Abstract
Serum concentrations of magnesium and manganese may be associated with increased chronic obstructive pulmonary disease exacerbation risk. However, associations with other aspects of asthma-chronic obstructive pulmonary disease overlap, pulmonary function test results and health status, have been studied less extensively. The aim of this study was to investigate the associations between serum concentrations of trace elements and T lymphocyte subsets, FeNO, and COPD-related questionnaire scores in individuals with ACO and the potential impact of these parameters on lung function. All the patients met the diagnostic criteria of ACO and were divided into two groups (group A, mild–moderate; group B, severe–very severe) by their specific characteristics. Pulmonary function testing and serum Mg and serum Mn and FeNO were measured. Four hundred sixty-five patients were screened, and 42 were included. Group A had significantly higher Mg and Fe concentrations than group B. No significant differences were seen in the serum concentration of any other trace element between the two groups. Serum Mg and Mn were correlated with FEV1% predicted (p < 0.01). Group A had a significantly higher FeNO concentration than group B (p = 0.005). The scores on CAT (p = 0.011) and mMRC (p = 0.008) in group A were lower than in group B. The low-FeNO group had a significantly lower concentration of serum Mg than the high-FeNO group (p = 0.03). Pulmonary function declined faster (p < 0.05) in the low-FeNO group than the high-FeNO group. Serum Mg concentration may indicate protective effects against lung function loss in ACO. This implies that FeNO might be a biomarker for identifying individuals with ACO who might benefit from inhaled corticosteroid therapy. Serum Mg and FeNO were associated with ACO severity. However, their role in guiding personalised treatment of individuals with ACO needs to be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD), which is a preventable and treatable disease, is gradually increasing in prevalence worldwide due to smoking habits and environmental pollution, accompanied by the increase in the elderly population. It has a significant effect on quality of life, symptoms, comorbidities, and the health care sector. Clinically, COPD is characterised by progressive dyspnoea, chronic cough, sputum production, and repeated episodes of acute worsening of respiratory status called COPD exacerbation (AECOPD) [1]. In 2014, asthma-COPD overlap (ACO) was jointly described by the Global Initiative for Asthma (GINA) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) as persistent airflow limitation with features of both asthma and COPD, and it was estimated to apply to approximately 10–20% of individuals with COPD [2]. They experience frequent exacerbations and have poor quality of life, a greater decline in lung function, and higher mortality than patients with asthma or COPD alone. ACO is a prevalent condition in middle-aged and older Chinese and Japanese. Better management and more research on ACO are needed [3, 4].
Individuals with COPD have a high risk of malnutrition that adversely affects their quality of life [5]. Malnutrition remains a major and widespread problem in individuals with moderate or severe COPD and affects the body composition and food intake of these patients. In some studies, there is a significant relationship between the stage of COPD and nutrient intake (energy, carbohydrate, fat, vitamin E, magnesium, and omega-3 fatty acids) [6]. Certain nutrients show protective effects against lung function loss, some mainly by improving average lung function and others by reducing the decline rate. Dietary interventions early in life may delay the decline of lung function [7, 8]. Another study showed that serum concentrations of Mg were not correlated with lung function [9].
Magnesium (Mg) plays an important role in neuromuscular, cardiovascular, and metabolic functions [10,11,12]. Studies show a possible association between Mg and both muscle strength and exercise performance [13, 14]. Serum Mg has also been associated with reduced lung function and an increased exacerbation rate in COPD [15,16,17,18].
Manganese (Mn) is a cofactor of many enzymes that have an effect on a number of physiological metabolic processes, such as human nutrition, and protect against lipid peroxidation [19]. The Mn-containing enzyme manganese superoxide dismutase is a principal antioxidant enzyme holding oxygen [20]. Moreover, excessive Mn is conducive to the generation of free radicals by its pro-oxidative properties [21]. Serum manganese (Mn) may be a beneficial biomarker of tissue oxygenation and mechanisms in COPD [22]. Serum concentrations of magnesium (serum Mg) and manganese (serum Mn) may be associated with increased exacerbation risk in chronic obstructive pulmonary disease (COPD).
However, associations with other aspects of asthma such as chronic obstructive pulmonary disease overlap (ACO), lung function and the CAT and T lymphocyte subsets, have been studied less extensively. The aim of this study was to investigate the associations between serum concentrations of trace elements and FeNO and various T lymphocyte subsets. Health status was evaluated by three questionnaires: COPD Assessment Test (CAT), Cough Clinical Symptom Score (CCSS), and Medical Research Council dyspnoea questionnaire (MRC) in individuals with ACO. We assessed the potential impact of all these parameters on lung function.
Materials and Methods
Inclusion and Exclusion Criteria
We collected data from elderly individuals with ACO who were hospitalised in the First Affiliated Hospital of Shantou University Medical College, Shantou City, Guangdong Province, People’s Republic of China, from January 2018 to December 2019. Inclusion criteria were an ACO diagnosis for at least 1 year prior to study commencement, age over 40 years, and unchanged usual drug therapy the last 4 weeks before inclusion. The exclusion criteria were (1) endocrine diseases, (2) systemic steroid therapy in the last 4 weeks, (3) other diseases, including as cancer, lung resection, known addiction to alcohol or drugs, and other lung diseases, such as active tuberculosis and lung fibrosis, and (4) no history of using medicines such as dietary supplementations (both vitamin and mineral supplementations) that affect the serum concentration of trace elements.
Experimental protocols were approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College. Written informed consent was obtained from every patient.
Methods
All the patients had diagnostic criteria that suggested ACO, including data from their medical history, symptoms, signs, chest computed tomography, and other imaging examinations, combined with the time of admission to hospital and past lung function testing results: FEV1/FVC (forced vital capacity rate of 1 s) < 70% after the application of bronchodilators. Airflow limitation is not fully reversible, but often, it has current or historical variability, for example, bronchodilator reversibility. The stages of individuals with ACO were determined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria by the responsible physician specialists. The patients with forced expiratory volume in 1 s (FEV1) ≥ 80%, FEV1 < 80% but ≥ 50%, FEV1 < 50% but ≥ 30%, and FEV1 < 30% or FEV1 < 50% + dyspnoea were defined to have mild, moderate, severe, and very severe ACO, respectively. These criteria were used to divide them into two groups: mild and moderate ACO (group A, 17 cases) and severe and very severe ACO (group B, 25 cases) [1, 2].
Pulmonary Function Testing
Pulmonary function was measured by routine spirometry (CHESTGRAPH HI-101, Chest M.I., Inc., Japan) before and after inhalation of 400 mg salbutamol via MDI following the guidelines recommended by the European Respiratory Society. The highest values from a minimum of three technically acceptable spirometric manoeuvres were used and are expressed as percentages.
Fractional Exhaled Nitric Oxide
FeNO was measured by chemiluminescence using an online nitric oxide monitor (Sunvou-P100, Wuxi, China) according to the European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines. It is expressed as parts per billion (ppb). The patients were instructed to avoid eating and drinking and not to perform any strenuous exercise within 2 h before the FeNO measurements. After inhaling ambient air to full lung capacity through a nitric oxide scrubber, the participants exhaled against expiratory resistance, excluding nasal air. Exhalation times were 10 s, with a 2-min analysis period. Repeated exhalations (2 values that agreed within 5% or 3 that agreed within 10%) were performed without a nose clip at a constant flow rate of 50 mL/s [23, 24].
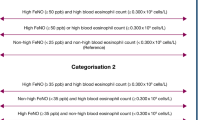
Both high (> 50 ppb) and low (< 25 ppb) FENO concentrations may be used to predict outcomes in patients with a definite history of asthma currently in remission and in whom withdrawal of ICS therapy is being undertaken [25]. One recent study used 25 ppb as the cutoff of FeNO based on the mean concentration of FeNO in the recruited patients with COPD. FeNO was associated with COPD severity and allergic airway inflammation in individuals with stable COPD [24].
Determination of Serum Trace Elements and T Lymphocyte Subsets
The doctor took 3–5 mL of venous blood in the morning. Serum magnesium, manganese, lead, zinc, ferrum, and copper were measured by tandem mass spectrometry (Agilent ICP-MS 7500a, USA).
-
T-
lymphocyte subsets of peripheral blood were assayed by flow cytometry. (Beckman Kurt Navios flow cytometry system, USA)
Health State and Three COPD-Related Questionnaires
All analyses were done as routine analyses at the hospital laboratory. Body height and weight were measured, and participants were interviewed about their smoking status and COPD status, including overall health status and respiratory symptoms. Health status was evaluated by three questionnaires: the COPD Assessment Test (CAT), Cough Clinical Symptom Score (CCSS), and Medical Research Council dyspnoea questionnaire (MRC). The CCSS self-report questionnaire measures self-reported cough clinical symptoms at daytime and nighttime [26]. CAT gives a quantitative self-reported measure for COPD-related quality of life [27]. MRC measures the severity of dyspnoea [28].
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 23.0. If data were normally distributed, the mean and standard deviation were calculated, and Student's t test was used for comparisons. Median and 25th and 75th percentiles were used when data were not normally distributed, and the Mann-Whitney U test was used for comparisons. p values less than 0.05 were considered statistically significant.
Correlation Analysis
We performed correlated regression analysis between Mg and Mn and the FEV1, COPD-related questionnaire scores and FeNO.
Results
Four hundred sixty-five patients were screened, and 42 were included. Thirty-eight men and four women participated in the study, and all participants were in southern China. The present study included 17 cases of mild to moderate ACO (group A) and 25 cases of severe to very severe ACO (group B). Demographic data of the study participants are given in Table 1.
Serum Trace Elements and T Lymphocyte Subsets
Table 2 shows serum concentrations of trace elements such as magnesium, manganese, lead, zinc, ferrum, and copper. Group A had significantly higher Mg (p = 0.011) and Fe (p = 0.043) than group B. The level of CD8+ cells in Group A were higher than in group B (p = 0.049). No significant differences were seen in the serum concentration of any other trace element, such as manganese, lead, zinc, and copper, between the two groups (p > 0.05) (Table 2).
Serum Mg was correlated with FEV1% predicted (correlation coefficient = 0.46, p = 0.002), as shown in Fig. 1. Serum Mn was correlated with FEV1% predicted (correlation coefficient = 0.399. p = 0.009), as shown in Fig. 2. Serum Mg and Mn were not correlated with other parameters (p > 0.05).
FeNO and the Three COPD-Related Questionnaires
Table 3 shows the concentration of FeNO and the scores on three questionnaires (CCSS, CAT, mMRC). Group A had a significantly higher FeNO concentration than group B (p = 0.005). The CAT (p = 0.011) and mMRC (p = 0.008) scores in Group A were lower than those in Group B. No significant differences in the Cough Clinical Symptom Score (CCSS) (p = 0.437) were found between group A and group B (Table 3).
Differences in serum Mg and PFT were examined between the high- and low-FeNO groups. The low-FeNO group had a significantly lower concentration of serum Mg than the high-FeNO group (p = 0.03). Compared with that in the high-FeNO group, pulmonary function declined faster (p < 0.05) in the low-FeNO group (Table 4).
Discussion
A review article has suggested that serum Mg could have an effect on lung function in patients with COPD. Certain micronutrients (vitamins and minerals) have potent antioxidant or methyl-donating properties and thus have received considerable interest. Probing into the effects of nutrients on lung function preservation has suggested several hypothetical intervention patterns. Intakes of vitamins and magnesium all show protective effects against lung function loss, some mainly by improving average lung function and others by reducing the decline rate [7, 8]. However, Sarah Hashim et al. found that the serum concentration of Mg was not associated with FEV1 but was associated with a healthy state in COPD. Serum Mg has also been associated with reduced lung function and an increased exacerbation rate in COPD [15,16,17,18]. In a small prospective observational study, Gumus A et al. found that serum magnesium concentration during the exacerbation period was the most significant predictor of frequency of AECOPD [17].
To our knowledge, this is the first study investigating the association between Mg and lung function in ACO. We found that severe to very severe individuals with ACO had a significantly lower Mg concentration (p = 0.011) than mild to moderate individuals with ACO. Serum Mg was correlated with FEV1% predicted (correlation coefficient = 0.46. p = 0.002), as shown in Fig. 1. Moreover, the scores of CAT (p = 0.011) and mMRC (p = 0.008) in Group A were lower than in Group B. It seems that severe and very severe individuals with ACO have worse health states. On the one hand, malnutrition is a common problem in individuals with moderate or severe COPD that affects the body composition and food intake of these patients. The percentage of patients who did not meet the daily recommended intake (RNI) is the highest for magnesium (93.8%) in individuals with COPD [17, 29, 30]. Mg-deficient participants report significantly more problems with mobility, usual activities, discomfort, and muscle strength and exercise performance [13, 14, 31]. On the other hand, lung elasticity is reduced in COPD lungs, which is largely due to chronically enhanced elastin degradation. An optimal formulation including magnesium has the proven ability to help regain lost lung function in individuals with COPD and could cause a major paradigm shift. Decelerating elastinolysis might be an attractive therapeutic target in this debilitating condition. Magnesium can be regarded as a natural calcium antagonist and has the proven ability to ameliorate vascular calcification [32].
An animal study has suggested that magnesium deficiency promotes elastin degradation. Janssen et al. hypothesise that inhibiting elastin calcification by means of magnesium supplementation might counteract both vascular calcification and elastin degradation in COPD [33]. Finally, two studies showed that intravenous magnesium sulphate used as an adjunct therapy to standard bronchodilators in AECOPD may improve lung function, exercise performance, and respiratory mechanics in the short term [34, 35]. This could be explained by the important role of Mg in muscle strength and elastin degradation. Our findings support this hypothesis. Therefore, dieticians should be aware of the characteristics of individuals with COPD to evaluate their Mg intake and plan nutritional strategies to improve the prognosis of the disease. Dietary interventions early in life may help the lung function reserve over the lifespan [17].
Manganese (Mn) is constituent of numerous enzymes that play an important role in a number of physiological metabolic processes, such as human nutrition, and protects against lipid peroxidation [19]. One study has investigated whether serum Mn may be a beneficial marker of tissue oxygenation and defence mechanisms before the clinical onset of COPD in smokers [22]. On the other hand, a randomised controlled trial showed that trace element status (Se, Mn, and Zn) is altered in individuals with COPD. Their supplementation achieved a reduction in the period those patients spent on mechanical ventilation [36]. In the present study, Serum Mn was a trace element parameter weakly associated with FEV1% predicted (correlation coefficient = 0.399. p = 0.009). However, a larger multicentre trial is required to confirm this preventive effect and to explore its applicability to other arenas of critical care.
The value of fractional exhaled nitric oxide (FeNO) in patients with chronic obstructive pulmonary disease (COPD) remains unclear. Liu X et al. found that a high FeNO concentration was associated with a lower moderate or severe exacerbation in the preceding 12 months. There were more patients with GOLD stage III–IV in the low-FeNO group than in the high-FeNO group. In individuals with stable COPD, FeNO was associated with COPD severity and allergic airway inflammation [24]. Our study showed a similar effect. Individuals with ACO with stage III–IV disease had significantly lower FeNO concentration than individuals with ACO with stage I–II disease (p = 0.005).
In our study, we used 25 ppb as the cut-off of FeNO based on previous investigations in individuals with COPD [24, 25]. Based on this 25 ppb cutoff of FeNO, our data showed that pulmonary function declined faster (p < 0.05) in the low-FeNO group than the high-FeNO group. Patients with pulmonary function declining faster had a significantly decreased positive proportion of FeNO by hyperventilation. Högman et al. found FeNO was associated with eosinophil inflammation and inhaled corticosteroid (ICS) use in ex-smoking COPD subjects, but not in smoking subjects, suggesting that the value of FeNO as an inflammatory marker is more limited in smoking subjects [37]. This implies that patients with worse pulmonary function might use much more ICS to relieve dyspnoea and be more likely to have smoked cigarettes. All of them reduce the FeNO concentration.
The association found between low FeNO values and low lung function requires further investigation. Fabbri et al. have shown that patients with historical evidence of asthma have eosinophilic airway inflammation in association with raised FeNO concentration [38]. Earlier, Papi et al. reported that increased sputum eosinophils and FeNO occur in individuals with COPD with greater degrees of bronchodilator reversibility [39]. This implies that FeNO might be a biomarker for identifying individuals with ACO who might benefit from inhaled corticosteroid therapy. However, the role of FeNO in guiding the personalised treatment of individuals with ACO needs to be further investigated.
However, there are certain limitations to this study. Individuals with ACO have about 9% of the individuals with COPD patients enrolled in the research. In 2014, GINA and GOLD summarised that it was estimated to apply to approximately 10–20% of individuals with COPD [2]. In urban China, the prevalence of ACO has been estimated by the China National Health and Wellness Surveys (CNHWS), which was 18.49% in those with COPD [4]. Individuals with ACO med the exclusion criteria were removed in the study. It may be the reason for the small proportion compared with previous reports. Because of the small sample size, the results require further investigation. The cross-sectional design makes it difficult to conclude the direction of any relationship between serum Mg and FEV1 over time. Moreover, some data (e.g., COPD-related questionnaires) were self-reported; therefore, a risk of recall bias cannot be excluded.
In conclusion, this study shows that serum Mg is related to spirometry values in ACO. Serum Mg concentration may indicate protective effects against lung function loss. Moreover, we found that the FeNO of mild to moderate individuals with ACO was significantly higher than that of severe to very severe patients. This implies that FeNO might be a biomarker for identifying individuals with ACO who might benefit from inhaled corticosteroid therapy. In individuals with ACO, Serum Mg and FeNO were associated with ACO severity. However, their role in guiding personalised treatment of individuals with ACO needs to be further investigated.
References
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, López Varela MV, Martinez F, Montes de Oca M, Papi A, Pavord ID, Roche N, Sin DD, Stockley R, Vestbo J, Wedzicha JA, Vogelmeier C (2019) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 53:1900164. https://doi.org/10.1183/13993003.00164-2019
Global Initiative for Asthma (GINA), Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2017) Diagnosis and initial treatment of asthma, COPD, and asthma–COPD overlap. Available from: https://ginasthma.org/
Barrecheguren M, Pinto L, Mostafavi-Pour-Manshadi SM (2020) Identification and definition of asthma-COPD overlap: the CanCOLD study. Respirology 25:836–849
Song P, Zha M, Xia W, Zhu Y (2020) Asthma-chronic obstructive pulmonary disease overlap in China: prevalence, associated factors and comorbidities in middle-aged and older adults. Curr Med Res Opin:1–9
Arslan M, Soylu M, Kaner G (2016) Başmısırlı, evaluation of malnutrition detected with the nutritional risk screening 2002 (NRS-2002) and the quality of life in hospitalized patients with chronic obstructive pulmonary disease. Hippokratia 20(2):147–152
Ahmadi A, Haghighat N, Hakimrabet M, Tolide-ie H (2012) Nutritional evaluation in chronic obstructive pulmonary disease patients. Pak J Biol Sci 15(10):501–505
Zhai T, Li S, Hu W, Leng S (2018) Potential micronutrients and phytochemicals against the pathogenesis of chronic obstructive pulmonary disease and lung cancer. Nutrients 10(7)
Leng S, Picchi MA, Tesfaigzi Y, Belinsky SA (2017) Dietary nutrients associated with preservation of lung function in Hispanic and non-Hispanic white smokers from New Mexico. Int J Chron Obstruct Pulmon Dis 12:3171–3181
Hashim Ali Hussein S, Nielsen LP, Konow Bøgebjerg Dolberg M, Dahl R (2015) Associated with better QoL in COPD: A cross-sectional study. Respir Med 109(6):727–733
Topf JM, Murray PT (2003) Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord 4(2):195
Rude RK, Oldham SB, Singer FR (1976) Functional hypoparathyroidism and parathyroid hormone end-organ resistance in human magnesium deficiency. Clin Endocrinol 5(3):209
Huang CL, Kuo E (2007) Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 18(10):2649
Dominguez LJ, Barbagallo M, Lauretani F, Bandinelli S, Bos A, Corsi AM et al (2006) Magnesium and muscle performance in older persons: the InCHIANTI study. Am J Clin Nutr 84(2):419
Brilla LR, Haley TF (1992) Effect of magnesium supplementation on strength training in humans. J Am Coll Nutr 11(3):326
Postnikova LB, Alekseeva OP, Kubysheva NI, Gorshkova TN, Ishanova OS (2004) Significance of biochemical parameters of saliva in the diagnosis of chronic obstructive pulmonary disease at exacerbation. Klin Lab Diagn 10:16
Ruljancic N, Popovic-Grle S, Rumenjak V, Sokolic B, Malic A, Mihanovic M et al (2007) COPD: magnesium in the plasma and polymorphonuclear cells of patients during a stable phase. COPD 4(1):41–47
Gumus A, Haziroglu M, Gunes Y (2014) Association of serum magnesium levels with frequency of acute exacerbations in chronic obstructive pulmonary disease: a prospective study. Pulm Med 2014:329476
McKeever TM, Lewis SA, Smit HA, Burney P, Cassano PA, Britton J (2008) A multivariate analysis of serum nutrient concentrations and lung function. Respir Res 9:67
Soldin OP, Aschner M (2007) Effects of manganese on thyroid hormone homeostasis. Neurotoxicology 28(5):951–956. https://doi.org/10.1016/j.neuro.2007.05.003
Jain RB, Choi YS (2015) Normal reference ranges for and variability in the levels of blood manganese and selenium by gender, age, and race/ethnicity for the general U.S. population. J Trace Elem Med Biol 30:142–152. https://doi.org/10.1016/j.jtemb.2014.12.004
Kim YJ, Kim YK, Kho HS (2010) Effects of smoking on trace metal levels in saliva. Oral Dis 16:823–830. https://doi.org/10.1111/j.1601-0825.2010.01698.x
Ates Alkan F, Karis D, Cakmak G, Ercan AM (2019) Analysis of the relationship between hemorheologic parameters, aluminum, manganese, and selenium in smokers. Biol Trace Elem Res 187(1):22–31
American Thoracic Society. European Respiratory Society (2005) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 171(8):912–930
Liu X, Zhang H, Wang Y, Huang K (2020) Fractional exhaled nitric oxide is sssociated with the severity of stable COPD. COPD:1–7
Taylor DR, Pijnenburg MW, Smith AD et al (2006) Exhaled nitricoxide measurements: clinical application and interpretation [J]. Thorax 61(9):817–827
Zhan W, Tang J, Chen X, Lai K (2019) Duration of treatment with inhaled corticosteroids in nonasthmatic eosinophilic bronchitis: a randomized open-label trial. Ther Adv Respir Dis 13:1753466619891520
Jones P, Harding G, Berry P et al (2009) Development and first validation of the COPD assessment test. Eur Respir J 34:648–654
Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J (1999) Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 54(7):581–586
Yılmaz D, Çapan N, Canbakan S, Besler HT (2015) Severe COPD in relation to fat-free mass index: a cross-sectional study. Nutr J 14:35
Ahmadi A, Haghighat N, Hakimrabet M, Tolide-ie H (2012) Nutritional evaluation in chronic obstructive pulmonary disease patients. Pak J Biol Sci 15(10):501–505
Hashim Ali Hussein S, Nielsen LP, Konow Bøgebjerg Dolberg M, Dahl R (2015) Serum magnesium and not vitamin D is associated with better QoL in COPD: a cross-sectional study. Respir Med 109(6):727–733
Janssen R, Fischer I, Wouters EF (2018) Inhalation therapy for repairing damaged elastin fibers and decelerating elastinolysis in chronic obstructive pulmonary disease. Expert Rev Respir Med 12(5):349–360
Janssen R (2017) Magnesium to counteract elastin degradation and vascular calcification in chronic obstructive pulmonary disease. Med Hypotheses 107:74–77
Mukerji S, Shahpuri B, Clayton-Smith B, Marsh E (2015) Intravenous magnesium sulphate as an adjuvant therapy in acute exacerbations of chronic obstructive pulmonary disease: a single centre, randomised, double-blinded, parallel group, placebo-controlled trial: a pilot study. N Z Med J 128(1425):34–42
Amaral AF, Gallo L, Vannucchi H, Martinez JA (2012) The effect of acute magnesium loading on the maximal exercise performance of stable chronic obstructive pulmonary disease patients. Clinics (Sao Paulo) 67(6):615–622
El-Attar M, Said M, El-Assal G, Ashour L (2009) Serum trace element levels in COPD patient: the relation between trace element supplementation and period of mechanical ventilation in a randomized controlled trial. Respirology 14(8):1180–1187
Högman M, Thornadtsson A et al (2019) Different relationships between FENO and COPD characteristics in smokers and ex-smokers. COPD 16(3-4):227–233
Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A (2003) Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167:418–424
Papi A, Romagnoli M, Baraldo S et al (2000) Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 162:1773–1777
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, M., Li, Q., Xiao, L. et al. Serum Magnesium and Fractional Exhaled Nitric Oxide in Relation to the Severity in Asthma-Chronic Obstructive Pulmonary Disease Overlap. Biol Trace Elem Res 199, 1771–1777 (2021). https://doi.org/10.1007/s12011-020-02314-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02314-5