Abstract
Rice is one of the most valuable nutrients in the diet of most people in the world. The aim of this study was to evaluate the effect of various pre-cooking (washing, soaking) and cooking processes (traditional and rinse) of rice on the amount of toxic and essential elements in the various brands of rice in Iran and assessing human health risks from their carcinogenic and non-carcinogenic effects. For this purpose, totally, 144 sample sizes were examined from three brand (Iranian (n = 48), Pakistani (n = 48), and Indian (n = 48)) in order to the amount of toxic and essential elements using inductively coupled plasma-optical emission spectrometry. The results showed that pre-cooking processes such as washing and soaking in the rinse method were significantly effective in removal toxic metals than the traditional method, so that the most changes were observed for potassium and aluminum metals. The estimated daily intakes of copper, magnesium, manganese, iron, and zinc in different cooking methods were 1.19–1.2%, 0.29–0.32%, 1.01–1.23%, 0.4–0.98%, and 0.9–1.32%, respectively. The Monte Carlo simulation results showed that the rank order of toxic metals of cooked rice based on target hazard quotients value was arsenic > chromium > cadmium > mercury > lead > aluminum, respectively. The result of cancer risk probability was lower than the safe risk limits (1E-4), representing no remarkable cancer risk probability that was due to ingestion of rice for adults and children in Iran. According to the this results, it is recommended to use the rinse method due to further reduction of metals especially toxic metals for rice samples, although the amount of essential elements was also removed by this method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice has been recognized as a staple worldwide food and is known as a very important source of carbohydrates and fibers and supplies the calories needed for more than half of the world’s population [1, 2]. Rice also is a good source of vitamins (especially B group), copper (Cu), magnesium (Mg), zinc (Zn), iron (Fe), calcium (Ca), potassium (K), manganese (Mn), phosphorus (P), selenium (Se), proteins, and slight amounts of fat, which has made rice a good food for all diets [3]. Rice consumption is very high in many countries, especially Asian countries such as India, Pakistan, Iran, and China. Various types of rice are produced and consumed in the world (mainly Iranian, Pakistani, and Indian rice etc.). Many studies have revealed that rice is the main part of the diet of most people in the world [4]. Rice consumption in Iran was about 3.2 million tons in 2017-2018 (for each person is about 36 kg per year). Since rice production is not enough for domestic consumption in Iran (~ 67% were produced domestically), therefore, part of Iran’s demand for rice is mainly imported from other countries such as India and Pakistan (~ 33% import). Types of rice that are consumed in Iran are mainly Iranian (~ 67%), Pakistani (~ 6.5%), and Indian (~ 23%) rice [3]. Therefore, regarding the importance of rice for nutritional value, the quality control and safety of this nutrient in terms of its contaminants, especially toxic and essential elements, are very important. Previous study reported that rice can be contaminated with various pollutants, including toxic metals such as aluminum (Al), chromium (Cr), arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) through water, soil, and climatic conditions; thus, considering the high consumption of rice by most different people of society, the assessment of toxic and essential elements impacts on the health of consumers is necessary [5,6,7,8].
Contamination of water, soil, and climate by contaminants especially toxic metals is a global environmental problem, particularly in agriculture. On the other hand, with the increasing population and agricultural and industrial activities, the concentration of some contaminants, such as toxic metals, has increased in the environment, which leads to the entry of these hazardous compounds into the food chain [9, 10]. Toxic metals may also be transported through soils and weather to water and food chain and intake by rice, wheat, grains, legumes, fruits, or vegetables. All people are exposed to toxic metals through various ways such as the diet daily and weather; however, the number of toxic metals received differs from place to place depending on the type of food and dietary habits, environmental conditions and pollution, and recycling of food products [11]. These metals and other elements can naturally be present in the food or as a result of human practices (during agricultural or industrial activities). The most important toxic metals that have adverse effects on people’s health include Pb, Hg, Cd, As, and Ni. [10, 12]. The toxicity of these metals is due to accumulation in the body biological tissues, occurring in all of the living organisms that are exposed to these metals [13]. Therefore, the development of strategies to reduce, prevent, or eliminate these contaminants from the environment and food has attracted the attention of many researchers.
In recent years, many studies have assessed the effects of several methods and processes of cooking on the reduction of toxic and essential elements in rice.
Numerous studies have shown that various cooking processes such as washing and rinsing rice before cooking or cooking with extra water can reduce the elements (toxic and essential) in cooked rice [14,15,16]. In Iran, rice is usually prepared in two methods: rinse cooking (boil the rice in high water and then remove water after the boiling process) and traditional cooking (faster method, boil the rice in less water without water removal until the cooking process is completed). Therefore, considering the abovementioned and high consumption of rice in Iran, the evaluation of different methods of cooking rice in reducing the elements in order to introduce a suitable method for removing the elements from rice is necessary.
Therefore, according to what has described, the main aim of this study was to evaluate and measure the amount of toxic and essential elements in consumable rice (Iranian, Pakistani, and Indian) in Iran, the effect of different methods of rice cooking (washing, soaking and rinsing, and traditional) on reducing and removing toxic and essential elements and introducing the best method for reducing toxic metals, and also assessing the health risks associated with consuming raw and cooked rice in order to characterize the risk difference in various types of rice tested.
Materials and Methods
Sampling
In this study, 48 sample of different brands were purchased (Iranian (n = 16 × 3), Pakistani (n = 16 × 3), and Indian (n = 16 × 3)) from the different regions of Tehran, Iran. All samples were labeled with letters. According to different cooking methods (traditional and rinsing), the total number of samples examined was 144 samples.
Chemicals and Reagent
All chemicals and stock standard solutions in analytical grade (purity > 99%) were purchased from Merck (Darmstadt, Germany). For dilutions, double-deionized water was used.
Sample Preparation
All rice samples (n = 48) were prepared in two different methods (traditional and rinsing), along with raw samples;
Raw Rice
The raw samples (200 g) was washed with distilled water 4 times.
Rinse Cooking Method
To prepare specimens in the rinsing method, raw rice added to Pyrex container containing 1000 mL distilled water. Afterwards, the containers, containing rice and distilled water were placed on a flame to achieve the boiling point for ~ 15 min. In the next step, the extra water was eliminated by colander, and semi-cooked rice samples were transferred to another flask for the final cooking stage (were cooked for 1 h at 105 °C) [17, 18].
Traditional Cooking Method
To prepare samples in the traditional method, raw rice added to Pyrex container containing 400 mL distilled water. Afterwards, the container containing rice and distilled water was boiled at high temperatures by flame. When the distilled water diminished in Pyrex container, the flame was reduced and the samples were cooked for ~ 1 h at 105 °C [17, 18].
Method Validation
The selectivity, function and linear ranges, detection limit (LOD), quantification limit (LOQ), and repeatability and reproducibility (precision) were applied for the quantitative analysis of elements. The standard addition method also used to investigate the Matrix effects. Regarding this, 200 μl of mixed standard solutions were added to the original samples (Mix standard CRM: 92091 Supelco LOT BCCB9855, TraceCERT®, elements, 10 mg/L in nitric acid, element standard CRM: 28941 Supelco, LOT BCCB8927, 1000 mg/L element in nitric acid).
Metals Analysis
Analysis of toxic and essential elements was performed using a simultaneous inductively coupled plasma-optical emission spectrometry, Spectro Arcos ICP-OES (Spectro, Germany). All the chemical reagents applied in this study were analytical grade and purchased from Merck Company (Darmstadt, Germany). All experiments were performed with three replications.
At the beginning of each experiment, the equipment was soaked in nitric acid solution (15%) for 24 h and rinsed three times with distilled water. To acidify digestion, the acidic mixture including 70% nitric acid (30 mL), 70% perchloric acid (10 mL), and sulfuric acid (5 mL) was exploited. At first, in order to acidic digestion, 5 g of each sample (raw and cooked) was weighed and mixed with the acidic solution according to the ASTM procedure (ASTM 1999). For complete mixing and homogenization of samples, a shaker was used for 30 min at room temperature. Then, the mixture was heated to reach a boiling point on a heater and heating treatment continued until 3 mL of clear extract remained. The extract filtered by the Whatman filter paper (No.41) in 25 mL volumetric balloons. The final volume of the solution was adjusted to 25 mL by distilled water and samples stored in the refrigerator before further analysis [19].
Human Health Risk Assessment
Non-carcinogenic Risk Assessment and Estimated Daily Intake
The estimated daily intake (EDI, mg/kg day) is used to determine the dose of oral exposure to harmful substances using the following equation [19, 20]:
Where EDI is the estimated daily intake (mg/kg day), C (metal concentration (mg/kg)), IR (ingestion rate, 110 g/n day), ED (exposure duration, 54 years), EF (exposure frequency, 365 days/year), BW (reference body mass, the mean weight of children and adults is between 15 and 70 kg, respectively), and AT (AT is the mean time (for both children and adults are 25,550 days, respectively) [21].
The human non-carcinogenic risk assessment was calculated by reference dose (RfD), which established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and the United States Environmental Protection Agency (USEPA). The hazard quotient (HQ) characterizes the health risk of non-carcinogenic indices for the toxic metals in rice samples calculated using the equation detailed by the USEPA [22]:
RFD refers to the oral reference dose (mg/kg day). RfD for Cd, Pb, Al, Cr, Mo, and As is 0.0005, 0.0035, 1, 0.003, 0.005, and 0.0001 mg/kg day, respectively. Considering the relatively high per capita, rice consumption in Iran, it is assumed that people in society to consume rice until the age of 70. The hazard index (HI) as a potential risk of harmful health effect toxicants in rice was calculated as the sum of HQ [23]:
If HI < 1, chronic risks is acceptable, whereas non-cancer risks are likely to occur in the target population HI > 1 [24].
Carcinogenic Risk Assessment
The carcinogenic risk was determined for any toxicant by Incremental Life time Cancer Risk (ILCR) according to the following equation [25, 26]:
Where EDI is the estimated daily intake (mg/kg day) over a lifetime, and SF is the cancer slope factor (SF) for As which is 1.5 kg day/mg.
EDI of Essential Elements
In order to evaluate the nutritional profile and the essential elements of rice consumption, it is necessary to calculate the EDI, which was calculated using the equation [27]:
Statistical Analysis
The data are presented as median, mean rank, minimum, and maximum. In this study, Mann-Whitney test was applied to determine the statistical significance for toxic and essential elements between the subjects as classified by type of rice (rinse versus traditional). Agglomerative Hierarchical Clustering (AHC) was performed to the classification of element groups and diagnose relations among them. Statistical analyses were carried out by the statistical package SPSS, version 11.5.1 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered as the statistical significance.
Results and Discussion
Method Validation
The concentration of elements was evaluated by measuring the wavelength. The baseline signals and their interferences at selected lines during the experiment were calculated (Table 1).
The recoveries of elements were recorded as 89 to 106%. LODs of the As, Cd, Hg, Pb, and Al were 0.179, 0.049, 0.351, 1.2, and 0.043 μg/kg, respectively. The LODs for Cr, Cu, Fe, Mn, Ni, and Zn were 0.30, 0.10, 0.16, 0.07, 0.30, and 0.30 μg/kg, respectively. The correlation factors were between 0.99943-0.99998 (Table 1).
Evaluation of Essential Elements
The main purpose of this study was to compare different methods of cooking rice and their effect on the amount of toxic and essential elements in rice. The results of changes in the amount of essential elements in rice samples by cooking methods (rinse and traditional) are shown in Table 2. The findings indicated that the mean of elements (Ca, Zn, Cr, Cu, Fe, K, Mg, Mn, and P) for traditional samples were greater than for rinse. Furthermore, there were no statistically significant differences in Ca, Cr, Cu, Fe, Mg, Mn, P, and Zn between the type of rice, P > 0.05. The most changes were related to the K element, which was significantly higher in the traditional samples (8103.2 μg/kg) than in the rinse samples (3085.4 μg/kg) P<0.001. The amount of essential elements in the rinse and traditional samples was as follows: P > Mg > Ca > K > Zn > Fe > Mn > Cu. As a result, the main differences were observed between K and Ca elements in two cooking methods. The Se was not detected in any of the samples.
These results demonstrated that the traditional cooking method could be more effective in preserving the essential elements in rice samples compared with the rinse method. This fact can be due to the large quantities of elements are lost in rinse samples due to the washing of surface layers of rice grains containing large amounts of elements during the pre-cooking. Therefore, more amounts of elements may be dissolved in water and removed [17]. Several studies have reported that the cooking process reduces the amount of elements in rice. Mihucz et al (2010) described that the cooking process (boiling method) decreased the amount of elements in the cooked samples than raw samples [14]. The main reason for reducing the elements in the rinse method is to remove boiled water in this method during pre-cooking compared with the traditional method. On the other hand, the water rate used in these two methods can be effective in reducing the content of the elements (usually rinse cooking 4:1 versus traditional cooking 2:1) [18, 28].
Today, the role and importance of essential elements in the health of humans and mammals are well known [29], so that the deficiency of any of the trace elements causes certain diseases such as dermatitis or gustatory decline (Zn deficiency) [30], anemia (Cu or Fe deficiency) [31],and cardiovascular disease (Mg deficiency) [32]. As regards, rice is an important food source and contains many essential elements for humans. Therefore, it can be recommended as a valuable dietary supplement for human. Recommended dietary allowances (RDAs) of essential elements have been recognized as levels necessary to prevent symptoms of deficiency diseases. The current RDAs for some trace elements may be insufficient to protect against genomic instability. So, in this case, it seems necessary to use dietary supplements. Various studies have reported that essential elements may be necessary for DNA vital functions such as synthesis, repair, methylation, gene mutation, chromosome breakage, chromosome segregation, gene expression, oxidative stress, necrosis, and apoptosis and to prevent degenerative diseases including cardiovascular disease, migraine disease, Alzheimer’s disease Parkinson disease, cancer, and premature aging [33,34,35]. On the other hand, most of these elements are cofactors of many enzymes that play a very important role in metabolic pathways [33, 36,37,38]. For example, Linder (2001) and Hartwig (2001), respectively, showed that Cu and Mg play an important role in genetic stability [39, 40]. In another study, Ames et al. (1998) have been shown that Zn and Fe have an important role in preventing DNA damage [41]. Laires et al. (2004) reported that Mg plays an important role in various physiological functions. It is critical in membrane integrity, muscle contraction, protein synthesis, energy metabolic processes, in mediation metabolism nervous tissue conduction, neuromuscular excitability, and hormone secretion [42].
According to the results of our study and other studies, the traditional cooking method is preferred for the maintenance of more nutritious elements than rinse cooking methods. So, considering that these essential elements are obtained through the diet and the importance of nutrients losses during the cooking process, therefore, choosing the proper method to maintain the nutritional value of food seems necessary.
Evaluation of Toxic Metals
The findings of toxic metal values in rice samples are reported in Table 3. The results of our study exhibited that different cooking processes (rinse versus traditional) can have a significant role in decreasing the toxic metals of rice samples. According to our study results, the median of Cr for the rinse samples (72.2 μg/kg) was greater than for traditional rice (64.3 μg/kg) and in other toxic metals (Al, As, Cd, and Pb) for the traditional samples were greater than for the rinse samples.
A Mann-Whitney test showed that there were no statistically significant differences in Al, As, Cd, Hg, and Pb between the type of rice, P > 0.05. However, the amounts of toxic metals were lower in the rinse samples than the traditional samples. As mentioned in the essential elements section above, this can be mainly for various reasons; the first reason is the removal of boiling water in the rinse method and washing the rice grains during the pre-cooking process dissolve a large amount of toxic metals in water and remove them; the second reason is the ratio of water used in two methods (usually rinse cooking 4:1 versus traditional cooking 2:1) and; the third reason is the duration of soaking the rice grains in the rinse method, so that, as the soaking time increases, due to the greater penetration of water in the grains and the further dissolution of metals in water, the amount of toxic metal removal will be higher [14, 18, 28, 43]. Changes in the amounts of toxic metals of As and Hg were not observed among the samples P > 0.05. The trend of toxic metal values in the rinse and traditional samples was observed as follows: Al > Cr > As > Cd > Pb.
According to our findings, the rinse cooking procedure is a more efficient method for eliminating toxic metals (As, Cd, Pb, Hg, and Al) in rice samples compared with the traditional cooking method, while the traditional cooking method is an effective method for preserving essential elements. In agreement with our study results, many studies have shown that rice washing and soaking before cooking have an important effect on reducing toxic metals such as As, Cd and Pb and Hg in cooked samples [14, 28, 43,44,45]. Naseri et al (2014) indicated that the amount of toxic metals, Cd, Pb, Cr, Ni, and Co in cooked rice was lower than raw samples (rinse cooking method ˂ boiling-cooking method ˂ raw rice) [46]. Some studies also reported an increase in the amount of inorganic—as in rice samples cooked (boiling method) in comparison with raw samples [47,48,49]. In another study, Perello et al (2008) investigated the effects of different cooking conditions (time, temperature, and medium of cooking) on the amount of toxic metals (As, Cd, Hg, and Pb). They reported that the cooking method and the high temperatures increased As, Cd, and Pb concentrations [50].
Other factors that can contribute to the accumulation and increase of metals (toxic and essential) in rice grains are the soil and water used to grow rice [51]. So, some studies have shown that some plants, including rice, can absorb some of the toxic metals such as Pb and As from the soil and water by root [51, 52]. Considering that rice consumption in Iran and other parts of the world are almost high and since the exposure to toxic metals such as Pb and Cd and As and Hg in low amounts for a long time have harmful effects on human health; hence, it is needed to use methods to reduce or remove toxic metals in rice grains. Several methods have been suggested to reduce the amount of toxic metals in rice grains that are included the use of liming (CaCO3) method in the removal of Cd [53], rinse and soak the rice grains before cooking for removal As, Cd, Pb, and Hg [18, 28, 43, 51], microwave cooking could be a feasible strategy for reducing Cd bioaccessibility [54], reducing bioaccessibility of Cd and As in rice by washing and cooking method [55], decreasing bioaccessibility Hg in cooked rice [56].
EDI of Essential Elements
The EDI of eight essential elements estimated from the rice (two method cooking) for an adult person of Tehran is reported in Table 4. The average EDI (mg/day) of Ca, Cu, Fe, K, Mg, Mn, P, and Zn for traditional cooked rice was 0.09, 1.2, 0.4, 0.01, 0.32, 1.23, 0.37, and 1.32, and for rinse cooking method, 0.01, 1.2, 0.4, 0.01, 0.32, 1.23, 0.37, and 1.32, respectively. In the case of the Zn element, rice provides the highest intake of this essential element in this study (1.32 mg/day). The mean percentage contribution to Reference Daily Intake for eight essential elements in two cooking methods for both males and females was lower than 100 percent. However, this amount in cooked rice with traditional type was more than rinse type. It is demonstrated that, after cooking by both traditional and rinsed cooking methods, all of the rice samples do not contain sufficient amounts of essential elements anymore on the subject of nutritional health benefits.
Human Health Risk Assessment
According to Table 5, among the studied toxic elements in rinsed cooked, As has the highest calculated 95th percentile of target hazard quotients (THQ) for children (0.6136) and adults (0.1855), and Al has the least calculated 95th percentile of THQ for children (0.0017) and adults (0.0005). The estimated mean of the calculated 95th percentile of THQ for studied toxic metals ranked in the following order As > Cr > Cd > Mo > Pb > Al, respectively.
In traditionally cooked rice, As has the highest calculated 95th percentile of THQ for children (2.3353) and adults (0.6883), and Al has the least calculated 95th percentile of THQ for children (0.0018) and adults (0.0005). The estimated mean of the calculated 95th percentile of THQ for studied toxic metals ranked in the following order As > Cr > Cd > Mo > Pb > Al, respectively.
Among all of the rice samples, 95 percent of THQ for Mo, Pb, Cd, and Al estimated below 1, and only As for children (2.3353) is far over the THQ threshold of 1, denoting that there is remarkable non-carcinogenic risk from ingesting to this toxic metals.
As Fig. 1 represents, As (68%) and Cr (20%) are two main contributors in total THQ (TTHQ), while the other toxic metals estimated to have less than 12% contribution. Therefore, according to the results mentioned, it can be concluded that the consumption of rinsed cooked type is safe for children and adults, but in traditional cooked type, As (88%) and Cr (7%) are two major contributors to the TTHQ, while other metals account for less than 5%.
The TTHQ in traditionally cooked rice in both adults and children was 2.9 times higher than in rinsed cooked rice. The risk of contaminants for human health is depended on various factors, such as per capita consumption of foodstuff, exposure time, toxicity, and body weight. Also, When the TTHQ value is higher than 10, adverse health effects are considered high for the exposed population. Hence, based on TTHQ value, the rice samples in rinsed cooked and traditional cooked collected from the market in Tehran are not adverse health effects for the exposed population.
Carcinogenic Risk
As has been classified by the United States Environmental Protection Agency (USEPA) as probably carcinogenic toxic metals (group A) to humans, and the chronic exposure to that could risk of developing some types of cancers. The result of cancer risk due to consumption of rice (As) was presented in Fig. 2.
The percentile 95% of the carcinogenic risk indexes in the rice samples (rinsed cooked and traditional cooked) for adults due to As were 2.66E-05 and 1.035E-04, and for children, 9.39E-05 and 3.48E-04, respectively (Fig. 2).
The characteristic of the cancer risk (CR) can be qualitatively described as follows; safe limit CR < 10 − 6; threshold risk limit CR > 10 − 4; considerable risk limit CR > 10 − 3. Comparison between percentile 95% carcinogenic risk assessment of As, the rice samples (rinsed cooked and traditional cooked) with regulatory standard (USEPA), illustrated that CR indexes for traditionally cooked in adults and children were higher than 10-4. Also, the carcinogenic risk assessment for As in the traditional cooked was higher than rinsed cooked because of the rinsed cooked methods applied for all rice samples could efficiently decrease the carcinogenic risk related to As to the acceptable level.
Classifications of Primary Groups Between Rice Types
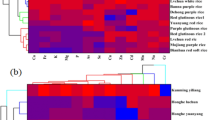
To obtain the best possible categorization of essential element concentrations and to identify relations between them, HCA was used according to cooking methods in studied rice samples and the findings of the analysis are stated as a dendrogram in Fig. 3. The axis Y displays the level of association among elements, where the lower the amount the more noteworthy the association. Most commonly multivariate statistical methods, hierarchical clustering, and principal component analysis are used where the clusters are organized in the pattern by starting with the most similar pair of the elements [57]. Two separate clusters of rinsed cooked were represented: cluster I containing K, Mn, Zn, Ca, Cu, Mg, and Zn, and cluster II including Cr and Fe. Cluster I was divided into two fundamental subgroups, the first including Ca, Mg, K, and P, whereas the rest of the elements i.e., Cu, Zn, and Mn consisted of a second separate subgroup. The strongest detected association was among Cr and Fe (similarity > 95%). In traditionally cooked rice samples, two separate clusters of rinsed cooked were represented: cluster I containing K, Mg, and P, and cluster II including Mn, Zn, Ca, Cu, Cr, and Fe. Cluster II was divided into two primary subgroups, the first containing Zn, Mn, Cu, Cr, and Ca, whereas the rest of the elements i.e., Cr and Fe consisted of a second different subgroup. The strongest detected association was among Cr and Fe (similarity > 95%).
Conclusion
Regarding the findings of this research, it can be concluded that the preparation, pre-cooking, and type of cooking method (traditional and rinse) can have a significant effect on the decrease of metals (toxic and essential), and consequently, they are associated with the risk of carcinogenicity and non-carcinogenicity. It was concluded that the best method for removing the highest amount of toxic metals whereas keeping the essential elements in maximum levels was the rinse method (washing the rice for 5 times followed by soaking for 5 h). However, in order to decrease the quantity of metals, especially toxic metals, in addition to efficient pre-cooking and cooking methods, good agricultural practices can also be effective. The findings of health risk assessment exhibited that the THQ values for the 95th percentile consumer were not at considerable non-carcinogenic risk for all of the toxic metals, except As in traditionally cooked rice for children. Also, the CR values for the 95th percentile were below safe limited for all of the rice samples except in traditionally cooked rice for children. Also, the carcinogenic risk assessment for As in the traditional cooked was higher than rinsed cooked because of the rinsed cooked methods applied for all rice samples could efficiently decrease the carcinogenic risk related to As to the acceptable level.
References
Fresco L (2005) Rice is life. J Food Compos Anal 4(18):249–253
Phuong T, Chuong P, Khiem DT (1999) Elemental content of Vietnamese rice. Part 1. Sampling, analysis and comparison with previous studies. Analyst 124(4):553–560
Kennedy G, Burlingame B (2003) Analysis of food composition data on rice from a plant genetic resources perspective. Food Chem 80(4):589–596
Naseri M, Vazirzadeh A, Kazemi R, Zaheri F (2015) Concentration of some heavy metals in rice types available in Shiraz market and human health risk assessment. Food Chem 175:243–248
Behrouzi R, Marhamatizadeh MH, Razavilar V, Rastegar H, Shoeibi S (2019) Effects of the pre-cooking process using acetic acid and citric acid on lead concentration in rice. Pol J Environ Stud 29(1):545–551
Halder D, Saha JK, Biswas A (2020) Accumulation of essential and non-essential trace elements in rice grain: possible health impacts on rice consumers in West Bengal, India. Sci Total Environ 706:135944
Abtahi M, Fakhri Y, Oliveri Conti G, Keramati H, Zandsalimi Y, Bahmani Z, Hosseini Pouya R, Sarkhosh M, Moradi B, Amanidaz N (2017) Heavy metals (As, Cr, Pb, Cd and Ni) concentrations in rice (Oryza sativa) from Iran and associated risk assessment: a systematic review. Toxin Rev 36(4):331–341
Roya AQ, Ali MS (2017) Heavy metals in rice samples on the Torbat-Heidarieh market, Iran. Food Addit Contam Part B Surveill 10(1):59–63
Velkova Z, Kirova G, Stoytcheva M, Kostadinova S, Todorova K, Gochev V (2018) Immobilized microbial biosorbents for heavy metals removal. Eng Life Sci 18(12):871–881
Sun Y, Zhou Q, Diao C (2008) Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresour Technol 99(5):1103–1110
Khan K, Naeem M (2006) Simultaneous determination of accumulated hazardous metals in hen’s eggs by atomic absorption spectroscopy. J Appl Sci 6(1):198–201
Siripongvutikorn S, Asksonthong R, Usawakesmanee W (2016) Evaluation of harmful heavy metal (Hg, Pb and Cd) reduction using Halomonas elongata and Tetragenococcus halophilus for protein hydrolysate product. FFHD 6(4):195–205
Gupta N, Yadav KK, Kumar V, Kumar S, Chadd RP, Kumar A (2018) Trace elements in soil-vegetables interface: translocation, bioaccumulation, toxicity and amelioration-a review. Sci Total Environ
Mihucz VG, Silversmit G, Szalóki I, De Samber B, Schoonjans T, Tatár E, Vincze L, Virág I, Yao J, Záray G (2010) Removal of some elements from washed and cooked rice studied by inductively coupled plasma mass spectrometry and synchrotron based confocal micro-X-ray fluorescence. Food Chem 121(1):290–297
Mwale T, Rahman M, Mondal D (2018) Risk and benefit of different cooking methods on essential elements and arsenic in rice. Int J Environ Res Public Health 15(6):1056
Sengupta M, Hossain M, Mukherjee A, Ahamed S, Das B, Nayak B, Pal A, Chakraborti D (2006) Arsenic burden of cooked rice: traditional and modern methods. Food Chem Toxicol 44(11):1823–1829
Adibi H, Mazhari M, Bidoki SK, Mahmoodi M (2014) The effect of washing and soaking on decreasing heavy metals (Pb, Cd and As) in the rice distributed in Kermanshah in 2011. J Kermanshah Univ Med Sci 17(10):628–636
Rezaei Malidareh R, Shokrzadeh M, Khasi B, Rouhi S, Zaboli F (2016) Survey and comparison of different processes effect, rinsing and baking on remaining amount of heavy metals lead and cadmium in cultivated Tarom rice in Qhaemshahr city paddies in northern Iran. JREH 2(1):52–59
Rezaei M, Ghasemidehkordi B, Peykarestan B, Shariatifar N, Jafari M, Fakhri Y, Jabbari M, Khaneghah AM (2019) Potentially toxic element concentration in fruits collected from Markazi Province (Iran): a probabilistic health risk assessment. Biomed Environ Sci 32(11):839–853
Peykarestan B, Rezaei M, Malekirad AA, Ghasemidehkordi B, Jabbari M, Shariatifar N, Basaki T, Barba FJ, Mousavi Khaneghah A (2020) The concentration and non-carcinogenic risk assessment of aluminium in fruits, soil, and water collected from Iran. Int J Environ Anal Chem:1–16
Madani-Tonekaboni M, Rafiei Nazari R, Mirzamohammadi S, Abdolshahi A, Abbasi-bastami N, Arabameri M (2019) Monitoring and risk assessment of lead and cadmium in milks from east of Iran using Monte Carlo simulation method. NFSR 6(2):29–36
Heshmati A, Sadati R, Ghavami M, Khaneghah AM (2019) The concentration of potentially toxic elements (PTEs) in muscle tissue of farmed Iranian rainbow trout (Oncorhynchus mykiss), feed, and water samples collected from the west of Iran: a risk assessment study. Environ Sci Pollut Res 26(33):34584–34593
Ghasemidehkordi B, Malekirad AA, Nazem H, Fazilati M, Salavati H, Shariatifar N, Rezaei M, Fakhri Y, Khaneghah AM (2018) Concentration of lead and mercury in collected vegetables and herbs from Markazi province, Iran: a non-carcinogenic risk assessment. Food Chem Toxicol 113:204–210
Cao S, Duan X, Zhao X, Wang B, Ma J, Fan D, Sun C, He B, Wei F, Jiang G (2015) Health risk assessment of various metal (loid) s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ Pollut 200:16–23
Sultana MS, Rana S, Yamazaki S, Aono T, Yoshida S (2017) Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ Sci 3(1):1291107
Antoine JM, Fung LAH, Grant CN (2017) Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol Rep 4:181–187
Song D, Zhuang D, Jiang D, Fu J, Wang Q (2015) Integrated health risk assessment of heavy metals in Suxian county, South China. Int J Environ Res Public Health 12(7):7100–7117
Hajeb P, Sloth JJ, Shakibazadeh S, Mahyudin N, Afsah-Hejri L (2014) Toxic elements in food: occurrence, binding, and reduction approaches. Compr Rev Food Sci Food Saf 13(4):457–472
Rezaei M, Farahani S, Karimi F, Eshghi N, Ghasemikhah R, Abbasi A, Mohammadpourfard I, Jafari M (2016) Essential elements content of hen egg-white in Markazi province (Iran). Toxin Rev 35(1–2):29–32
Kay RG, Tasman-Jones C, Pybus J, Whiting R, Black H (1976) A syndrome of acute zinc deficiency during total parenteral alimentation in man. Ann Surg 183(4):331–340
Graham G, Cordano A (1969) Copper depletion and deficiency in the malnourished infant. Johns Hopkins Med J 124:139–150
HA SEELING (1974) Magnesium interrelationships in ischemic heart disease: a review. Am J Clin Nutr 27:59–79
Hemmati-Dinarvand M, Valilo M, Kalantary-Charvadeh A, Sani MA, Kargar R, Safari H, Samadi N (2019) Oxidative stress and Parkinson’s disease: conflict of oxidant-antioxidant systems. Neurosci Lett 709:134296
Gupta U, Gupta S (2014) Sources and deficiency diseases of mineral nutrients in human health and nutrition: a review. Pedosphere 24(1):13–38
Nattagh-Eshtivani E, Sani MA, Dahri M, Ghalichi F, Ghavami A, Arjang P, Tarighat-Esfanjani A (2018) The role of nutrients in the pathogenesis and treatment of migraine headaches. Biomed Pharmacother 102:317–325
McDowell LR (2003) Minerals in animal and human nutrition, vol Ed. 2. Elsevier Science BV, Amsterdam
Majewski M, Kozlowska A, Thoene M, Lepiarczyk E, Grzegorzewski W (2016) Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J Physiol Pharmacol 67(1):3–19
Soetan K, Olaiya C, Oyewole O (2010) The importance of mineral elements for humans, domestic animals and plants-a review. Afr J Food Sci 4(5):200–222
Linder MC (2001) Copper and genomic stability in mammals. Mutat Res 475(1–2):141–152
Hartwig A (2001) Role of magnesium in genomic stability. Mutat Res 475(1–2):113–121
Ames BN (1998) Micronutrients prevent cancer and delay aging. Toxicol Lett 102:5–18
Laires MJ, Monteiro CP, Bicho M (2004) Role of cellular magnesium in health and human disease. Front Biosci 9(262):76
Fakhri Y, Bjørklund G, Bandpei AM, Chirumbolo S, Keramati H, Pouya RH, Asadi A, Amanidaz N, Sarafraz M, Sheikhmohammad A (2018) Concentrations of arsenic and lead in rice (Oryza sativa L.) in Iran: a systematic review and carcinogenic risk assessment. Food Chem Toxicol 113:267–277
Khan SI, Ahmed AM, Yunus M, Rahman M, Hore SK, Vahter M, Wahed M (2010) Arsenic and cadmium in food-chain in Bangladesh—an exploratory study. J Health Popul Nutr 28(6):578
Chakravarty I, Sinha R, Ghosh K (2003) Arsenic in food chain-study on both raw and cooked food. Arsenic contamination: Bangladesh perspective Dhaka: ITN-Bangladesh, Bangladesh University of Engineering and Technology:227–240
Naseri M, Rahmanikhah Z, Beiygloo V, Ranjbar S (2018) Effects of two cooking methods on the concentrations of some heavy metals (cadmium, lead, chromium, nickel and cobalt) in some rice brands available in Iranian market. JCHR 4(2). https://doi.org/10.22034/jchr.2018.544068
Bae M, Watanabe C, Inaoka T, Sekiyama M, Sudo N, Bokul MH, Ohtsuka R (2002) Arsenic in cooked rice in Bangladesh. Lancet 360(9348):1839–1840
Laparra JM, Vélez D, Barberá R, Farré R, Montoro R (2005) Bioavailability of inorganic arsenic in cooked rice: practical aspects for human health risk assessments. J Agric Food Chem 53(22):8829–8833
Signes A, Mitra K, Burlo F, Carbonell-Barrachina AA (2008) Contribution of water and cooked rice to an estimation of the dietary intake of inorganic arsenic in a rural village of West Bengal, India. Food Addit Contam 25(1):41–50
Perelló G, Martí-Cid R, Llobet JM, Domingo JL (2008) Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. J Agric Food Chem 56(23):11262–11269
Malidareh HB, Mahvi AH, Yunesian M, Alimohammadi M, Nazmara S (2014) Admium, lead and arsenic content in polished white rice (Oryza sativa L.) in Ghaemshahr city (North of Iran). Middle-East J Sci Res 20(12):1709–1714
Rahman MA, Hasegawa H (2011) High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci Total Environ 409(22):4645–4655
Chen H, Zhang W, Yang X, Wang P, McGrath SP, Zhao F-J (2018) Effective methods to reduce cadmium accumulation in rice grain. Chemosphere 207:699–707
Wang C, Duan H-Y, Teng J-W (2014) Assessment of microwave cooking on the bioaccessibility of cadmium from various food matrices using an in vitro digestion model. Biol Trace Elem Res 160(2):276–284
Zhuang P, Zhang C, Li Y, Zou B, Mo H, Wu K, Wu J, Li Z (2016) Assessment of influences of cooking on cadmium and arsenic bioaccessibility in rice, using an in vitro physiologically-based extraction test. Food Chem 213:206–214
Liao W, Wang G, Li K, Zhao W, Wu Y (2019) Effect of cooking on speciation and in vitro bioaccessibility of Hg and As from Rice, using ordinary and pressure cookers. Biol Trace Elem Res 187(1):329–339
Ghelichkhani G, Modaresi MH, Rashidi L, Shariatifar N, Homapour M, Arabameri M (2019) Effect of the spray and freeze dryers on the bioactive compounds of olive leaf aqueous extract by chemometrics of HCA and PCA. Journal of Food Measurement and Characterization:1–13
Acknowledgments
This study was approved and supported by the Tehran University of Medical Science (TUMS), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shariatifar, N., Rezaei, M., Alizadeh Sani, M. et al. Assessment of Rice Marketed in Iran with Emphasis on Toxic and Essential Elements; Effect of Different Cooking Methods. Biol Trace Elem Res 198, 721–731 (2020). https://doi.org/10.1007/s12011-020-02110-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02110-1