Abstract
This study aimed to investigate the effect of selenium and magnesium nanoparticles and their combination on growth, serum biochemical and immune parameters, serum hepatic enzymes, and digestive enzymes in Asian Sea bass (Lates calcarifer), with an average weight of 32.78 ± 1.16 g for 42 days. After 4 weeks of adaptation to experimental conditions, 96 fish were randomly distributed equally in 12 cylindrical 300-l fiberglass tanks. Four treatment groups included control, 4 mg nano-selenium (Nano-Se), 500 mg nano-magnesium (Nano-Mg), and a combined treatment of 4 mg Nano-SE + 500 mg Nano-Mg treatments examined in this study. The fish fed twice a day as satiation. The results showed fish fed with combined treatment showed the highest rate of body weight gain and the specific growth rate. Digestive enzymes were significantly different between experimental treatments (p < 0.05), whereas amylase did not show a significant difference between experimental treatments (p > 0.05). The results of this study showed that serum levels of glucose, cholesterol, triglyceride, and protein indices did not show any significant difference (p > 0.05). The immunoglobulin, IgM, C3, and ACH50 indexes did not differ significantly between the experimental treatments (p > 0.05). But cortisol and lysozyme were significantly different between the experimental treatments (p < 0.05). Serum levels of SGPT, SGOT, ALP, and LDH decreased in comparison with the control group (p < 0.05). The results of the present study showed that adding Nano-Se and Nano-Mg to the diet of L. calcarifer had a positive effect on the growth and non-specific immune system with no disturbance in serum biochemical and hepatic enzymes parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In an aquaculture farm, more than 50% of current expenses are related to food and nutrition. Diet quality and quantity are among the topics that can have a significant impact on growth rate and productivity. Also combining the right amounts of nutrients in a balanced diet improves this process [1, 2].
Selenium is a rare and essential micronutrient for humans and animals, and plays a significant role in the antioxidant system, regulating thyroid hormone metabolism, and cellular growth [3]. Selenium also plays a key role in the growth mechanism, fertility, and immune system of farmed organisms. Selenium can be found naturally in foods and organic matter. Fish meal and seafood are also the best sources of selenium. However, some species, such as tuna, have poor biological access to selenium. Magnesium is an important cofactor for several enzymatic reactions. This trace element involves in activity of phosphokinase and phosphate hydrolysis to transfer phosphate groups. Also magnesium involves in activity of phosphokinase and pyrophosphatase for transfer pre-phosphate groups. It also activates the saturated fatty acids acetyl coenzyme A (thiokinase) and activates the synthesis of amino acids. The enzymatic function of magnesium is well established. Magnesium is also vital for skeletal tissue metabolism, osmotic regulation, and neuromuscular transmission [4]. Although smaller nanoparticles can absorb more and more easily from the intestinal wall, so, Nano-Mg can exacerbate the positive effects of magnesium and reduce the negative effects of magnesium [5]. With the advancement of nanotechnology, Nano-Se is widely considered for animal and fish nutrition because nanometer particles exhibit new properties such as high surface activity, high catalytic coefficient, strong adsorption capacity, and low toxicity [6, 7]. Nano-Se has been reported to have comparable efficacy with selenium and selenium-methylselenocysteine in regulating the mechanism of selenium enzymes but has been significantly reduced [7].
The Asian Sea bass (Lates calcarifer), also is known as Barramundi in Australia. L. calcarifer is a member of the Centropomidae family. This fish has a euryhaline and reported to spread from the west of the Indian Ocean to the Pacific Ocean from the Persian Gulf to China, Taiwan, Papua New Guinea, and northern Australia. Barramundi divided into the category of catadromous, which during the breeding season, leaves its habitat in freshwater and brackish water and migrates to seawater [8,9,10,11]. In latest years, Barramundi farming in low salinity or freshwater is expanding in southwestern are in Iran. Given the different physiological role of selenium and magnesium in farmed organisms, in the present study, the possibility of using Nano-SE and Nano-Mg in Asian Sea bass was investigated. This study aimed to investigate the effects of Nano-Se and Nano-Mg, individually and in combination together on growth, digestive enzymes, immune system, serum hepatic enzyme, and serum biochemical parameters of L.calcalifer.
Material and Methods
Experimental Trials
A commercial diet (Faradaneh Co.) for Asian Sea bass prepared as the basic diet. This diet contained 0.65 mg/kg selenium, and 207.3 mg/kg magnesium. The biochemical composition of the basic diet (Table 1) was analyzed according to the standard methods [12]. The basic diet grinded and mixed with the Nano-Se (purity: 99.95%, particle size: 30–45 nm; prepared from Iranian Nanomaterials Pioneers Co.) and/or Nano-Mg (purity: 98%, particle size: 20 nm; prepared from Iranian Nanomaterials Pioneers Co.). Then, water was gradually added to the mixture to produce firm dough. The resulted firm dough mixed completely using a mixer for 10 min. The experimental diets were as follows: (1) control treatment group: without any nanoparticle supplements, (2) Nano-Se treatment group: 4 mg Nano-Se/kg food, (3) Nano-Mg treatment group: 500 mg Nano-Mg/kg food, and (4) combination treatment group: 4 mg Nano-Se/kg food + 500 mg Nano-Mg/kg food [5, 13]. Then, the mixture pelleted and dried at 25 °C. The dried pellets garbled into suitable pellet size and stored at refrigerator before use.
The Nano-Mg and Nano-Se mean particle sizes confirmed by a scanning electron microscopy [14].
The practical and rearing section of this experiment was conducted in the wet laboratory. The Juveniles of L. calcarifer were transferred from a local farm located in Khorramshahr, Iran to the wet laboratory. The fish adapted to freshwater (2 ppt) by gradually reducing the salinity level (initial salinity, 25 ppt) over 1-week period. Then, the fish acclimatize to the experimental condition and fed with the basal diet for 14 days. At the start of the trials, 10 fish (mean weight: 32.78 ± 0.16 g) distributed in each of 12 fiberglass 300-L tanks. The fish fed with experimental diets two times per day at 10:00 and 17:00 as satiation for 6 weeks. During the trials, the water quality parameters checked and recorded features maintained as daily (temperature 28.6 ± 0.8 °C, pH 7.2 ± 0.2, and dissolved oxygen 7.8 ± 0.2 mg/L). Natural photoperiod was considered during the experiment. The rate of water exchange was 20% every 2 days.
Growth Parameter Analyses
At the end of the experiment, all animals in feeding trials, weighed for growth measurement. Growth performance was measured based on the standard formulae: Weight gain (WG) = 100 × (final weight − initial weight)/(initial weight); Specific growth rate (SGR) = 100 × ln (final weight) − ln (initial weight)/days; Survival rate = 100 × (final fish number/initial fish number).
Sampling
At the end of the experimental period and 1 day before sampling, the fish did not feed. Initially, the fish captured and transferred to the sampling site and anesthetized with 200 ppm Eugenol [15]. Blood sampling was performed with 2.5-ml syringe from caudal vein, and blood samples were collected to 1.5-ml microtubes and centrifuged at 3000 rpm for 10 min and sera collected. Serum samples stored at − 80 °C until serum analyses.
Serum Biochemical Parameter Analyses
Serum levels of total protein, glucose, albumin, triglyceride, cholesterol, LDL, HDL, calcium, phosphorus, magnesium, urea, and creatinine were measured by the photometric method using biochemical auto analyzer (Mindray BS-200, China) with Diagnostic Kits (Pars Azmoon, Iran) [16, 17]. Serum levels of globulin calculated by deducting total serum albumin from total serum protein [18].
Immunological Parameter Assay
Serum Lysozyme Activity Assay
This measurement quantified using turbidity assay and based on the method recommended by Ellis (1990) [19]. To perform this analyses, 200 μl of Micrococcus lysodeikticus suspension (0.2 mg/ml sodium phosphate buffer 0.6 M, pH: 6.2) with 10 μl of serum samples in 96-well ELISA plates, mixed, and the optical absorption of the samples read after 1, and 6 min using an ELISA plate reader at 530 nm. PBS used as blank. Each unit of enzyme activity calculated as the amount of enzyme that reduces the absorption by 0.001 per min/ml of serum, in comparison with standard curves of egg white lysozyme concentrations (prepared by Sigma).
Complement Activity Assay
Serum C3 and C4 complement activities measured by a quantitative diagnostic kit (Pars Azmoon, Iran). In this experiment, the concentration of C3 and C4 determined by photometric measurement of the reaction between the antibodies sensitive to C3 and C4 in the kit and the serum C3 and C4 antigen, and assayed on the wavelength of 340 nm. C3 and C4 calculated in U/L [16].
ACH50 (Alternative complement activity) measured by the method described by Sunyer and Tort (1995) [20]. Serum complement volume determined based on 50% hemolysis, and the ACH50 units per ml of the sample, calculated. ACH50 activity measured by hemolysis of rabbit erythrocytes. Rabbit erythrocytes were washed using veronal buffer three times. Their cells adjusted by the Neubauer slides per ml of buffer to 2 × 108 cells/ml. First, the optical absorption of 100% rabbit lysate determined by adding 100 μL of the above suspension to 3.4 ml of distilled water. Then the serum samples diluted 100 times with the above buffer, and the volumes were prepared in a sterile test tube, and volume of all tubes adjusted to 250 μL by the buffer. Finally, 100 μl of rabbit erythrocyte was added to all tubes, and the mixture was placed at 20 °C for 90 min. As a final point, 3.15 ml of 0.85% sodium chloride solution was added to each tube. The tubes were centrifuged at 1600g for 10 min at 4 °C (Model 5417R, Eppendorf Co., Germany), and optical absorption of supernatant read at 414 nm. A volume of serum that causes 50% hemolysis (k) determined and used to calculate the ACH50 activity of the samples: ACH50, U/ml) = 0.5 × k.
Serum Total Immunoglobulin Level Assay
Total serum immunoglobulin level determined by Amar et al. method [21]. For this purpose, 100 μl of serum sample (diluted 1: 100 in phosphate buffer solution) incubated in 12% (V/V) polyethylene glycol solution for 2 h at room temperature. Immunoglobulin molecules precipitated by centrifugation (5000 rpm at 4 °C for 10 min). Then, serum protein levels measured by the Biuret method. Total immunoglobulin level calculated based on subtraction of serum protein after immunoglobulin participation, from serum protein level before immunoglobulin participation. The results presented as mg/ml.
Measurement of Serum Cortisol
To measure cortisol, a colorimetric method [22] and a special cortisol measuring kit of Pars Azmoun company were used using Eliza Reader device (Mindary MR-69A, China) with a wavelength of 450 nm.
Serum Hepatic Enzyme Analyses
Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) measured by quantitative diagnostic kits (Pars Azmoon, Iran) [23].
Digestive Enzyme Activity Analyses
The intestine of the fish homogenized into buffer solution (KCl 50 mM, Tris-HCl 50 mM, and CaCl 20 mM, pH: 7.5) at 9:1 (v/w) ratio using a homogenizer (IKA, Ultra-turrax®, USA). The homogenates centrifuged at 12,000 rpm for 10 min at 4 °C. Then, the supernatant separated and poured into 1.5-ml microtubes and administered for enzyme analyses. The protein content of the extract assayed by the Bradford method (1976) [24]. Activity of trypsin, chymotrypsin, lipase, α-amylase, and alkaline phosphatase (ALP) measured in intestine tissue extract. Trypsin activity evaluated with Nα-Benzoyl-L-arginine ethyl ester (BAEE). For this purpose, the intestine tissue extract incubated for 2 min at 25 °C in 2 mL of the Tris buffer (pH: 8.1). Chymotrypsin activity evaluated using N-Benzoyl-L-tyrosine ethyl ester (BTEE) as substrate. For chymotrypsin activity assay, the tissue extract incubated for 2 min in 2 mL of Tris buffer (pH: 7.8). The activity of trypsin and chymotrypsin enzyme evaluated at wavelength of 253, and 256 nm, respectively [25]. Lipase, amylase, and ALP activities evaluated using photometric diagnostic kits (Pars Azmoon, Tehran, Iran), by using a biochemical auto-analyzer (Mindray BS-200, China) at a wavelength of 580, 405, and 405 nm, respectively [26].
Data Analyses
Each tank considered as a test unit, and the results reported as mean ± standard error. In this study, all statistical calculations performed using SPSS software version 20. One-way ANOVA used to compare the means and the Duncan test to examine the presence or absence of a significant difference between the treatments. The significance level in this study considered to be less than 0.05.
Results
Growth Parameters
The effect of different levels of Nano-SE and Nano-Mg on growth performance was presented in Table 2. At the beginning of the experimental period, there was not any significant difference among the four experimental treatments in terms of initial mean weight (p > 0.05). At the end of the experimental period, fish fed with combined selenium nanoparticles and magnesium nanoparticles showed the highest rate of body weight gain (131.33 ± 26.58%) and the specific growth rate (2.08 ± 0.28%/day) (p < 0.05). Also, the survival rate did not significantly differ between experimental treatments. Although, the results showed that Se and Mg nanoparticles (individually or in combination together) improve survival rate.
Serum Biochemical Parameters
The effect of supplementation of selenium and magnesium nanoparticles on serum biochemical composition was presented in Table 3. According to the results of measuring these parameters, there was no significant difference in experimental treatments and control groups for glucose, cholesterol, triglycerides, and protein levels (p > 0.05). The lowest amount of calcium level was observed in the combined treatment and showed a significant difference with other treatments. The highest amount of phosphorus was observed in the control treatment and there was a significant difference with other treatments (p < 0.05). The highest amount of albumin was in the Nano-Se treatment, and the lowest amount was seen in the combined treatment, which showed a significant difference with other treatments (p < 0.05).
Serum Hepatic Enzyme Analyses
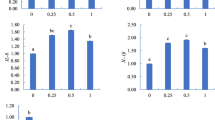
According to the results obtained at the end of the experiment, the highest levels of SGOT, ALP, and LDH were observed in the control treatment (p < 0.05). Also, there was no significant difference between SGPT levels in different treatments (p < 0.05) (Table 4).
Serum Immune Parameters
The results of this study showed that there was no significant difference between globulin, IgM, C3, and ACH50 and different treatments (p > 0.05). However, in the case of lysozyme, the highest amount was observed in the combined treatment and the lowest in the control treatment (p < 0.05). Cortisol has been shown to be the most abundant in control treatments and Nano-Mg, respectively, and the lowest was in Nano-Se treatment and combined treatment (p < 0.05) (Table 5).
Digestive Enzyme Activity
In this study, the lowest amount of ALP was observed in the combined treatment and was significantly different from other treatments (p < 0.05). The highest amount of lipase was observed in Nano-Mg treatment, which showed a significant difference with other treatments (p < 0.05). The maximum amount of trypsin enzyme was observed in the treatment of Nano-Mg and the minimum in the Nano-SE treatment (p < 0.05). The highest levels of the chymotrypsin were observed in the Nano-Mg treatment, and the lowest levels were observed in the control treatment (p < 0.05). Also, the amount of amylase did not show a significant difference between the four experimental treatments (p > 0.05) (Table 6).
Discussion
Growth Parameters
In the growth parameters, the highest values were observed in the combined treatment of Nano-Se and Nano-Mg (p > 0.05). BW% and SGR indicating the nutritional status of fish [1]. Given these high characteristics in the Se nanoparticle group and the combined group of Mg and Se nanoparticles, it can be said that these treatments had better growth conditions than the control treatment. The results coincided with the results of some other studies on hybrid striped bass: Morone chrysops x Morone saxatilis [27], Rachycentron canadum [28], Carassius gibelio [29], Cyprinus carpio [30], Lates calcarifer [31], Carassius auratus [32], and Lates calcarifer [33].
Due to the properties of Se nanoparticles (high surface activity, high catalytic coefficient, strong adsorption capacity, and low toxicity) [6, 7] and comparable better efficiency to Se and Se-methyl selenocysteine in regulating the mechanism of Se-enzymes [7], the increase in growth in treatments supplemented with Nano-Se and Nano-Mg is quite justified. Mg is an important cofactor for many enzymatic reactions and involves the transfer of phosphate and pre-phosphate groups. It also activates the saturated fatty acids acetyl coenzyme A (thiokinase) and activates the synthesis of amino acids. The enzymatic function of Mg is well established. Mg is also vital for skeletal tissue metabolism, osmotic regulation, and neuromuscular transmission [4]. A closer look reveals that the level of Nano-Mg used in this study is much lower than the Mg levels used in other researches. Nanoparticles, despite their smaller particles, can absorb more and more easily from the intestinal wall, intensifying the positive effects of Mg and reducing the negative effects of Mg [5]. Also, although Barramundi is a marine catadromous species, this fish adapted and reared in freshwater in the current study. The basic diet used for this experiment contained an appropriate Mg concentration (207.3 mg Mg/kg food), which is similar to the previous report for dietary levels of Mg requirement in some freshwater fish species (200–700 mg Mg/kg food) [33]. This specifies that the Mg content of the basic diet was adequate for the Mg requirement of Asian Sea bass when reared in freshwater.
The possible reason for such a beneficial role is the increase in activity of growth hormones by Mg nanoparticles [34]. Reigh et al. showed the critical effects of magnesium on appetite and growth performance [35]. Mg plays a critical role in the normal metabolism of lipids, proteins, and carbohydrates as a cofactor in a large number of enzymatic and metabolic reactions [36]. Mg influences cell division and plays a critical role as a cofactor in pathways of many enzymes involved in cell metabolism and in energy production [37]. In acclimation to low salinity media, more protein would be required to the hemolymph as a source of amino acids to maintain the osmotic pressure, which reduced growth because of the loss of amino acids from muscle [38]. Therefore, in this study, supplementation of Mg to diets improved growth performance and feed efficiency of fish, perhaps owing to the sparing action of Mg on nutrient consumption in osmotic regulation. Also, the higher growth of tilapia fed the selenium-supplemented diet can be explained by the fact that selenium is an important micronutrient that is needed for the better growth performance and feeding parameters of fish. Its supplementation enhances erythrocytes production and prevents high quantity fat accumulation in the liver tissue [39]. Also, it is an important constituent of the deiodinase enzyme, which is necessary for the proper functioning of the thyroid hormones [40], and the thyroid hormones are required for growth hormone secretion from the pituitary gland [41]; thus, selenium is indirectly involved in the secretion of the growth hormone [42].
Gastric Enzyme Activity
At the end of the trial period, the highest and lowest levels of the activity of the ALP were observed in the treatment of Nano-Se and the Nano-Se and Nano-Mg combination. The highest activity rates of trypsin, chymotrypsin, and lipase were found in the treatment of Nano-Mg. Also, the activity of amylase did not show a significant difference between the four experimental treatments. The level of digestive enzyme activity depends on several aspects such as the intrinsic properties of the enzyme, its production, and secretion [43]. Adkins and Ewan (1984) examined the effect of selenium on pancreatic pig digestive enzymes [44]. The results showed that supplementing selenium did not have any effect on trypsin, chymotrypsin, amylase, and lipase activity in the pancreas.
Serum Biochemical Parameters
Serum biochemical parameters in fish may change under the influence of physiological factors such as age, size, sex, reproductive stage, and health status [45]. These factors also changed under the influence of external factors such as season, water temperature, environmental conditions, food, stress, types of pollution, and diseases [46]. Any changes in albumin, globulin, and total plasma protein levels can use as a clinical indicator in monitoring the health of the immune system, liver, and kidneys [47]. In the present study, among the measured biochemical parameters, glucose, cholesterol, triglycerides, and total protein at the end of the experiment did not have a significant difference between the experimental treatments (p > 0.05). The highest serum urea levels were observed in the treatment of 500 mg Nano-Mg, and the lowest urea was observed in 4 mg Nano-Se. Uric acid, which produced in fish from endogenous and exogenous purine nucleotides as well as catabolism of proteins by purines, converted to urea in the liver and a lesser extent in the kidneys and excreted through gills. Due to the positive effects of Se on the cell membrane of body tissues and the inhibition of the activity of enzymes such as glutathione peroxidase [48], especially in liver tissue, low urea levels can be related to Se’s ability to reduce liver damage and subsequently, decreased urea levels. Also, due to the effect of Mg as a cofactor in enzymes complicated in protein metabolism [49], high urea levels in Nano-Mg-treated treatments are justifiable. Serum calcium levels have shown a significant decrease in combined treatment in comparison with other treatments (p > 0.05). The balance between salts and minerals in aquatic food can indirectly affect the serum levels of mineral elements [50]. Also, serum phosphorus levels in all nutritional treatments of Nano-Se and Nano-Mg had a significant decrease compared to the control treatment, which can be found in the imbalance of minerals in the diet, and competition between Ca and Mg absorption from intestinal mucosa and the effect on phosphorus absorption from intestinal mucosa is stated [50]. The lowest serum albumin levels were observed in the combined treatment. The optimal amount of Se is a protective agent against heavy metals through the synthesis of glutathione peroxidase. However, high levels of Se can have negative effects on physiological indicators [51]. Changes in plasma protein concentrations, especially albumin, are proportional to changes in total plasma Ca concentrations. In the opposite direction of increasing albumin in plasma, it is linked with an increase in plasma Ca concentration [49]. Butler et al. also showed a significant positive correlation between Ca and albumin [52], which coincided with the results obtained in the current study. Total protein, albumin, triglycerides, glucose, and cholesterol in the present study were not affected by the nanoparticles in the diet, which could be due to possible causes such as test duration, nanoparticle size, the dose used and type of nanoparticles, which coincided with the results reported by Longbaf Dezfooli et al. [33].
Serum Hepatic Enzyme Activity
At the end of the trial period, the highest levels of SGOT, ALP, and LDH were observed in the control treatment group. Also, there was not any significant difference between SGPT levels and different treatments. Serum SGPT and SGOT belong to plasma inactive enzymes that are naturally present in the hepatic cells, heart, kidneys, and other organs [53]. Wells et al. reported that increased SGPT and plasma SGOT activity probably provided information about liver damage or liver dysfunction [54]. In the current study, similar to Zhang et al. study, serum SGOT enzyme activity did not increase with the use of Mg and Se nanoparticles in the diet [5], which indicates a better process of liver activity by adding these nanoparticles, and this may be related to the increased antioxidant performance of these nanoparticles, membrane stabilization, and cell damage prevention [55]. Increased SGPT and creatine kinase activity indicate the induction of transamination processes that occur as amino acid input to the tri-carboxylic acid cycle. Kouba et al., similar to the current study, observed the highest SGOT activity rate in the control group [56] and, contrary to the present study, the highest SGPT and LDH activity in 1 g Nano-Se-consuming treatments with microalgae and 0.3 g of Se. Ashouri et al., in contrast to the current study, obtained the highest levels of SGOT, SGPT, and ALP activity in Cyprinus carpio serum fed 2 mg of Nano-Se per kg of food [30]. A significant increase in serum ALP, SGOT, and SGPT activity is thought to be the body’s response to stressors [57]. Therefore, it can be stated that the health status of the liver in fish that used nanoparticles was better than the control group.
Serum Immune Responses
The results of the current study showed that at the end of the experimental period, the amount of globulin, IgM, C3, and ACH50 did not differ significantly between different treatments. The results of this study are inconsistent with the results of Longbaf Dezfooli et al. in Lates calcarifer [33]. Lovell and Wang reported a significant increase in antibody production and survival rate in catfish infected with the Edwardsiella ictaluri upon feeding with dietary selenium levels [58]. Ashouri et al. measured the biochemical characteristics of blood by adding different levels of Se nanoparticles to a common carp diet [30]. At the end of the experimental period, the highest biochemical index of globulin obtained in the treatment of 2 g of Se per kg of diet. Impairment of globulin, IgM, C3, and ACH50 parameters between different researches can be due to possible reasons such as the length of the test period, the size of the nanoparticles, the dose used, and the type of nanoparticles. The highest amount of lysozyme was observed in the combined treatment and showed a significant difference with other treatments. Dietary Se is essential for optimal immune response, although mechanisms are not fully understood, but Se affects innate and acquired immune systems [59, 60]. The highest levels of cortisol were observed in the control treatment and showed a significant difference. Sathya et al. stated in their study that adding Se to the buffalo diet reduced oxidative stress and plasma cortisol levels [61]. Increasing the lipid peroxidation under stress conditions stimulates the stress axis and leads to an increase in cortisol concentrations [62]. Decreases in lipid peroxidation following the use of vitamin E and Se, as well as a further reduction in MDA, can also reduce cortisol concentrations [61].
Se plays an important role in the formation of several important types of selenoproteins such as glutathione peroxidase and thioredoxin reductase [63] and protects the body against oxidative stress [64]. Wang et al. found that dietary Mg supplementation could improve the antioxidant capacity of juvenile grass carp by increasing antioxidase activities [65]. Likewise, in this study, supplementation of dietary Mg might promote growth performance by reducing oxidative stress of Japanese seabass fed oxidized oil diets. Also, numerous researches reported that Mg could reduce oxidative stress and free radicals in organisms [66]. An increase in supplemented Se led to an increase in stress tolerance, as shown by the decreased cortisol levels. Se plays an important role to prevent oxidative stress and, in chinook salmon (Oncorhynchus tshawytscha) has been found to induce a loss of whole-body Se. Se supplementation has been reported to allow salmonids to better cope with stressful conditions caused by handling [67].
Conclusion
In general, the administration of combination of Se and Mg nanoparticles improve growth indices. However, the growth parameters indicated that there is not any significant difference between nano-selenium treatment and combined treatment. Also, Mg nanoparticles improve growth indices, but there is not any significant difference with control group. The results of this study specified that the Mg content of the basic diet was adequate for the Mg requirement of Asian sea bass when reared in freshwater. Thus, the results confirmed that extra levels of magnesium in diet could not significantly affect growth and other analyses parameters during the period of the study. Also, using Se and Mg nanoparticles (individually or in combination together) improves survival rate. The supplementation of Se and Mg nanoparticles had no negative effect on glucose, cholesterol, triglycerides, and protein. The results showed that the use of Se and Mg nanoparticles in the diet had positive effects on the non-specific immune system, serum biochemical composition, hepatic enzymes, and digestive enzymes of L. calcarifer. Finally, this research presented that combination of Se and Mg nanoparticles may be proposed as a nutrient additive to L. calcarifer farms.
References
Hosseinzadeh Sahhafi H, Nafari Yazdi M (2014) Growth performance of rainbow trout (Oncorhynchus mykiss) with respect to nutritional factors in north Iran (Haraz River). Iran J Fish Sci 13(3):509–521
Karimi M, Mousavi SM, Zolgharnain H, Zakeri M (2020) Dietary montmorillonite as growth promoter and immunomodulator in rainbow trout (Oncorhynchus mykiss). Chemosphere 252:126459. https://doi.org/10.1016/j.chemosphere.2020.126459
Eisler R (2000) Handbook of chemical risk assessment: health hazards to humans, plants, and animals, vol 3. CRC Press, Boca Raton
Houston AH (1985) Erythrocytic magnesium in freshwater fishes. Magnesium 4:106–128
Zhang CX, Huang F, Li J, Wang L, Song K, Mai KS (2016) Interactive effects of dietary magnesium and vitamin E on growth performance, body composition, blood parameters and antioxidant status in Japanese seabass (Lateolabrax japanicus). Aquac Nutr 22:708–722
Wang HL, Zhang JS, Yu HQ (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med 42:1524–1533
Zhang JS, Wang XF, Xu TW (2008) Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in mice. Toxicol Sci 101:22–31
Rimmer MA, Russell DJ (1998) Aspects of the biology and culture of Lates calcarifer. In: De Silva SS (ed) Tropical Mariculture. Academic Press, London, pp 449–476
Rimmer MA (2003) Barramundi. In: Lucas JS, Southgate PC (eds) Aquaculture: farming aquatic animals and plants. Blackwell Publishing, Oxford, pp 364–381
Morshedi V, Nafisi Bahabadi M, Azodi M, Modaresi M, Cheraghi S (2015) Effects of dietary probiotic (Lactobacillus plantarum) on body composition, serum biochemical parameters and liver enzymes of Asian sea bass (Lates calcarifer, Bloch 1790). J Mar Sci Technol 14(2):1–14
Ghavam Pour A (2018) Introduction in sea bass aquaculture, classification and history, Iranian Fisheries Research Center p 42
AOAC (2005) Official methods of analysis of the association of official analytical chemists international. USA: Maryland
Ilham I, Siddik MA, Fotedar R (2016) Effects of organic selenium supplementation on growth, accumulation, haematology and histopathology of juvenile barramundi (Lates calcarifer) fed high soybean meal diets. Biol Trace Elem Res 174(2):436–447
Dekani L, Johari SA, Joo HS (2019) Comparative toxicity of organic, inorganic and nanoparticulate zinc following dietary exposure to common carp (Cyprinus carpio). Sci Total Environ 656:1191–1198
Mousavi SM, Majdi Nasab E, Yavari V, Rajabzadeh Ghatrami E, Razi Jalali M (2012) Effects of two anaesthetic regimes, MS-222 and eugenol, on plasma biochemical profile in Barbus sharpeyi. Comp Clin Pathol 21:859–863
Johnson AM, Rohlfs EM, Silverman LM (1999) Proteins. In: Burtis CA, Ashwood ER (eds) Tietz textbook of clinical chemistry, 3rd edn. W. B. Saunders company, Philadelphia, pp 477–540
Stavros C, Angela GE, Pascal D (2006) Fishmeal replacement by alfalfa protein concentrate in sharp snout sea bream Diplodus puntazzo. Fish Sci 72(6):1313–1315
Kumar A, Vihan VS, Rana R, Kumar V (2005) Blood biochemical changes in some important parasitic infestations in goats for clinical appraisal. Indian J Small Rumin 11:156–160
Ellis AE (1990) Lysozyme Assays. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinkel WB (eds) Techniques in Fish Immunology. SOS Publications, Fair Haven, pp 101–103
Sunyer JO, Tort L (1995) Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Vet Immunol Immunopathol 45:333–345
Amar EC, Kiron V, Satoh S, Okamoto N, Watanabe T (2000) Effects of dietary b-carotene on the immune response of rainbow trout (Oncorhynchus mykiss). Fish Sci 66:1068–1075
Watts NB, Tindall GT (1988) Rapid assessment of corticotropin reserve after pituitary surgery. JAMA 259(5):708–711
Sahu S, Das BK, Mishra BK, Pradhan J, Sarangi N (2007) Effect of Allium sativum on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. J Appl Ichthyol 23:80–86
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7(72):248–254
Hummel BCW (1959) A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol 37:1393–1399
Tietz N, Shuey D (1993) Lipase in serum- the elusive enzyme: an overview. Clin Chem 39(5):746–756
Fjr J, Peng L, Gatlin DM (2009) Selenium nutrition of hybrid striped bass (Morone chrysops × M. saxatilis) bioavailability, toxicity and interaction with vitamin E. Aquac Nutr 15(2):160–165
Liu K, Wang XJ, Ai Q, Mai K, Zhang W (2010) Dietary selenium requirement for juvenile cobia (Rachycetron canadum). Aquac Res 41(10):594–601
Han D, Xie S, Liu M, Xiao X, Liu H, Zhu X, Yang Y (2011) The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac Nutr 17(3):e741–e749
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29
Ilham I, Fotedar R (2016) Growth, enzymatic glutathione peroxidase activity and biochemical status of juvenile barramundi (Lates calcarifer) fed dietary fermented soybean meal and organic selenium. Fish Physiol Biochem 43(3):775–790
Seiedi J, Kalbassi MR (2017) Effects of different levels of diet nano selenium (Nano-Se) on growth and gonad quality indices and seminal plasma antioxidants in male goldfish (Carassius auratus gibelio). Aquat Physiol Biotechnol 5(2):68–80
Longbaf Dezfouli M, Ghaedtaheri A, Keyvanshokooh S, Salati AP, Mousavi SM, Pasha Zanoosi H (2019) Combined or individual effects of dietary magnesium and selenium nanoparticles on growth performance, immunity, blood biochemistry and antioxidant status of Asian seabass (Lates calcarifer) reared in freshwater. Aquac Nutr 25:1422–1430. https://doi.org/10.1111/anu.12962
Jayarambabu N, Siva Kumari B, Venkateswara Rao K, Prabhu YT (2016) Enhancement of growth in maize by biogenic- synthesized Mgo nanoparticles. Int J Pure Appl Zool 4(3):262–270
Reigh RC, Robinsonan EH, Brown PB (1991) Effects of dietary magnesium on growth and tissue magnesium content of blue Tilapia Oreochromis aureus. J World Aquacult Soc 22(3):192–200
Lall SP (2002) The minerals. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press Inc, San Diego, pp 259–308
Van der Velden JA, Kolar ZI, Flik G (1991) Intake of magnesium from water by freshwater tilapia fed on a low-Mg diet. Comp Biochem Physiol 99:103–105
Rosas C, Cuzon G, Gaxiola G, Le Priol Y, Pascual C, Rossignyol J, Contreras F, Sanchez A, Van Wormhoudt A (2001) Metabolism and growth of juveniles of Litopenaeus vannamei: effect of salinity and dietary carbohydrate levels. J Exp Mar Biol Ecol 259:1–22
Khan KU, Zuberi A, Nazir S, Fernandes JB, Jamil Z, Sarwar H (2016) Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk J Zool 40(5):704–712
Kohrle J, Gartner R (2009) Selenium and thyroid. Best Pract Res Clin Endocrinol Metab 2(3):815–827
Valcavi R, Zini M, Portioli I (1992) Thyroid hormones and growth hormone secretion. J Endocrinol Investig 1(5):313–330
Swain P, Das R, Das A, Padhi SK, Das KC, Mishra SS (2018) Effects of dietary zinc oxide and selenium nanoparticles on growth performance, immune responses and enzyme activity in rohu, Labeo rohita (Hamilton). Aquac Nutr 00:1–9
Yan T, Teo LH, Sin YM (1996) Effects of metals on α-amylase activity in the digestive gland of the green mussel, Perna viridis L. Bull Environ Contam Toxicol 56(4):677–682
Adkins RS, Ewan RC (1984) Effect of supplemental selenium on pancreatic function and nutrient digestibility in the pig 1. J Anim Sci 58(2):351–355
Luskova V (1995) Determination of normal values in fish. Acta Univ Carol Biol 39:191–200
Witeska M (1998) Changes in selected blood indices of common crap after acute exposure to cadmium. Acta Vet Brno 67:289–293
John PJ (2007) Alteration of certain blood parameters of freshwater teleost Mystus vittatus after chronic exposure to metasystox and sevin. Fish Physiol Biochem 33:15–20
Lee S, Lee JH, Bai SC (2008) Effects of different levels of dietary selenium (Se) on growth, tissue Se accumulations and histopathological changes in black seabream, Acanthopagrus schlegeli. Asian-Australas J Anim Sci 21:1794–1799
Nelson DL, Cox MM (2001) Lehninger Principles of Biochemistry, Sixth Edition, NewYork. Freeman, W.H
Hursky O, Pietrock M (2012) Chemical contaminants and parasites: assessment of human health risks associated with consumption of whitefish (Coregonus clupeaformis) from two boreal lakes in northern Saskatchewan, Canada. Sci Total Environ 97:103–110
Rosmini MR, Perlo F, Pérez-Alvarez JA, Pagán-Moreno MJ, Gago-Gago A, López-Santoveña F, Aranda-Catalá V (1996) TBA test by an extractive method applied to ‘Paté’. Meat Sci 42:103–110
Butler SJ, Payne RB, Gunn IR, Burns J (1984) Correlation between serum ionized calcium and serum albumin concentration in two hospital populations. J Clin Res 289:948–950
Hadi A, Shokr A, Alwan S (2009) Effects of aluminum on the biochemical parameters of fresh waterfish Tilapia zillii. J Appl Sci 3:33–41
Wells R, McIntyre R, Morgan A, Davie P (1986) Physiological stress responses in big game fish after exposure: observation on plasma chemistry and blood factors. Comp Biochem Physiol A Physiol 64:565–571
Niu H, Jia Y, Hu P, Meng Z, Lei J (2014) Effect of dietary vitamin E on the growth performance and nonspecific immunity in sub-adult turbot (Scophthalmus maximus). Fish Shellfish Immunol 41:501–506
Kouba A, Velíšek J, Stará A, Masojídek J, Kozák P (2014) Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response, and accumulation in common barbel (Barbus barbus). Biomed Res Int 2014:1–8
Bitiren M, Karakılçık AZ, Zerin M, Aksoy N, Musa D (2004) Effects of selenium on histopathological and enzymatic changes in experimental liver injury of rats. Exp Toxicol Pathol 56(1):59–64
Lovell RT, Wang CL (1997) Comparison of organic and inorganic sources of selenium for growth and health of channel catfish. In: Lyons TP, Jacques KA (eds) Biotechnology in the feed industry. Proceedings of Alltech’s 13th annual symposium, pp. 165-179
Turner RJ, Finch JM (1991) Selenium and the immune response. Proc Nutr Soc 50:275–285
Kiremidjian-Schumacher L, Roy M (1998) Selenium and immune function. Z Ernahrungswiss 37:50–56
Sathya A, Prabhakar S, Sangha SPS, Ghuman SPS (2007) Vitamin E and selenium supplementation reduces plasma cortisol and oxidative stress in dystocia-affected buffaloes. Vet Res Commun 31(7):809–818
Gupta SK, Kumar H, More T (2004) Assessment of oxidative stress in dairy cows supplemented prepartum with vitamin E and selenium with reference to retention of fetal membranes. J Anim Reprod 25:19–22
Yu JC, Tang HY, Yu JG (2002) Bacteriearal and photocatalytic activities of TiO2 thin films prepared by sol–gel and reverse micelle methods. J Photochem Photobiol A Chem 3:211–219
Burk RF, Hill KE (1993) Regulation of selenoproteins. Annu Rev Nutr 13:65–81
Wang D, Hu J, Irons DR, Wang J (2011) Synergistic toxic effect of nano-TiO2 and As (V) on Ceriodaphnia dubia. Sci Total Environ 409:1351–1356
Khosravi-Katuli K, Prato E, Lofrano G, Guida M, Vale G, Libralato G (2017) Effects of nanoparticles in species of aquaculture interest. Environ Sci Pollut Res 24:17326–17346
Rider S, Davies S, Jha A, Fisher A, Knight J, Sweetman J (2009) Supra-nutritional dietary intake of selenite and selenium yeast in normal and stressed rainbow trout (Oncorhynchus mykiss): implications on selenium status and health responses. Aquaculture 295(3–4):282–291
Acknowledgements
The authors of this paper express their appreciation to the research council of the Khorramshahr University of Marine Science and Technology for their financial support of this research project.
Data Availability Statement
Research data are not shared.
Funding
This study was funded by Khorramshahr University of Marine Science and Technology (grant number: 1397).
Author information
Authors and Affiliations
Contributions
Hamed Deilamy Pour: investigation, methodology; Seyed Mohammad Mousavi: conceptualization, supervision, writing-review and editing, project administration, resources; Mohammad Zakeri: data curation, validation, methodology; Saeed Keyvanshokooh: investigation, methodology, resources, Preeta Kochanian: data curation, validation
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors declare that they have no conflict of interest.
Ethics Approval
The authors of the manuscript declared that the experimental conditions were standard and ethical statements about experimental animals were observed during the experimental. The minimum samples were collected and the animals were anesthetized before any sampling.
Consent to Participate
All authors of the manuscript are aware from submission of this manuscript.
Consent for Publication
I hereby accept liability for the scientific integrity of the manuscript contents. I hereby declared that this manuscript is an original research article which has not been submitted or published in other journals. Also, my study does not involve human subjects. This manuscript is resulted from Research Project under financial support of Khorramshahr University of Marine Science and Technology, Khorramshahr, Iran.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deilamy Pour, H., Mousavi, S.M., Zakeri, M. et al. Synergistic Effects of Selenium and Magnesium Nanoparticles on Growth, Digestive Enzymes, Some Serum Biochemical Parameters and Immunity of Asian Sea Bass (Lates calcarifer). Biol Trace Elem Res 199, 3102–3111 (2021). https://doi.org/10.1007/s12011-020-02421-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02421-3