral cortex and the CA1 hippocampal area of the rat brain. The analysis was done on three randomly selected regions using image J 1.52v software. The average value (cell number) of each region was calculated and graphed.
Statistical Analysis
Graph pad prism (version 5.1) was used for all statistical analyses. The data were analyzed using one-way ANOVA followed by the Newman-Keuls test, except in the body weight analysis where repeated measures (RM) ANOVA was used. Data are presented as mean ± SD of five observations. p values ˂ 0.05 were considered statistically significant.
Results
Effect of CAE on Body Weight
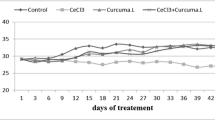
Chronic AlCl3 treatment caused a gradual decline in body weight that was prevented by co-treatment with CAE (150 and 300 mg/kg b.w.), and was partially reversed when CAE (300 mg/kg b.w.) was given for just the last 2 weeks of AlCl3 treatment. The effect of treatment on changes in animal body weight was found significant (F(4, 28) = 15.33, p < 0.0001) by RM-ANOVA analysis (Fig. 2). The body weight of AlCl3-treated rats decreased continuously throughout the experimentation and post-hoc analysis exhibited a significant (p < 0.001) difference between the Control and AlCl3-treated group after 60 days of treatment. In CAE(150; 300) co-administered groups, body weight was significantly (p < 0.001) greater than the AlCl3-treated group and was nearly similar to the Control group. In CAE300, 15 days-treated group, the body weight decreased initially (for 6 weeks) when only AlCl3 was given, and the reduction pattern was found similar to the AlCl3-treated group. After the 6th week, upon CAE co-administration, a gradual increase in body weight was noticed (for the last 15 days); t-test analysis at 60 days revealed that body weight was significantly greater (p ˂ 0.0125) in the CAE300, 15 days-treated group as compared to the AlCl3-treated group.
Effect of AlCl3 and CAE on body weight of rats. All animals received AlCl3 (100 mg/kg b.w./day) for 60 days except Control group which received normal saline only. CAE was co-administered at a dose of 150 mg/kg b.w./day in CAE150 group and at 300 mg/kg b.w./day in CAE300 group for 60 days. In CAE300,15d group, CAE was co-administered at a dose of 300 mg/kg b.w./day for last 15 days only. Data was analyzed by repeated measures ANOVA followed by Newman-Keuls test and presented as mean ± SD (n = 5). ###p < 0.001 compared with Control group, ***p < 0.001 compared with AlCl3 group
Effect of CAE on Memory
Chronic AlCl3 treatment for 60 days induced behavioral impairments that were protected by co-treatment with CAE (150 and 300 mg/kg b.w.), and was moderately improved when CAE was given just for the last 2 weeks of AlCl3-treatment (Fig. 3A and B). One-way ANOVA revealed a significant effect of treatment on SAS (F(4, 20) = 28.32, p < 0.0001) and coping behavior (F(4, 20) = 14.34, p < 0.0001). Furthermore, post hoc analysis showed a significantly (p < 0.001) lower SAS and coping behavior in the AlCl3-treated rats, compared with the Control rats. On the contrary, in CAE(150; 300; 300, 15 days) co-treated groups, SAS (p < 0.01) and coping behavior (p < 0.05) were significantly greater than in the AlCl3-treated group. Furthermore, the difference between the Control and CAE(150; 300) co-treated groups were insignificant (p > 0.05).
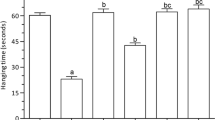
Effect of AlCl3 and CAE on behavioral performance of rats in Y-maze and open field test. All animals received AlCl3 (100 mg/kg b.w./day) for 60 days except Control group which received normal saline only. CAE was co-administered at a dose of 150 mg/kg b.w./day in CAE150 group and at 300 mg/kg b.w./day in CAE300 group for 60 days. In CAE300,15d group, CAE was co-administered at a dose of 300 mg/kg b.w./day for last 15 days only. Changes in spontaneous alternation score (A) and coping behavior (B) in Y-maze and ambulation number (C) in open field test were assessed. Data was analyzed by one-way ANOVA followed by Newman-Keuls test and presented as mean ± SD (n = 5). ###p < 0.001 compared with Control group, ***p < 0.001, **p < 0.01, and *p < 0.05 compared with AlCl3 group
There was a significant effect of treatment on ambulation number as revealed by the one-way ANOVA (F(4, 20) = 14.82, p < 0.0001). Newman-Keuls test revealed a significantly lower ambulation number (p < 0.001) in AlCl3-treated rats than in Control rats (Fig. 3C). In CAE(150; 300; 300, 15 days) co-treated groups, the ambulation number was significantly greater (p < 0.01) than in the AlCl3-treated group. Furthermore, the difference between Control and CAE(150; 300) co-treated groups were insignificant (p > 0.05).
Effect of CAE on Oxidative Stress in the Cerebrum and Cerebellum of Rat Brain
Chronic AlCl3 treatment for 60 days produced oxidative stress in the cerebrum that was prevented by co-treatment with CAE and was substantially reversed when CAE was given just for the last 2 weeks of AlCl3-treatment (Fig. 4A, B, and C). One-way ANOVA result showed a significant effect of treatment on MDA content (F(4, 20) = 26.5, p < 0.0001)). Newman-Keuls post hoc analysis showed that MDA content was significantly (p < 0.001) higher in AlCl3-treated rat cerebrum in comparison to the Control group. Furthermore, it was observed that co-administration of CAE(150; 300; 300, 15 days) reduces the MDA content significantly (p < 0.001) as compared with AlCl3-treated group (Fig. 4A). The difference in MDA content between Control and CAE300-treated group was insignificant (p > 0.05).
Effect of AlCl3 and CAE on oxidative stress parameters in the rat brain. All animals received AlCl3 (100 mg/kg b.w./day) for 60 days except Control group which received normal saline only. CAE was co-administered at a dose of 150 mg/kg b.w./day in CAE150 group and at 300 mg/kg b.w./day in CAE300 group for 60 days. In CAE300,15d group, CAE was co-administered at a dose of 300 mg/kg b.w./day for last 15 days only. MDA content, SOD, and catalase activity were assessed in supernatant (S2) fraction prepared from the cerebrum (A, B, and C) and cerebellum (D, E, and F) of different groups. Data was analyzed by one-way ANOVA followed by Newman-Keuls test and presented as mean ± SD (n = 5). ###p < 0.001 compared with Control group, ***p < 0.001, **p < 0.01, and *p < 0.05 compared with AlCl3 group
Furthermore, One-way ANOVA result showed a significant effect of treatment on SOD (F(4, 20) = 18.21, p ˂ 0.0001) and catalase (F(4, 20) = 43.58, p < 0.0001) activity. Newman-Keuls post hoc test revealed that SOD and catalase activity were significantly (p < 0.001) higher in AlCl3-treated rats as compared with the Control group (Fig. 4B and C). However, in CAE(150; 300; 300, 15 days) co-treated rats, the SOD and catalase activity were significantly (p < 0.001) low as compared with the AlCl3-treated group. Differences in SOD activity between Control and CAE(150; 300; 300, 15 days) co-treated groups were insignificant (p > 0.05). In the catalase assay, the difference between Control and CAE300-treated group was insignificant (p > 0.05).
The chronic AlCl3 treatment also caused oxidative stress in the cerebellum, while CAE treatment attenuated this effect (Fig. 4D, E, and F). One-way ANOVA showed a significant effect of treatment on MDA content (F(4, 20) = 10.24, p < 0.0001). Post hoc analysis revealed a significantly (p < 0.001) greater MDA content in AlCl3-treated rats compared to the Control rats (Fig. 4D). However, MDA content was significantly lower in CAE(150; 300; 300, 15 days)-treated rats as compared with AlCl3-treated rats (p < 0.05). Differences between Control and CAE(150; 300) co-treated groups were insignificant (p > 0.05).
One-way ANOVA result showed a significant effect of treatment on SOD (F(4, 20) = 9.025, p ˂ 0.0002) and catalase activity (F(4, 20) = 21.06, p ˂ 0.0001). Post hoc analysis revealed a significantly (p < 0.001) higher SOD and catalase activity in AlCl3-treated rats compared to the Control rats. After CAE(150; 300; 300, 15 days) co-administration, a significantly lower SOD (p < 0.01) and catalase activity (p < 0.001) was noticed in comparison to the AlCl3-treated group (Fig. 4E and F). Differences in SOD value between Control and varying doses of CAE co-treated groups were insignificant (p > 0.05). The difference in the catalase activity between Control and CAE300-treated group was insignificant (p > 0.05).
Effect of CAE on AChE Activity in the Cerebrum and Cerebellum of Rat Brain
Chronic treatment of AlCl3 for 60 days resulted in elevated AChE activity both in the cerebrum and cerebellum, which was prevented by co-treatment with CAE, and was considerably reversed when CAE was given for just the last 2 weeks of AlCl3-treatment. One-way ANOVA result showed a significant effect of treatment on AChE activity (F(4, 20) = 15.96, p < 0.0001) in the cerebrum. Newman-Keuls post hoc test showed a significantly (p < 0.001) higher AChE activity in AlCl3 treated group compared to the Control group, whereas co-treatment of varying doses of CAE(150; 300; 300, 15 days) resulted in the significantly (p ˂ 0.01) lower AChE activity in comparison with the AlCl3-treated group (Fig. 5A). Differences between Control and CAE(150; 300) co-treated groups were insignificant (p > 0.05).
Effect of AlCl3 and CAE on AChE activity in the cerebrum and cerebellum of rat brain. All animals received AlCl3 (100 mg/kg b.w./day) for 60 days except Control group which received normal saline only. CAE was co-administered at a dose of 150 mg/kg b.w./day in CAE150 group and at 300 mg/kg b.w./day in CAE300 group for 60 days. In CAE300,15d group, CAE was co-administered at a dose of 300 mg/kg b.w./day for last 15 days only. AChE activity was measured in supernatant (S2) fraction prepared from the cerebrum (A) and cerebellum (B) of different groups. Data was analyzed by one-way ANOVA followed by Newman-Keuls test and presented as mean ± SD (n = 5). ###p < 0.001 compared with Control group, ***p < 0.001, **p < 0.01, and *p < 0.05 compared with AlCl3 group
One-way ANOVA showed a significant effect of treatment on AChE activity (F(4, 20) = 20.3, p ˂ 0.0001) in the cerebellum. Post hoc analysis revealed significantly (p < 0.001) higher AChE activity in AlCl3-treated group compared to the Control group. In CAE(150; 300; 300, 15 days) co-administrated groups, the AChE activity was significantly (p ˂ 0.001) lower compared to the AlCl3-treated group (Fig. 5B). The differences between Control and CAE(150; 300) co-treated groups were insignificant (p > 0.05).
Effect of CAE on Histological Changes in the Cerebral Cortex and Hippocampal Area of the Rat Brain
Chronic AlCl3 treatment for 60 days induced histological changes in the cortex and hippocampal region that were prevented by co-treatment with CAE and was partially reversed when CAE was given for just the last 2 weeks of AlCl3-treatment. Figure 6 (upper panel) shows the effect of CAE on histological changes in the cerebral cortex of different experimental groups. No visible histological abnormalities were noted in the cortex of the Control group. The AlCl3-treated group showed marked histological abnormalities in the cerebral cortex irregular cells with indistinct cell margins, pyknosis, karyolysis, pericellular edema, karyorrhexis, and vacuolated cytoplasm. All these findings indicated that AlCl3 induces neurodegeneration in the cortex region of the rat brain. CAE(150; 300; 300, 15 days) co-treatment improved the histology considerably in the area of the cerebral cortex as compared with AlCl3-treated group. In the CAE300-treated group, the architecture was almost identical to the Control group; most neuronal cells were intact and healthy. Virtually, no abnormal features like pericellular edema, nuclear pyknosis, indistinct cell margin, and karyorrhexis were noticed. Small focal areas of neuropil vacuolation were observed in CAE300, 15 days-treated group.
Effect of AlCl3 and CAE on histological changes in the cerebral cortex (upper panel) and CA1 hippocampal area (lower panel) of rat brain. All animals received AlCl3 (100 mg/kg b.w./day) for 60 days except Control group which received normal saline only. CAE was co-administered at a dose of 150 mg/kg b.w./day in CAE150 group and at 300 mg/kg b.w./day in CAE300 group for 60 days. In CAE300,15d group, CAE was co-administered at a dose of 300 mg/kg b.w./day for last 15 days only. Sagittal brain Sects. (20 µm) were collected on slides followed by H&E staining. Slides were examined under light microscope at 10 × with a scale bar of 50 µm. CAE supplementation attenuated architectural changes induced by AlCl3-treatment. Arrows show karyolysis in the cortex and hippocampus region
The lower panel of Fig. 6 shows the effect of CAE on histological changes in the CA1 hippocampal area of different experimental groups. The Control group showed the intact nucleus with a distinct nuclear membrane in the CA1 region of the hippocampus. The cells were arranged tightly and linearly in a circular fashion. However, irregular cells with minimal cytoplasm and diffused morphology, indistinct outer membrane, and degraded nucleus were observed in the AlCl3-treated group; typical closely packed linear arrangements of the cells were disrupted. CAE(150; 300; 300, 15 days) co-administration for 60 days markedly diminished the structural anomalies caused by AlCl3. Cellular architecture in CAE(150; 300) co-treated groups appeared almost normal, but in CAE300, 15 days-treated group, some cellular damages were still present.
Figure 7 shows the cell count in the H&E-stained sections of the cerebral cortex and CA1 area of the hippocampus. One-way ANOVA showed a significant effect of treatment on cell count (normal cells) in the cortex (F(4, 10) = 32.46, p < 0.0001) and in the CA1 hippocampal region (F(4, 20) = 19.31, p < 0.0001). Post hoc analysis showed a significant (p < 0.001) reduction in cell count in the cortex and in the CA1 hippocampal area of the AlCl3-treated rats compared to the Control rats. However, in CAE(150; 300; 300, 15 days) co-administered groups, a significant amelioration in cell count was observed in the cortex (p < 0.05) as well as in the CA1 hippocampal area (p < 0.01) in comparison to AlCl3-treated group. The difference between Control and CAE300-treated group was insignificant (p > 0.05) in the CA1 and in the cortical region.
Effect of AlCl3 and CAE on neuronal changes in the rat brain. All animals received AlCl3 (100 mg/kg b.w./day) for 60 days except Control group which received normal saline only. CAE was co-administered at a dose of 150 mg/kg b.w./day in CAE150 group and at 300 mg/kg b.w./day in CAE300 group for 60 days. In CAE300,15d group, CAE was co-administered at a dose of 300 mg/kg b.w./day for last 15 days only. Sagittal brain Sects. (20 µm) were collected on slides followed by H&E staining. Cell counting was done on three randomly selected regions using image J 1.52v software. Graph exhibits cell count of cerebral cortex and CA1 hippocampal area of different experimental groups. Data are expressed as mean ± SD. One-way ANOVA followed by Newman-Keuls test. ###p < 0.001 compared with Control group, ***p < 0.001, **p < 0.01, and *p < 0.05 compared with AlCl3 group
Discussion
In this study, the neuroprotective effect of CAE was investigated in the AlCl3-induced memory-impaired rat model. Al is a non-redox trivalent cation and has been recognized as a causal factor in various neurological disorders because of its neurotoxicity [38].
We found a significant decrease in body weight of animals treated with AlCl3 and a significant increase in body weight following CAE supplementation. This protective effect of CAE on body weight is consistent with an earlier study [39]. Moreover, the improvement in body weight after CAE administration is an indication of a positive link between physical and mental health [40].
In the Y-maze test, we found that administration of AlCl3 resulted in a marked reduction in the SAS, which indicates impaired spatial working memory in rats. Rats normally exhibit a preference to explore novel locations, and those with compromised working memory do not remember the alternative sequence of arm entry, resulting in a reduction in the SAS [29]. Our data are consistent with an earlier report where exposure to Al reduced SAS in rats [41]. Exposure to AlCl3 also decreased entry into the novel arm, reflecting a lack of curiosity to explore the new environment. Reduced coping behavior in AlCl3-treated rats suggests that Al toxicity causes anxiety in animals. Importantly, CAE supplementation (150 and 300 mg/kg) in AlCl3-treated rats caused a notable increase in both SAS and coping behavior. This behavioral improvement indicates the retention of spatial working memory and anxiolytic effects. The similar memory-enhancing effect of CAE was observed in the AD rat model [24].
Ambulation number was significantly reduced in the OFT after AlCl3-treatment: a decrease in locomotor activity has been associated with anxiety in animals [42]. These results from the OFT are also supported by the coping behavior data of Y-maze test [43]. CAE supplementation showed substantial improvement in the OFT locomotor activity compared to AlCl3-treated rats. Rather et al. had shown that asiatic acid, one of the phytoconstituents of CA, improved the exploratory movement of rats in the OFT after chronic exposure to Al [44].
Numerous studies suggest that oxidative damage plays a critical role in a number of neurological conditions [14, 45, 46], and the accumulation of AlCl3 in the brain is associated with oxidative stress [4, 5, 47]. AlCl3 does not act as a pro-oxidant directly, but assists in iron- or noniron-induced lipid peroxidation [48, 49]. Therefore, AlCl3 indirectly causes oxidative damage to membrane lipids, which can severely affect membrane properties and functions. MDA is a biomarker of lipid peroxidation. In the present study, MDA levels increased significantly in the cerebrum and cerebellum after AlCl3 exposure, which is consistent with previous reports [44, 50]. Co-administration of CAE with AlCl3 lowered the extent of lipid peroxidation, suggesting a reduction in oxidative stress. Other work has also shown that asiatic acid protects the cortical and hippocampal region of the rat brain from lipid peroxidation induced by AlCl3 [44]. Under the influence of oxidative stress, enzymes such as SOD and catalase form the primary line of defense against free radicals in biological system [51]. In the present study, a significant increase in SOD and catalase activity occurred in the cerebrum and cerebellum following exposure to AlCl3. In normal physiological conditions, SOD metabolizes superoxide anion (O2−) and releases hydrogen peroxide (H2O2) [52]; catalase clears excess H2O2 [53]. Therefore, a rise in free radical production may account for the increased activity of SOD and catalase. The similar effect of AlCl3 exposure has been reported by different laboratories [27, 54, 55]. CAE stabilizes mitochondrial functions by reducing the formation of oxidative products [56], and importantly, the CAE treatment in the present study was associated with normalization of the MDA content, and catalase and SOD activity in the cerebrum and cerebellum, suggesting a reduction in oxidative stress. The decline in oxidative stress due to CAE supplementation in this study may be due to its strong free radical scavenging and iron chelation properties [57].
This study shows that AlCl3 increases AChE activity in the cerebrum and cerebellum of the rat brain, which declines after CAE supplementation. An increase in brain AChE activity after Al exposure [58, 59] and a reduction in brain AChE activity after CAE administration have also been observed previously [25]. It seems that the memory-enhancing effect of CAE, as seen in behavioral studies (Fig. 3), may be due to its AChE inhibition potential, which can be attributed to the presence of active phytoconstituents such as asiatic acid [18]. Inhibition of AChE activity is critical to the development of anti-AD medications.
Studies have shown a close relationship between oxidative stress and increased AChE activity, and increased AChE activity is associated with a decline in acetylcholine concentration, which reduces cholinergic neurotransmission [29, 60, 61]. Cholinergic neurotransmission plays an important role in the pathophysiology of memory impairment, as the cholinergic pathways have neural projections to the cerebral cortex and hippocampus [62].
Histological examination of the cerebral cortex and hippocampus revealed that AlCl3 mediates gradual pathological changes that include disorganization in the pyramidal cellular arrangement, dense cytosolic staining, nuclear degradation, pyknosis, and karyorrhexis. These findings corroborate former studies [44, 63] and could be due to the increased free radical formation after AlCl3 exposure that eventually causes oxidative damage and neurodegeneration. Interestingly, after CAE administration for 60 days, the pathological changes improved markedly. In addition, a significant reduction in the number of cells was noted after AlCl3 administration, and CAE co-administration reduced the loss of neuronal cells; the result was also supported by a parallel study on AD model of rat [24]. Thus, co-administration of CAE with AlCl3 may alleviate the structural abnormalities and neurodegeneration, thereby maintaining the normal architecture of the regions examined. This protective property of CAE can be attributed to its free radical scavenging activity, which removes the excess free radical burden and protects the entire cellular environment from oxidative damage.
The behavioral, biochemical, and histological results of this study clearly show that CAE at a dose of 150 or 300 mg/kg b.w. for 60 days effectively protects the brain from AlCl3 intoxication. Insignificant difference between the Control and CAE300-treated groups in all set of experiments shows that CAE at a dose of 300 mg/kg b.w. was effective in protecting the AlCl3-induced brain damages. CAE attenuates AlCl3-induced oxidative stress and AChE activity that consecutively ameliorates cellular damage, neurodegeneration, and cholinergic activity, ultimately leading to improved cognitive function. Moreover, the results of the CAE300, 15 days-treated group show that CAE (300 mg/kg b.w.) treatment for 15 days only can significantly reverse the AlCl3-induced toxicity in rat brain and thus can be used therapeutically to treat AlCl3-induced neurotoxicity.
Conclusion
CAE not only protects but also aids to recover from AlCl3-induced cognitive deficit, oxidative stress, cholinergic dysfunction, and neurodegeneration in rat brain. Hence, CAE could be used as an antioxidant, anti-cholinesterase, memory enhancer, and neuroprotective agent. Further research is needed to identify the active phytoconstituents and their mode of action for a complete understanding of the molecular mechanisms involved.
Data Availability
Not applicable.
References
Exley C (2013) Human exposure to aluminium. Environ Sci Process Impacts 15(10):1807–1816
Grochowski C et al (2019) Increased aluminum content in certain brain structures is correlated with higher silicon concentration in alcoholic use disorder. Molecules 24(9):1721
Mold M et al (2018) Aluminium in brain tissue in autism. J Trace Elem Med Biol 46:76–82
Yuan C-Y, Lee Y-J, Hsu G-SW (2012) Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J Biomed Sci 19(1):51
Gómez M et al (2008) Aluminum exposure through the diet: metal levels in AβPP transgenic mice, a model for Alzheimer’s disease. Toxicology 249(2–3):214–219
Becaria A, Campbell A, Bondy S (2002) Aluminum as a toxicant. Toxicol Ind Health 18(7):309–320
Wu Z et al (2012) Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiol Aging 33(1): 199. e1–199. e12.
Al-Amin MM et al (2016) Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Eur J Pharmacol 777:60–69
Auti ST, Kulkarni YA (2019) Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol. https://doi.org/10.3389/fneur.2019.00399
Kosari S et al (2012) Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav Brain Res 235(1):98–103
Batool Z et al (2016) Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res Bull 120:63–74
Wang B et al (2010) Effects of chronic aluminum exposure on memory through multiple signal transduction pathways. Environ Toxicol Pharmacol 29(3):308–313
Ribes D et al (2010) Impaired spatial learning and unaltered neurogenesis in a transgenic model of Alzheimer’s disease after oral aluminum exposure. Curr Alzheimer Res 7(5):401–408
Firdaus Z, Singh TD (2021) An insight in pathophysiological mechanism of Alzheimer’s disease and its management using plant natural products. Mini Rev Med Chem 21(1):35–57
Gray NE et al (2018) Centella asiatica: phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev 17(1):161–194
Gohil KJ, Patel JA, Gajjar AK (2010) Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci 72(5):546
Krishnamurthy RG et al (2009) Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res 87(11):2541–2550
Rather MA et al (2018) Neuroprotective role of Asiatic acid in aluminium chloride induced rat model of Alzheimer’s disease. Front Biosci (Schol Ed) 10:262–275
Gupta R, Flora S (2006) Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J Appl Toxicol 26(3):213–222
Rao SB, Chetana M, Devi PU (2005) Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol Behav 86(4):449–457
Mohandas Rao K, Muddanna Rao S, Gurumadhva Rao S (2006) Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Evidence-Based Complementary and Alternative Medicine 3(3): 349–357
Chiroma SM et al (2019) Centella asiatica protects d-galactose/AlCl3 mediated Alzheimer’s disease-like rats via PP2A/GSK-3β signaling pathway in their hippocampus. Int J Mol Sci 20(8):1871
Chiroma SM et al (2019) Protective effects of centella asiatica on cognitive deficits induced by D-gal/AlCl3 via inhibition of oxidative stress and attenuation of acetylcholinesterase level. Toxics 7(2):19
Chiroma SM et al (2019) Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: behavioral and ultrastructural approaches. Biomed Pharmacother 109:853–864
Amjad S, Umesalma S (2015) Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain-histopathological, and biochemical approach. J Molec Biomark Diag 6(1):1
Firdaus Z et al (2020) Centella asiatica prevents D-galactose-induced cognitive deficits, oxidative stress and neurodegeneration in the adult rat brain. Drug Chem Toxicol. https://doi.org/10.1080/01480545.2020.1833907
Singla N, Dhawan D (2014) Zinc modulates aluminium-induced oxidative stress and cellular injury in rat brain. Metallomics 6(10):1941–1950
Kumar A, Prakash A, Dogra S (2011) Centella asiatica attenuates D-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int J Alzheimer’s Dis. https://doi.org/10.4061/2011/347569
Foyet HS et al (2015) Emilia coccinae (SIMS) G extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in scopolamine-treated rats. BMC Complement Altern Med 15(1):333
Poimenova A et al (2010) Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience 167(3):741–749
Lee M-S et al (2013) Novel antidepressant-like activity of propolis extract mediated by enhanced glucocorticoid receptor function in the hippocampus. Evidence-Based Complement Alternat Med. https://doi.org/10.1155/2013/217853
Wills E (1965) Mechanisms of lipid peroxide formation in tissues role of metals and haematin proteins in the catalysis of the oxidation of unsaturated fatty acids. Biochimica et Biophysica Acta (BBA)-Lipids Lipid Metabol 98(2): 238–251
Kumar B, Kuhad A, Chopra K (2011) Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology 214(4):819–828
Aebi H (1974) Catalase. In: Bergmeyer, HU (ed) Methods of Enzymatic Analysis, 2nd edn. Verlag Chemie/Academic Press Inc., Weinheim/NewYork, 673-684. https://doi.org/10.1016/B978-0-12-091302-2.50032-3
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Ellman GL et al (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2): 88IN191–9095
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Yokel RA (2002) Brain uptake, retention, and efflux of aluminum and manganese. Environ Health Perspect 110(suppl 5):699–704
Wei L et al (2018) Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int Immunopharmacol 60:1–8
Rao MK, Rao MS, Rao GS (2007) Treatment with Centalla asiatica (Linn) fresh leaf extract enhances learning ability and memory retention power in rats. Neurosciences 12(3):236–241
Bazzari FH, Abdallah DM, El-Abhar HS (2019) Chenodeoxycholic acid ameliorates AlCl3-Induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules 24(10):1992
Carola V et al (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134(1–2):49–57
Li M et al (2020) Differentially expressed genes in the brain of aging mice with cognitive alteration and depression-and anxiety-like behaviors. Front Cell Develop Biol 8:814
Rather MA et al (2019) Asiatic acid attenuated aluminum chloride-induced tau pathology, oxidative stress and apoptosis via AKT/GSK-3β signaling pathway in wistar rats. Neurotox Res 35(4):955–968
Manoharan S et al (2016) The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: a mini review. Oxidat Med Cell Longevity. https://doi.org/10.1155/2016/8590578
Arimon M et al (2015) Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol Dis 84:109–119
Nayak P, Sharma SB, Chowdary NVS (2010) Augmentation of aluminum-induced oxidative stress in rat cerebrum by presence of pro-oxidant (graded doses of ethanol) exposure. Neurochem Res 35(11):1681–1690
Exley C (2004) The pro-oxidant activity of aluminum. Free Radical Biol Med 36(3):380–387
Zatta P et al (2002) Aluminium (III) as a promoter of cellular oxidation. Coord Chem Rev 228(2):271–284
Mathiyazahan DB, Thenmozhi AJ, Manivasagam T (2015) Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: a behavioural, biochemical and molecular approach. J Funct Foods 16:423–435
Benzi G et al (1989) Age-related effect induced by oxidative stress on the cerebral glutathione system. Neurochem Res 14(5):473–481
Jyoti A, Sethi P, Sharma D (2007) Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J Ethnopharmacol 111(1):56–62
Reddy PH (2006) Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem 96(1):1–13
Bhalla P, Dhawan D (2009) Protective role of lithium in ameliorating the aluminium-induced oxidative stress and histological changes in rat brain. Cell Mol Neurobiol 29(4):513–521
Esparza JL et al (2005) Melatonin reduces oxidative stress and increases gene expression in the cerebral cortex and cerebellum of aluminum-exposed rats. J Pineal Res 39(2):129–136
Prakash A, Kumar A (2013) Mitoprotective effect of Centella asiatica against aluminum-induced neurotoxicity in rats: possible relevance to its anti-oxidant and anti-apoptosis mechanism. Neurol Sci 34(8):1403–1409
Mohd Salim R et al (2013) Statistical analysis of metal chelating activity of Centella asiatica and Erythroxylum cuneatum using response surface methodology. Biotechnol Res Int. https://doi.org/10.1155/2013/137851
Fadl N et al (2013) Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum Exp Toxicol 32(7):721–735
Jayant S, Sharma B, Sharma B (2016) Protective effect of transient receptor potential vanilloid subtype 1 (TRPV1) modulator, against behavioral, biochemical and structural damage in experimental models of Alzheimer’s disease. Brain Res 1642:397–408
Maurer SV, Williams CL (2017) The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol 8:1489
Kaizer RR et al (2005) Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J Inorg Biochem 99(9):1865–1870
H Ferreira-Vieira T et al (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14(1): 101–115
Moneim AEA (2012) Evaluating the potential role of pomegranate peel in aluminum-induced oxidative stress and histopathological alterations in brain of female rats. Biol Trace Elem Res 150(1–3):328–336
Acknowledgements
We are thankful to Prof. Kathy W. Nordeen (Brain and Cognitive Sciences, University of Rochester, NY, USA) for her valuable suggestions and for editing the manuscript.
Funding
This work was supported by a fellowship grant to ZF from the Council of Scientific and Industrial Research (CSIR), India.
Author information
Authors and Affiliations
Contributions
ZF and TDS conceptualized and designed the study and prepared the manuscript. ZF carried the protocol and DY and SKS helped in behavioral and histological studies. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Central Animal Ethical Committee of the Banaras Hindu University (letter number—Dean/2017/CAEC/719, 30.03.2017).
Consent for publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Firdaus, Z., Kumar, D., Singh, S.K. et al. Centella asiatica Alleviates AlCl3-induced Cognitive Impairment, Oxidative Stress, and Neurodegeneration by Modulating Cholinergic Activity and Oxidative Burden in Rat Brain. Biol Trace Elem Res 200, 5115–5126 (2022). https://doi.org/10.1007/s12011-021-03083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03083-5