Abstract
Mycogenic synthesis of medically applied zinc oxide (ZnO) and copper oxide (CuO) nanoparticles (NPs) were exploited using Penicillium chrysogenum. The biogenesis and capping processes of the produced nano-metals were conducted by functional fungal extracellular enzymes and proteins. The obtained ZnO-NPs and CuO-NPs were characterized. Also, the antibacterial activity and minimum inhibitory concentration (MIC) values of ZnO-NPs and CuO-NPs were determined. Also, antibiofilm and antifungal activities were investigated. Results have demonstrated the ability of the bio-secreted proteins to cape and reduce ZnO and CuO to hexagonal and spherical ZnO-NPs and CuO-NPs with particle size at 9.0–35.0 nm and 10.5–59.7 nm, respectively. Both ZnO-NPs and CuO-NPs showed high antimicrobial activities not only against Gram-positive and Gram-negative bacteria but also against some phytopathogenic fungal strains. Besides this, those NPs showed varied antibiofilm effects against different microorganisms. Quantitative and qualitative analyses indicated that CuO-NPs had an effective antibiofilm activity against Staphylococcus aureus and therefore can be applied in diverse medical devices. Thus, the mycogenic green synthesized ZnO-NPs and CuO-NPs have the potential as smart nano-materials to be used in the medical field to limit the spread of some pathogenic microbes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is the multidisciplinary science concerned with the creation and modification of materials in a nano-size range between 1 and 100 nm. Nanotechnology is greatly affected by various disciplines especially physics, chemistry, and biology [1,2,3,4,5]. This technology provides great opportunities by changing the properties of materials significantly when they are in nano-size. When the dimension of the materials is getting closer to nano-size, quantum physics plays an important role instead of conventional laws of physics which adds different and unique properties to the material [6, 7]. Nowadays, biological synthesis (plant–algae–fungi–actinomycetes, bacteria, and viruses) method of NPs is preferred because it is safe, clean, cheap, and easily scaled up procedure for well-built scale NPs [8,9,10].
The fungal-intermediated green formation of NPs has many advantages including simple and easy scaling up, easy processing, economic viability, biomass processing, and recovery of considerable surface distances with the optimal outgrowth of mycelia [11,12,13,14]. Furthermore, from an economic overview, the utilization of fungal biomass filtrate containing different metabolites as a green approach for the fabrication of metallic NPs is preferred over other biological methods [6, 8, 15, 16]. Up to now, numerous metal oxides as CuO and ZnO nanoparticles have been bio-synthesized utilizing fungal cell extract [6, 17]. Such metal oxides are generally recognized as safe materials by the US food and drug administration as they are non-toxic and biocompatible for human beings and animals [18, 19]. ZnO-NPs have unique characteristics including electrical conductivity, UV-protection, photochemical beside antiviral, anticancer, antifungal, and antibacterial activities [12, 20, 21]. CuO-NPs have received great attention by numerous researchers for its chemical and biological demeanor which can be returned to their morphology [22,21,22,25]. CuO-NPs have been utilized in diverse biological applications like drug delivery and anticancer [26], antifungal, antioxidant, and antibacterial activities [27].
One of the major problems facing the medical field is the infectious diseases that lead to the death of many people worldwide [28]. The wrong use of antibiotics and the lack of capabilities and scientific tools to develop such drugs had led to the emergence of mutations and generations of microbes that are resistant to such drugs [29]. Moreover, the bacterial cells that emerged inside the biofilm matrix exhibit more resistance to the available antimicrobial therapy in addition to hosting the immune responses. Such a complex network enhances the ability of bacteria to gain resistance against a wide range of antibiotics, and thus makes traditional antibiotic therapy inactive [30]. Therefore, it was necessary to search for new treatment strategies such as the use of nanotechnology to provide a new vision in treatment and limiting the spread of pathogens. To fulfill this target, this work was conducted to synthesize and characterize ZnO-NPs and CuO-NPs through employing the metabolites (proteins) secreted by Penicillium chrysogenum. Studying the antibacterial, antifungal, and antibiofilm activities of the biosynthesized ZnO-NPs and CuO-NPs to be applied as smart nano-materials to be used in the medical field and limit the spread of some pathogenic microbes such as Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli (bacterial strains), Fusarium solani, Fusarium oxysporum, Sclerotium sclerotia, and Aspergillus terreus (fungal strains) will be investigated.
Materials and Methods
Materials
Zinc acetate dihydrate (Zn(CH3COO)2·2H2O) and Copper acetate monohydrate (Cu(CH3COO)2·2H2O) were purchased from Sigma-Aldrich for chemicals and used as precursors for preparation ZnO and CuO nanoparticles.
Fungal Strain
Penicillium chrysogenum MF318506 strain was used to synthesis ZnO-NPs and CuO-NPs. This strain was characterized and identified as described previously [11].
Synthesis of ZnO-NPs and CuO-NPs by Penicillium chrysogenum Filtrate
Penicillium chrysogenum MF318506 was grown up in 250 mL Erlenmeyer flask containing 100 mL Czapek Dox (CD) fermentative broth medium containing (g/L): 2.0 NaNO3, 0.5 MgSO4.7H2O, 0.5 KCl, 0.5 KH2PO4, 0.001 FeSO4, and 20.0 sucrose for 7 days at pH 6.0, 30 °C, and shaking at 150 rpm. The fungal biomass was separated by filtration method at the end of the incubation period using Whatman filter paper No. 1. The biomass filtrate of P. chrysogenum was used for ZnO-NPs and CuO-NPs formation as the following: Two (2 millimole) of zinc acetate and copper acetate were incubated separately and mixed with biomass filtrate of P. chrysogenum at 30 °C for 48 h on the shaker at 150 rpm [6, 31]. White and green dark colors were appeared for ZnO-NP and CuO-NP synthesis at the end incubation period, respectively. The ZnO-NPs and CuO-NPs were separated and dried at 80 °C for 48 h. The ZnO-NP and CuO-NP products were eventually collected and subjected for further investigation.
Characterization of ZnO-NPs and CuO-NPs
Biosynthesis of ZnO-NP and CuO-NP colloids were routinely monitored using UV–vis spectra on JASCO V-630 UV-VIS Spectrophotometer (JASCO INTERNATIONAL CO., LTD) at wavelengths of 200–800 nm to detect intense absorption peak which related to surface plasmon excitation. The particle shape and size of the biosynthesized ZnO-NPs and CuO-NPs were preliminarily characterized using transmission electronic microscope (TEM) (JEOL-2100) measurements through drop coating process in which a drop of solution containing NPs was placed on the coated-carbon copper grids and kept under vacuum desiccation for overnight before loading them onto a specimen holder. Moreover, the functional groups present in myco-synthesized NPs molecules were analyzed using Fourier transform infrared (FTIR) spectroscopy (JASCO, FT/IR- 6100). The samples NPs were mixed with KBr and filled into discs at high pressure. These discs were scanned in the range of 400 to 4000 cm−1 to obtain FTIR spectra. The elemental composition of biosynthesized ZnO-NPs and CuO-NPs was investigated by EDX analysis (JEOL JSM- 6510 LV). The crystalline structure of ZnO-NPs and CuO-NPs was characterized by XRD analysis (XRD, X PERT PRO-PAN Analytical) [1, 6, 24, 32].
Biological Activities of the Biosynthesized ZnO-NPs and CuO-NPs
In Vitro Antibacterial Activity and MIC Determination
Antimicrobial activities of the biosynthesized ZnO-NPs and CuO-NPs were evaluated according to CLSI guideline using agar well diffusion assay by Muller Hinton agar plates versus human pathogenic bacterial strains: Staphylococcus aureus ATCC23235 and Bacillus subtilis ATCC6051 which represent Gram-positive bacteria and Pseudomonas aeruginosa ATCC9027, Salmonella typhimurium ATCC14028, and Escherichia coli ATCC8739 which represent Gram-negative bacteria. The diameter of inhibition zone was determined by millimeter (mm) [33, 34]. Micro-dilution method in micro-titer plates (MTP) performed to determine the minimum inhibitory concentration (MIC) of active ZnO-NPs and CuO-NPs against tested pathogenic strains. Accordingly, ZnO-NPs and CuO-NPs were diluted in 1% DMSO at various concentrations (0.009–5.0 mg/ml, w/v) and tested against the pathogenic strains. Briefly, 1: 100 (v/v) of overnight cultures of the test strains were added to 200 μl of Muller Hinton broth media distributed in the wells of MTP with/without ZnO-NPs and CuO-NPs. The plates were then incubated with shaking (120 rpm) for 24 h at 37 °C. MIC was determined as the lowest concentration of ZnO-NPs and CuO-NPs which inhibit 100% of pathogenic strains [35]. All experiments were performed in triplicates.
In Vitro Biofilm Inhibition Assay
To assess the ability of ZnO-NPs and CuO-NPs to inhibit or prevent bacterial biofilm formation, micro-titer plate assay was used against Staphylococcus aureus ATCC29213 and Pseudomonas aeruginosa ATCC9027, a known biofilm-producing strain. Different concentrations (0.01–3.0 mg/mL) of ZnO-NPs and CuO-NPs were loaded in flat bottom MTP containing Tryptic-Soy broth media (TSB) supplemented with 1% glucose and mixed well. Overnight cultures of the tested strains were separately diluted at 1:100 (v/v) in TSB (5 × 105 CFU/ml), loaded into the wells, and then the plates were incubated at 37 °C for 48 h [36]. After incubation period, the planktonic cells were transferred without disturbing the biofilm and cell growth was read by ELIZA reader (Tecan Elx800, USA) at 620 nm. The plates were washed three times with sterilized phosphate-buffered saline (PBS) pH 7.2 to remove excess planktonic cells; then biofilms were fixed with 200 μl of methanol 95% for 10 min. Crystal violet (0.1%, w/v) was added and the plates were incubated for 15 min at room temperature. After the incubation, the crystal violet was removed, and the wells were gently washed with sterile distilled water to remove excess stain. Finally, the adhered biofilm bounded crystal violet was examined and photographed by an inverted microscope (Olympus Ck40 Japan) ×40 then, eluted in acetic acid (30%), and the absorbance was measured at 540 nm by ELIZA reader (Tecan, Elx800- USA). The treated wells were compared with that of the untreated control. All experiments were performed in triplicates.
In Vitro Antifungal Activity
The antifungal activity of ZnO-NPs and CuO-NPs was assessed by disc diffusion method versus phytopathogens fungi as Fusarium solani, Fusarium oxysporum, Sclerotium sclerotia, and Aspergillus terreus that obtained from the culture collection of Chemistry of Natural and Microbial Products, NRC, Egypt. Briefly, sterile discs of 0.7-mm diameter were placed into the potato dextrose agar (PDA) medium that contained the phytopathogenic strains [17]. The discs were loaded with 100 μl of ZnO-NPs and CuO-NPs at a concentration of 10 mg/mL and placed on the plates, separately. The plates were incubated for 5 days at 30 °C. Antifungal activity was evaluated by measuring the inhibition zone towards each strain being tested in triplicates.
Statistical Analysis
The means of three replications and standard error (SEr) were calculated for all the results obtained. The data were subjected to analysis of variance means by sigma plot 12.5 program.
Results and Discussions
Biosynthesis of ZnO-NPs and CuO-NPs Using P. chrysogenum
Recently, the green biosynthesis of oxides nanoparticles has gained great importance being suggested as a possible safe and eco-friendly alternative to chemical and physical synthetic methods. Proteins secreted by fungal strains have effective roles in the reduction of ZnO and CuO to ZnO-NPs and CuO-NPs. respectively, besides stabilizing the formed nanoparticles. In this context, P. chrysogenum filtrate was used as a bioreactor for green synthesis of ZnO-NPs and CuO-NPs through harnessing bioactive macromolecules such as proteins and enzymes secreted therefrom [11]. The appearance of white and green dark colors after contacting of biomass filtrate with zinc acetate and copper acetate at the end of reaction indicated the formation of ZnO-NPs and CuO-NPs, respectively. After calcination as described in “Materials and Method,” ZnO-NPs and CuO-NPs were obtained as a powder.
Characterizations of ZnO-NPs and CuO-NPs
Biosynthesis of ZnO-NPs and CuO-NPs was indicated by UV-visible spectroscopy as represented in Fig. 1. It was shown that the maximum absorption peak of the formed ZnO-NPs and CuO-NPs were occurred at 380 and 335 nm, respectively, due to their surface plasmon resonance. This observation is in agreement with the previous studies on the biosynthesis of ZnO-NPs and CuO-NPs, which indicates that the characterization peaks of ZnO-NPs and CuO-NPs were at 380 nm and 337 nm, respectively [37, 38]. Another report showed that the maximum absorption peaks of ZnO-NPs and CuO-NPs were 301 and 290 nm, respectively [39]. Moreover, the absorption bands for ZnO-NPs and CuO-NPs in Fig. 1 are slightly symmetrical with sharp in width at wavelength 335 and 380 nm, respectively. These could refer to the formation of ZnO-NPs and CuO-NPs with poly-dispersed narrow sizes particles.
TEM measurement was carried out to determine the approximate size and morphology of the biosynthesized ZnO-NPs and CuO-NPs. Figure 2 (A) and (B) indicates that ZnO-NPs and CuO-NPs with hexagonal and spherical shape were successfully formed with dispersed narrow sized particles surrounding with capping by active metabolites using P. chrysogenum filtrate. The average size of the formed ZnO-NPs and CuO-NPs are in the range of 9.0–35.0 nm and 10.5–59.7 nm, respectively. Figure 2 (A1) and (B1) shows the area selected electron diffraction (SAED) patterns of the ZnO-NPs and CuO-NPs, respectively. SAED pattern indicates good sharp rings that reveal the poly-crystalline nature of the ZnO-NPs and CuO-NPs. In previous literature, the size of ZnO-NPs and CuO-NPs biosynthesized by fungi is quite different. Gao et al. used Aspergillus niger for the mycosynthesis of ZnO-NPs with rod and cluster shapes, and the particle size distribution range was 80–130 nm [40]. Saravanakumar et al. reported that the particle size of CuO-NPs synthesized by Trichoderma asperellum ranged from 10 to 190 nm and it was almost spherical in shape [41]. Other reports showed various shapes of ZnO-NPs (hexagonal, nano-rod, and spherical) and CuO-NPs (spherical) with various sizes (10–42 nm, 8–38 nm, 10–45 nm, and 49.2 nm) that were synthesized by Fusarium keratoplasticum, Aspergillus niger and Aspergillus terreus, and Penicillium chrysogenum, respectively [6, 12, 17].
The SEM images of the myco-synthesized ZnO-NPs and CuO-NPs are shown in Fig. 3 (A) and (B). The SEM pictures showed randomly distributed ZnO-NPs and CuO-NPs with particle agglomeration. Other reported illustrated that the particles of metal oxide were predominantly spherical and aggregates into larger particles with no well-defined morphology [41, 42]. EDX examination was also investigated to confirm the purity and elemental composition of ZnO-NPs and CuO-NPs synthesized by P. chrysogenum as illustrated in Fig. 3 (A1) and (B1). Results showed the presence of zinc and oxygen elements. The highest peaks in the spectrum are related to the most concentrated elements. The ratio percentages of Zn and O were 58.3% and 20.0%, for ZnO-NPs, respectively, while CuO-NPs exhibited Cu and O with weight percentages 46.4% and 18.3%, respectively. The peaks for other elements such as C, Na, and K may appear from the filtrate of P. chrysogenum. This is in accordance with the earlier reports of the EDS analysis of ZnO-NPS and CuO NPs [23, 41, 43].
FTIR spectra obtained from the P. chrysogenum filtrate and green synthesized ZnO-NPs and CuO-NPs were shown in Fig. 3 (C). FTIR measurements were carried out to distinguish imaginable interactions between metal oxides (ZnO and CuO) and metabolites of P. chrysogenum, which may be accountable for capping, formation, stabilization, and dispersion of ZnO-NPs and CuO-NPs in colloidal solution. FT-IR analysis for P. chrysogenum filtrate showed the maximum bands at 1639.2 cm−1 that is a definite indicator of amide I and amide II linkages of peptides and band at 3238.86 cm−1 that corresponds to (N–H) stretching group from the previous results [11]. The recorded FT-IR spectra from the biosynthesized ZnO-NPs and CuO-NPs showed peaks at 602.64 and 564.07 cm−1, respectively, that are attributed to the ZnO and CuO stretching vibration mode. In addition, absorption bands in the range 400 to 700 cm−1 may be referred to the metal-oxygen (M−O) stretching mode [12, 44, 45]. While intense absorption peaks have appeared at 3319.85, 2927.41, 1696.08, 1533.13, 1364.39, 1013.40, and 868.77 cm−1. The peak at 3319.85 cm−1 corresponds to O–H stretching group of phenols and alcohol and may be due to the N–H asymmetric stretch mode of amines [6]. The band at 2927.41 cm−1 corresponds to the stretching of CH3 groups of the protein. The bands at 1696.08 and 1533.13 cm−1 correspond to the binding vibrations of amide I band of protein with N–H stretching’s [43]. The bands observed at 1364.39 and 1013.40 cm−1 are assigned to C–N stretching vibrations of aromatic and aliphatic amines [39]. While band at 868.77 cm−1 may be correlated with C–H and C=C alkene [32]. This refers to the coordinating bonds that occurred between metal oxides (ZnO and CuO) and metabolites secreted by P. chrysogenum. From the above results, it is evident that the metabolites secreted in biomass filtrate by P. chrysogenum have the main role in NP formation and size reduction of ZnO and CuO in well-stabilized nano-form.
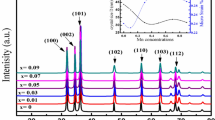
The XRD patterns for ZnO-NPs and CuO-NPs are represented in Fig. 4. From XRD analysis, CuO-NPs exhibited a monoclinic structure. The typical diffraction peaks observed at 2θ = 31.72° (100), 34.47 (002), 36.25 (101), 47.71 (102), 56.59 (110), 62.98 (103), and 67.78 (112) confirmed the hexagonal phase crystals of ZnO [6], while the diffraction peaks of CuO-NPs observed at 2θ° of 36.44°, 42.37°, 61.41°, and 73.37° were ascribed to (111), (200), (220), and (311) planes of the CuO crystalline form, respectively [17]. The crystallite particle sizes of the biosynthesized ZnO-NPs and CuO-NPs were assessed using Scherrer’s equation [46]. In this regard, the average particle size of ZnO-NPs obtained from XRD analysis was ranged from 12.4 to 18.9 nm, while the average particle size of CuO-NPs was ranged from 12.7 to 42.6 nm.
In Vitro Antibacterial Activity of ZnO-NPs and CuO-NPs
One of the great importances of ZnO-NPs and CuO-NPs is their activities against pathogenic microbes. Therefore, ZnO-NPs and CuO-NPs biosynthesized using P. chrysogenum were firstly investigated for their antibacterial activity versus both Gram-positive and Gram-negative bacteria. In general, the inhibitory action for biosynthesized ZnO-NPs and CuO-NPs which represented by the diameter of the clear zone was more effective against Gram-positive than Gram-negative bacteria. The diameters of clear zones formed by ZnO-NPs (5 mg/mL) were 16.33 ± 0.88, 13.5 ± 0.28, 12.43 ± 0.23, 11.06 ± 0.34, and 11.13 ± 0.41 mm for Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli and Salmonella typhimurium, respectively. As well, the diameters of clear zones formed due to CuO-NPs (5 mg/mL) were 22 ± 0.57, 16.26 ± 0.63, 13.6 ± 0.4, 11.93 ± 0.52, and 11.66 ± 0.33 mm, respectively (Fig. 5). The results indicated that CuO-NPs have better inhibitory effects versus Gram-positive and Gram-negative pathogenic-bacteria than those caused by ZnO-NPs. The inhibitory action for biosynthesized ZnO-NPs and CuO-NPs may be due to the reaction of nanoparticles with bacterial protein by combining the thiol (-SH) group and hence leads to inactivation of proteins and bacterial growth. Therefore, it is compulsory to detect the MIC to ZnO-NPs and CuO-NPs for each bacterial strain (Table 1). To this end, the inhibitory effect of different concentrations of ZnO-NPs and CuO-NPs (0.009–5.0 mg/mL) were investigated. Results showed that the MIC for ZnO-NPs were 2 mg/mL against Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa, and 3 mg/mL against Bacillus subtilis and Salmonella typhimurium, while the MIC for CuO-NPs were 1.0, 1.5, 2, and 3 mg/mL against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, and Salmonella typhimurium, respectively. Thus, CuO-NPs are more effective than ZnO-NPs against Gram-positive bacteria being exhibited the lowest inhibitory concentration at 1.0 and 1.5 mg/mL against Staphylococcus aureus and Bacillus subtilis, respectively. Salem and Fouda, explained the mechanism of nanoparticles in bacterial growth reduction through the interaction of NPs with phosphorous moieties in DNA, which lead to inactivation of DNA replication and hence inhibition of enzymes functions [8]. Also, it can inhibit respiratory enzymes of bacterial cells and stop ATP production which leads to cell death [47]. Besides, electrostatic attractions between positive charge of NPs and negative charge of bacterial surface lead to several changes as membrane detachment, cytoplasmic shrinkage, and ultimately membrane of cell rupture [24]. Hydrogen peroxide (H2O2) produced by metal oxide in the presence of water atmosphere plays an important role in the decomposition and death of the bacterial cell when it interacts with the surface of the bacterial cell [48].
In Vitro Anti-Biofilm Activity of ZnO-NPs and CuO-NPs
In this work, the antibiofilm activity of nanoparticles exhibited varied effects against different microorganisms (Fig. 6). Accordingly, CuO-NPs exhibited the highest effect against biofilm formation of Staphylococcus aureus without affecting the bacterial growth when performed at concentrations under MIC value. CuO-NPs at 0.3, 0.15, 0.07, 0.03, and 0.01 mg/mL had reduced the biofilm formation by 95, 94.1, 94.4, 85.9, and 68.8%, respectively, without affecting bacterial growth (Fig. 6a). On the other hand, CuO-NPs did not affect biofilm formations by a Gram-negative bacterium, Pseudomonas aeruginosa (Fig. 6c). Inverted microscopic analysis was carried out to evaluate the effect of nanoparticles on the surface topology of the biofilm matrix. The results showed the presence of biofilm matrix in the positive-control sample, while CuO-NPs-treated wells exhibited reduced surface colonization and biofilm matrix in Staphylococcus aureus. The images of light microscopic analysis confirmed that the CuO-NPs had achieved the whole dispersion of biofilm style by loosening the micro-colonies in the sample treated at 3.0 and 1.5 mg/mL (Fig. 7). Both the results from quantitative and qualitative analyses confirm that the CuO-NPs inhibited the biofilm fashioning of Staphylococcus aureus at the first stage. On the other hand, ZnO-NPs had exhibited no considered effect against biofilm formation of both Gram-positive (Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa) strains (Fig. 6b and d). Another report showed that biologically synthesized metal oxides inhibited the activities of biofilm figuration at its irreversible adherence stage. Interestingly, inhibition of primary stage biofilm activity was observed at the MIC values [49]. Kaweeteerawat et al. evaluated that the mechanism of metal oxide NPs in biofilm inhibition may consequence from NPs interacting with cell membranes of bacteria and stimulating oxidative stress [50]. Previous study explained the anti-bioflim activity according SEM images which showed deformation, external cell roughness, and cell wall’s shrinkage of bacterial cells. Moreover, biofilm formation and viable cells were reduced [51].
Light inverted microscopic images of S. aureus biofilms grown with various concentrations of CuO-NPs: a 0.0 mg/mL, represent the positive control; b negative control; c, d 3.0 and 1.5 mg/mL above the MIC value; e 0.7 mg/mL; f 0.3 mg/mL; g 0.15 mg/mL; h 0.07 mg/mL; i 0.03 mg/mL; and j 0.01 mg/mL. At concentrations from 0.01 to 0.3 mg/mL (f–j) bacteria have appeared as scattered cells and cannot aggregated together to perform normal biofilm
In Vitro Antifungal Activity of ZnO-NPs and CuO-NPs
The antifungal activity of the biosynthesized ZnO-NP and CuO-NPs against four phytopathogenic fungi (Fusarium solani, Fusarium oxysporum, Sclerotium sclerotia, and Aspergillus terreus) were investigated. ZnO-NP and CuO-NPs were proved to have antifungal activity against all the phytopathogenic fungi at 10 mg/mL (Fig. 8). The diameters of clear zones formed by the effect of ZnO-NPs were 12.33 ± 0.88, 11.83 ± 1.36, 23 ± 1.15, and 14 ± 0.5 mm for Fusarium solani, Fusarium oxysporum, Sclerotium sclerotia, and Aspergillus terreus, respectively, while CuO-NPs exhibit stronger antifungal activity where the inhibition zone diameters were 31.66 ± 0.88, 22.66 ± 0.66, 26.83 ± 0.60, and 28.66 ± 1.76 mm for Fusarium solani, Fusarium oxysporum, Sclerotium sclerotia, and Aspergillus terreus, respectively. ZnO-NP and CuO-NPs have also showed activities against phytopathogenic fungal strains Alternaria solani, Aspergillus niger, Fusarium oxysporum, Pythium ultimum, and Alternaria alternate [32, 52]. Nanomaterial inhibited the fungal growth by affecting cellular functions, which caused distortion in fungal hyphae and prevented the expansion of conidia and conidiophores and consequently lead to cell death [53].
Conclusion
In this study, a promising biogenic ZnO and CuO nanoparticles were produced by P. chrysogenum. They showed obvious ability to inhibit several pathogens including Gram-positive and Gram-negative bacteria, as well as some phytopathogenic fungi. CuO-NPs at 0.3, 0.15, 0.07, 0.03, and 0.01 mg/mL had reduced the biofilm formation by 95, 94.1, 94.4, 85.9, and 68.8%, respectively, without affecting bacterial growth. The obtained biosynthesized ZnO-NPs and CuO-NPs are smart nano-materials that exhibited the potential to be applied in the medical field and limit the spread of some pathogenic microbes.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Change history
25 September 2020
A Correction to this paper has been published: https://doi.org/10.1007/s12011-020-02391-6
References
Elfeky AS, Salem SS, Elzaref AS, Owda ME, Eladawy HA, Saeed AM, Awad MA, Abou-Zeid RE, Fouda A (2020) Multifunctional cellulose nanocrystal /metal oxide hybrid, photo-degradation, antibacterial and larvicidal activities. Carbohydr Polym 230:115711. https://doi.org/10.1016/j.carbpol.2019.115711
Salem SS, Fouda MM, Fouda A, Awad MA, Al-Olayan EM, Allam AA, Shaheen TI (2020) Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J Clust Sci:1–11. https://doi.org/10.1007/s10876-020-01794-8
Alsharif SM, Salem SS, Abdel-Rahman MA, Fouda A, Eid AM, El-Din Hassan S, Awad MA, Mohamed AA (2020) Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon 6(5):e03943. https://doi.org/10.1016/j.heliyon.2020.e03943
Shaheen TI, Salem SS, Zaghloul S (2019) A new facile strategy for multifunctional textiles development through in situ deposition of SiO2/TiO2 nanosols hybrid. Ind Eng Chem Res 58(44):20203–20212. https://doi.org/10.1021/acs.iecr.9b04655
Sharaf OM, Al-Gamal MS, Ibrahim GA, Dabiza NM, Salem SS, El-ssayad MF, Youssef AM (2019) Evaluation and characterization of some protective culture metabolites in free and nano-chitosan-loaded forms against common contaminants of Egyptian cheese. Carbohydr Polym 223:115094. https://doi.org/10.1016/j.carbpol.2019.115094
Mohamed AA, Fouda A, Abdel-Rahman MA, Hassan SE-D, El-Gamal MS, Salem SS, Shaheen TI (2019) Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal Agric Biotechnol 19:101103. https://doi.org/10.1016/j.bcab.2019.101103
Mohmed AA, Fouda A, Elgamal MS, El-Din Hassan S, Shaheen TI, Salem SS (2017) Enhancing of cotton fabric antibacterial properties by silver nanoparticles synthesized by new egyptian strain fusarium keratoplasticum A1-3. Egypt J Chem 60:63–71. https://doi.org/10.21608/ejchem.2017.1626.1137
Salem SS, Fouda A (2020) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02138-3
Hassan SE-D, Salem SS, Fouda A, Awad MA, El-Gamal MS, Abdo AM (2018) New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J Radiat Res Appl Sci 11(3):262–270
Fouda A, Abdel-Maksoud G, Abdel-Rahman MA, Eid AM, Barghoth MG, El-Sadany MA-H (2019) Monitoring the effect of biosynthesized nanoparticles against biodeterioration of cellulose-based materials by Aspergillus niger. Cellulose 26(11):6583–6597
Abd El Aty AA, Mohamed AA, Zohair MM, Soliman AA (2020) Statistically controlled biogenesis of silver nano-size by Penicillium chrysogenum MF318506 for biomedical application. Biocatal Agric Biotechnol 25:101592. https://doi.org/10.1016/j.bcab.2020.101592
Fouda A, El-Din Hassan S, Salem SS, Shaheen TI (2018) In-vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized zinc oxide nanoparticles for medical textile applications. Microb Pathog 125:252–261. https://doi.org/10.1016/j.micpath.2018.09.030
Fouda A, Hassan SE-D, Abdo AM, El-Gamal MS (2019) Antimicrobial, Antioxidant and Larvicidal activities of spherical silver nanoparticles synthesized by endophytic Streptomyces Spp. Biol Trace Elem Res 195:707–724
Amr Fouda, Gomaa Abdel-Maksoud, Mohamed Ali Abdel-Rahman, Salem S. Salem, Saad El-Din Hassan, Mohamad Abdel-Haleem El-Sadany, (2019) Eco-friendly approach utilizing green synthesized nanoparticles for paper conservation against microbes involved in biodeterioration of archaeological manuscript. International Biodeterioration & Biodegradation 142:160-169
Asem Mohmed, Saad Hassan, Amr Fouda, Mamdouh Elgamal, Salem Salem, (2017) Extracellular Biosynthesis of Silver Nanoparticles Using Aspergillus sp. and Evaluation of their Antibacterial and Cytotoxicity. Journal of Applied Life Sciences International 11 (2):1-12
Soliman AM, Abdel-Latif W, Shehata IH, Fouda A, Abdo AM, Ahmed YM (2020) Green approach to overcome the resistance pattern of Candida spp. using biosynthesized silver nanoparticles fabricated by Penicillium chrysogenum F9. Biol Trace Elem Res:1–12. https://doi.org/10.1007/s12011-020-02188-7
El-Batal AI, El-Sayyad GS, Mosallam FM, Fathy RM (2020) Penicillium chrysogenum-mediated mycogenic synthesis of copper oxide nanoparticles using gamma rays for in vitro antimicrobial activity against some plant pathogens. J Clust Sci 31(1):79–90
Xiong HM (2013) ZnO nanoparticles applied to bioimaging and drug delivery. Adv Mater 25(37):5329–5335
Zhong Q, Tian J, Liu T, Guo Z, Ding S, Li H (2018) Preparation and antibacterial properties of carboxymethyl chitosan/ZnO nanocomposite microspheres with enhanced biocompatibility. Mater Lett 212:58–61
Pirhashemi M, Habibi-Yangjeh A, Pouran SR (2018) Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J Ind Eng Chem 62:1–25
Yadavalli T, Shukla D (2017) Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomedicine 13(1):219–230
Jamdade DA, Rajpali D, Joshi KA, Kitture R, Kulkarni AS, Shinde VS, Bellare J, Babiya KR, Ghosh S (2019) Gnidia glauca-and plumbago zeylanica-mediated synthesis of novel copper nanoparticles as promising antidiabetic agents. Adv Pharmacol Sci 2019. https://doi.org/10.1155/2019/9080279
Asghar MA, Yousuf RI, Shoaib MH, Asghar MA (2020) Antibacterial, anticoagulant and cytotoxic evaluation of biocompatible nanocomposite of chitosan loaded green synthesized bioinspired silver nanoparticles. Int J Biol Macromol 160:934–943. https://doi.org/10.1016/j.ijbiomac.2020.05.197
Asghar MA, Asghar MA (2020) Green synthesized and characterized copper nanoparticles using various new plants extracts aggravate microbial cell membrane damage after interaction with lipopolysaccharide. Int J Biol Macromol 160:1168–1176. https://doi.org/10.1016/j.ijbiomac.2020.05.198
Asghar MA, Zahir E, Asghar MA, Iqbal J, Rehman AA (2020) Facile, one-pot biosynthesis and characterization of iron, copper and silver nanoparticles using Syzygium cumini leaf extract: as an effective antimicrobial and aflatoxin B1 adsorption agents. PLoS One 15(7):e0234964. https://doi.org/10.1371/journal.pone.0234964
Valodkar M, Jadeja RN, Thounaojam MC, Devkar RV, Thakore S (2011) Biocompatible synthesis of peptide capped copper nanoparticles and their biological effect on tumor cells. Mater Chem Phys 128(1–2):83–89
Zangeneh MM, Ghaneialvar H, Akbaribazm M, Ghanimatdan M, Abbasi N, Goorani S, Pirabbasi E, Zangeneh A (2019) Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J Photochem Photobiol B Biol 197:111556
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451(7181):990–993
Khan ST, Musarrat J, Al-Khedhairy AA (2016) Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: current status. Colloids Surf B: Biointerfaces 146:70–83
Waters EM, Rowe SE, O'Gara JP, Conlon BP (2016) Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLoS Pathog 12(12):e1006012
Harne S, Sharma A, Dhaygude M, Joglekar S, Kodam K, Hudlikar M (2012) Novel route for rapid biosynthesis of copper nanoparticles using aqueous extract of Calotropis procera L. latex and their cytotoxicity on tumor cells. Colloids Surf B: Biointerfaces 95:284–288. https://doi.org/10.1016/j.colsurfb.2012.03.005
Hassan SED, Fouda A, Radwan AA, Salem SS, Barghoth MG, Awad MA, Abdo AM, El-Gamal MS (2019) Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J Biol Inorg Chem 24(3):377–393. https://doi.org/10.1007/s00775-019-01654-5
Weinstein MP (2019) Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute,
Abdelhameed RM, Abu-Elghait M, El-Shahat M (2020) Hybrid three MOFs composites (ZIF-67@ ZIF-8@ MIL-125-NH2): enhancement the biological and visible-light photocatalytic activity. J Environ Chem Eng 8(5)7:104107. https://doi.org/10.1016/j.jece.2020.104107
Desouky SE, El-Gamal MS, Mohammed AF, Abu-Elghait MA (2014) Determination of some virulence factors in Staphylococcus spp. isolated from clinical samples of different Egyptian patients. World Appl Sci J 32:731–740
Hamed A, Abdel-Razek AS, Araby M, Abu-Elghait M, El-Hosari DG, Frese M, Soliman HS, Stammler HG, Sewald N, Shaaban M (2020) Meleagrin from marine fungus Emericella dentata Nq45: crystal structure and diverse biological activity studies. Nat Prod Res:1–9. https://doi.org/10.1080/14786419.2020.1741583
Santhoshkumar J, Kumar SV, Rajeshkumar S (2017) Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technol 3(4):459–465. https://doi.org/10.1016/j.reffit.2017.05.001
Sathiyavimal S, Vasantharaj S, Bharathi D, Saravanan M, Manikandan E, Kumar SS, Pugazhendhi A (2018) Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J Photochem Photobiol B Biol 188:126–134
Maruthupandy M, Zuo Y, Chen J-S, Song J-M, Niu H-L, Mao C-J, Zhang S-Y, Shen Y-H (2017) Synthesis of metal oxide nanoparticles (CuO and ZnO NPs) via biological template and their optical sensor applications. Appl Surf Sci 397:167–174
Gao Y, Anand MAV, Ramachandran V, Karthikkumar V, Shalini V, Vijayalakshmi S, Ernest D (2019) Biofabrication of zinc oxide nanoparticles from Aspergillus niger, their antioxidant, antimicrobial and anticancer activity. J Clust Sci 30(4):937–946
Saravanakumar K, Shanmugam S, Varukattu NB, MubarakAli D, Kathiresan K, Wang M-H (2019) Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J Photochem Photobiol B Biol 190:103–109
Janaki AC, Sailatha E, Gunasekaran S (2015) Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 144:17–22. https://doi.org/10.1016/j.saa.2015.02.041
Ganesan V, Hariram M, Vivekanandhan S, Muthuramkumar S (2020) Periconium sp.(endophytic fungi) extract mediated sol-gel synthesis of ZnO nanoparticles for antimicrobial and antioxidant applications. Mater Sci Semicond Process 105:104739
Mahmoud BG, Khairy M, Rashwan FA, Foster CW, Banks CE (2016) Self-assembly of porous copper oxide hierarchical nanostructures for selective determinations of glucose and ascorbic acid. RSC Adv 6(18):14474–14482
Fardood ST, Ramazani A, Moradi S, Asiabi PA (2017) Green synthesis of zinc oxide nanoparticles using arabic gum and photocatalytic degradation of direct blue 129 dye under visible light. J Mater Sci Mater Electron 28(18):13596–13601
Gawade V, Gavade N, Shinde H, Babar S, Kadam A, Garadkar K (2017) Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J Mater Sci Mater Electron 28(18):14033–14039
Aref MS, Salem SS (2020) Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal Agric Biotechnol 27:101689. https://doi.org/10.1016/j.bcab.2020.101689
Abdalkarim SYH, Yu H-Y, Song M-L, Zhou Y, Yao J, Ni Q-Q (2017) In vitro degradation and possible hydrolytic mechanism of PHBV nanocomposites by incorporating cellulose nanocrystal-ZnO nanohybrids. Carbohydr Polym 176:38–49. https://doi.org/10.1016/j.carbpol.2017.08.051
Ashajyothi C, Harish KH, Dubey N, Chandrakanth RK (2016) Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: a nanoscale approach. J Nanostruct Chem 6(4):329–341. https://doi.org/10.1007/s40097-016-0205-2
Kaweeteerawat C, Ivask A, Liu R, Zhang H, Chang CH, Low-Kam C, Fischer H, Ji Z, Pokhrel S, Cohen Y, Telesca D, Zink J, Mädler L, Holden PA, Nel A, Godwin H (2015) Toxicity of metal oxide nanoparticles in Escherichia coli correlates with conduction band and hydration energies. Environ Sci Technol 49(2):1105–1112. https://doi.org/10.1021/es504259s
Wong CW, Chan YS, Jeevanandam J, Pal K, Bechelany M, Abd Elkodous M, El-Sayyad GS (2020) Response surface methodology optimization of mono-dispersed MgO nanoparticles fabricated by ultrasonic-assisted sol–gel method for outstanding antimicrobial and antibiofilm activities. J Clust Sci 31(2):367–389. https://doi.org/10.1007/s10876-019-01651-3
Abdelhakim HK, El-Sayed E, Rashidi FB (2020) Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J Appl Microbiol 128:1634–1646
He L, Liu Y, Mustapha A, Lin M (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166(3):207–215
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, A.A., Abu-Elghait, M., Ahmed, N.E. et al. Eco-friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol Trace Elem Res 199, 2788–2799 (2021). https://doi.org/10.1007/s12011-020-02369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02369-4