Abstract
This study aimed to evaluate the mean concentration of cadmium (Cd), chromium (Cr), copper (Cu), nickel (Ni), and lead (Pb) in the meat and offal of cow and sheep. Also, the estimated daily intake (EDI) and health risk of these metals were calculated. Analysis of metals was undertaken by the use of an inductively coupled plasma-optic emission spectroscopy (ICP-OES). All samples were contaminated with all metals. Principal component analysis (PCA) showed a clear differentiation of cow and sheep in both the kidney and liver samples. In the liver and kidney, level of Cd, Cu, and Pb were positively correlated. The highest target hazard quotients (THQs) were calculated for Pb. Cd level in cow kidney had the highest carcinogenic rate (CR). Although, hazard index (HI) was lower than one, consumption of muscle especially in children should be noticed in both national and international consumers due to higher level of HI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals contaminate the environment by anthropogenic or natural activity such as mining, use of fertilizer, pesticides, and atmospheric deposition [1]. Classification of trace metals into essential and toxic ones is so hard because it mainly depends on dose of exposure [2]. Some metals, despite their essential role in coenzymes, may show toxic effects in excess amount (e.g., copper (Cu), chromium (Cr), and nickel (Ni)) [3], since, they are not biodegradable and simply aggregated in the living organisms such as cow and sheep [4]. The elevated level of toxic metals in environment leads to contamination of foods including red meat and meat organs [1].
Food consumption is one of the major routes of human exposure to heavy metals [1]. Recently, by change of lifestyle and more demand for fast foods which mainly contain red meat, consumption of meat increased during recent decades in developing countries. Therefore, consumption of foods containing metal residues raised a major public health hazard due to chronic intake of heavy metals. It may lead to neurological problems, headache, and liver dysfunction [5, 6], due to accumulation of metals in the liver and deposition in neurons [7].
Meat is an important part of usual diet of people and contains some essential trace elements but may also contain heavy metal residues. The liver and kidney are two examples of soft tissues with the capacity of bioaccumulation of metals including cadmium (Cd), Cr, Cu, Ni, and lead (Pb) [8]. It was mentioned that residues in meat and edible organs may relate to pollution of the grazing area and drinking water [9]. According to Shahryari et al. [10], Cu concentration in the samples of ground water and distribution network of Birjand, Iran, was 0.0046 ± 0.87 mg/L and 0.0 ± 0.0 (zero) mg/L, respectively. Cr amount was 0.0916 ± 0.12 and 0.0514 ± 0.02 mg/L in the samples of ground water and distribution network of this region, respectively. 51.3% of the samples of distribution network and 67.9% of the ground water of the city had unallowable amount (> 0.05 mg/L) of Cr [10].
The liver and kidney are a good source of essential micronutrients in the human diet [11]. Moreover, the liver and kidney had lower price and consist more portion of daily diet of poor people. Despite the low rate of adsorption of Cd through diet, it is deposited in the kidney and liver with a long half-life of 10–30 years. Chronic exposure to Cd through diet associated with the risk of malignancy in the breast [12], lung, endometrium, and bladder [13]; renal dysfunction/failure; and demineralization of the bone. The European Commission (EC) and Joint FAO/WHO Expert Committee on Food Additives (JECFA) set the maximum residue levels (MRLs) for Cd in the meat, liver, and kidney of cow and sheep as 0.05, 0.5, and 1.0 mg/kg wet/weight (w/w), respectively [14].
Dietary exposure to lead is mainly through contamination of food during production or processing. Chronic Pb intoxication leads to colic, constipation and anemia [12]. Elevated level of Pb associated with delayed cognitive development in children and increased the incidence of hypertension and cardiovascular disease in adults [14]. MRLs of Pb were set as 0.1 and 0.5 mg/kg for meat and edible offal, respectively [14, 15]. According to a recent review made by European Food Safety Authority (EFSA), MRLs of Cu were set as 3, 400 and 10 mg/kg in the bovine meat, liver, and kidney, respectively. In the sheep muscle, liver, and kidney, MRLs were determined as 3, 150, and 6 mg/kg, respectively [16]. Ni is widely distributed in the surface of earth and presence of Ni in food resulted from human activity and also natural sources [17]. Regarding Cr and Ni in meat, Codex and WHO did not set any MRLs. A national standard of Brazil has set a 0.1 μg/g for Cr in meat, and an allowable limit of 0.5 mg/kg for nickel in meat was set by Russia [18].
The EU Scientific Panel on Contaminants in the Food Chain (CONTAM) reported that in the food group “meat and meat products and offal”, 3.6%, 3.7%, and 1% of the meat, liver, and kidney samples exceeded the MLs, respectively [13].
The precise measurement of the metals in the animal tissues may help to elucidate the role of metals in animal’s body and introduce as biological indicator to determine the environmental pollution [8, 9]. Since meat and edible offal comprise a major part of Iranian diet, the heavy metal concentration in them can correlate significantly with the heavy metal intake of consumer [12].
Birjand (32° 53′ 00″ N; 59° 12′ 00″ E) is a town in the Southeast of Iran, located about 100 km east of the capital city, Tehran. It had a population about 261,324 [19] and some well-known mining such as granite, chromite, and copper.
This study aimed to evaluate the mean concentrations of heavy metals in the meat and edible offal of beef and sheep; to reveal the accumulation features of metals in beef and sheep; and to estimate the daily intake (EDI), target hazard quotient (THQ), and carcinogenic rate (CR) of toxic metals through consumption of these food animals in Birjand, Southeast of Iran. Furthermore, to the best of our knowledge, this is the first report regarding the heavy metal concentrations in the muscle and edible organs of cow and sheep distributed in this region. The muscle is selected as the central site of metal uptake in the present study.
Methods
Sampling
From January 2017 until September 2017, a total of 102 samples from the liver, kidney, and muscle of beef (51) and sheep (51) were collected from different butcheries and retails in five different regions of Birjand, Southeast of Iran, according to clustered random sampling. Fifty-eight and 44 samples were taken in cold (winter) and warm (summer) seasons, respectively. All of the collected samples were from the animals that freshly produced in the region. The samples were labeled and immediately transferred to laboratory and kept at − 20 °C until analysis.
Sample Preparation and Metal Analysis
All of the chemicals and standards were purchased as analytical grade (Merck KGaA, Darmstadt, Germany). Nitric acid (10% solution) was used to wash all laboratory equipments and containers. Prior to analysis, the samples were thawed and washed with deionized water. One gram (g) of each sample was placed into a 50-mL volumetric flask; 5 ml of HNO3 (65%) and 15 ml of HCL were added to the sample. A volumetric flask was kept at room temperature for 24 hours (h). Then, the sample was placed on a heater block at 105 °C for 2 to 3 h until full acid digestion. The digested sample was left to cool and filtered by Whatman filter paper (Ashless no. 42). The filtrate was made up to 50 mL with deionized water. An inductively coupled plasma-optic emission spectroscopy (ICP-OES) (Spectro Arcos, Germany) was used for quantification of Pb, Cr, Ni, Cu, and Cd. The calibration of instrument was performed by use of a multi-elemental standard solution of the respective metals (to diagram standard curves before metal analysis). Standard solutions of each metal were prepared using a 1000-mg/L standard stock solution. Working calibration multi-elemental 2-ppm standards of Pb, Cd, Cr, Cu, and Ni were prepared by serial dilution of stock solutions (100 ppm). The standard curve was obtained by injection of standards and blank solution into ICP-OES. Concentrations of metals were stated in milligrams per kilogram (ppm) wet/weight (mg/kg w/w). A blank solution and reference solution were also treated the same procedure as the samples prior to analysis.
Quality Control
The detection limit (ppb) of Cd, Cr, Cu, Ni, and Pb was 1, 1, 2, 3, and 3, respectively. Furthermore, a recovery study of the analytical method was done in selected samples by spiking measured samples with the different aliquots of a multi-elemental standards and then reanalyzed the samples and by use of certified reference material, fish protein DORM-3 (National Research Council, Canada) and DOLT-4 (dogfish liver, the National Research Council, Canada). All experiments were performed in triplicate. In order to test the reliability of instruments, after every 10 samples, a blank and known standard were run (relative standard deviation, RSD ≤ 3%). Acceptable recoveries were in the range of 95–103% for measured metals.
Estimated Daily Intake of Toxic Metals
The EDI of toxic metals (Cd, Cr, Cu, Ni, and Pb) was determined by the following equation:
where MC is the mean concentration of metal in food (μg/g, on fresh weight basis); FDC relates to the daily consumption of specified food according to ISIRI 12968 (g/person/d); BW is the average weight of body. The local inhabitants consumed an average offal (liver/kidney) and muscle of 3 and 30 g/day for adult (60 kg in BW) and children (30 kg in BW), respectively. The EDI of metal were compared with the tolerable daily intakes (TDI) of metals recommended by the US EPA [20] and ISIRI [21].
Target Hazard Quotient
The THQ showed the potential health risks from the consumption of polluted food. The THQ is a ratio between the exposed and the reference dose (RfD). RfD shows the oral reference dose that is an assumption of the safe daily exposure of a contaminant. RfD values of Cd, Cr, Cu, Ni, and Pb are 0.001, 0.003, 0.04, 0.02, and 0.004 (μg/g bw/day), respectively [2, 22].
The THQ was measured according to the method presented in the US EPA Region III risk-based concentration table [22] and in Wang et al. [23], according to the following equation:
where EFr is frequency of exposure (365 days/year); ED is the duration of exposure (72 years); FIR is food ingestion rate (g/person/d); MC is the mean level of metal in food (μg/g, on fresh weight basis); RfD is the oral reference dose (mg/kg/d); BW is the average body weight, adult (60 kg); children (30 kg); AT is the mean exposure time (365 days/year × number of exposure years, assuming 72 years in this study).
If the measured THQ is less than 1, the consumer will expose no health risk.
Hazard Index
The potential risk through the consumption of more than one heavy metal in meat and offal was evaluated by the hazard index (HI). All of the hazard quotients were summed to measure the HI [20].
where Σ HQ is the summation of hazard quotients of metals and HQ Cd, HQ Cr, HQ Cu, HQ Ni, and HQ Pb are the hazard quotients for cadmium, chromium, copper, nickel, and lead, respectively. In the case of hazard index greater than 1, a potential adverse effect existed [24].
Carcinogenic Risk
CR is a likelihood of person for developing cancer over the period of life due to contact with a potential carcinogen. Cancer risk due to exposure to Pb and Cd was determined using cancer slope factor (CSF), provided by US EPA [25] with the following equation:
where CSF is the carcinogenic slope factor of 0.0085 (mg/kg/day)−1 for Pb and 0.38 (mg/kg/day)−1 for Cd set by USPEA [20]. EDI is the estimated daily intake of heavy metals. E-4 (1 in 10,000) to E-6 (1 in 1,000,000) is the acceptable range of CR.
Statistical Analysis
The data was analyzed using SPSS (version16). The distribution’s normality of the analyzed samples was tested by the Kolmogorov-Smirnov test. The comparison of Cd and Pb with MRL recommended by WHO and EU was done through one sample t test. The mean concentration of metals in two seasons was compared by use of independent t test. A two- way analysis of variance was used to assess the interaction of animal and tissue (p < 0.05). If there was any interaction between animal and tissue, one-way analysis of variance (ANOVA) and independent t test were adjusted to compare the concentration of metals in different tissues and two animals, respectively (p < 0.05). The means were separated using Tukey’s test. R software (version 3.5) was used to perform Principal Component analysis (PCA).
Results and Discussion
Levels of Heavy Metals
Concentration of Cd, Cr, Cu, Ni, and Pb in the muscle, liver, and kidney of cow and sheep were determined (Table 1S). All of the metals were detected in all of the samples. Meanwhile, the mean concentration of lead and cadmium were below than MRL level defined by WHO and EU in all the samples (p < 0.001).
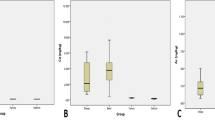
Mean concentration of metals in warm (summer) and cold (winter) seasons were compared in all the samples. Just in the samples of sheep liver, the mean concentration of Cd in cold season was significantly higher than warm season (p < 0.05). All of the other samples did not have any significant difference in the mean concentration of detected metals in the two seasons (Fig. 1).
Cadmium
The mean concentration of cadmium in the muscle, liver, and kidney samples of cow and sheep was lower than the MRL recommended by WHO and EU, 0.05, 0.5, and 1 mg/kg (p < 0.001) [14]. Cd was detected in all 102 samples. There was no interaction between animal and tissues (p > 0.05) in two-way analysis. There was no difference between two animals (p > 0.05), but a significant difference was seen between tissues (p < 0.001). Tukey’s follow-up test for tissues showed that mean concentration of Cd in kidney was significantly higher than the muscle (p < 0.001) and liver (p < 0.001), respectively (Table 1).
Due to mainly excretion of Cd through urine, the kidney may have some upper level of Cd [7, 26]. Also, it has been related to some free protein–thiol groups in this organ that bind the heavy metal [27]. Exposure of food animals to Cd mainly occurred through municipal waste, discharges from nickel–cadmium-based battery electroplating, and plastic and paint wastes due to careless discarding of these items [1].
Absorption of Cd from respiratory system is higher than gastrointestinal (GI) system, in vitro. However, GI adsorption is affected by diet and nutritional status. The accumulation of Cd in the kidney leads to renal dysfunction [22].
Chromium
A significant interaction existed between animal and tissue (p < 0.001). The highest and lowest levels of chromium were measured in cow’s kidney and liver, respectively (Table 1S). The mean concentration of Cr in the muscle of these two animals did not have any significant difference (t test; p < .014) but the liver and kidney had highly significant difference (t test; p < .001). There was a significant difference between the tissues of cow. For example, the kidney had a higher significant level of Cr than the muscle (Tukey’s test; p < 0.001) and the liver (t test; p < 0.001), respectively. The muscle and liver had similar amount. In sheep tissues, the liver had a higher significant amount of Cr than the muscle (Tukey’s test; p = .008) and the kidney (Tukey’s test; p < .029). The kidney and muscle had similar level (Tukey’s test; p < .873) (Table 1S).
Copper
Two-way analysis showed no interaction between animal and tissues (p > 0.05). Also, there was no difference between the two animals (p > 0.05). Tukey’s follow-up test for tissues showed that mean concentration of Cu was significantly higher in the liver than in the muscle (p < 0.001) and kidney (p < 0.001) (Table 1). The liver and kidney damage were seen in high intake of copper [28].
Nickel
A significant interaction was seen between animal and tissue (p < 0.001) in two-way analysis. The highest concentration of Ni detected in cow kidney was higher than 0.5 mg/kg [18]. But, the least amount of this metal was detected in cow and sheep muscle (Table 1S). The mean concentration of Ni in sheep liver was significantly higher than cow liver (t test; p < .003), and in cow kidney, Ni was significantly higher than sheep kidney (t test; p < 0.001). Tukey’s test showed a highly significant difference in comparison of the muscle by the kidney (p < 0.001) and the liver by the kidney (p < 0.001) of cow samples. In comparison of sheep samples, the mean concentration of Ni in the muscle was significantly lower than the liver (p < 0.001) and the liver was significantly higher than the kidney (p < .016). According to EC 2006, respiratory problems are caused by higher amount of intakes of Ni [14]. Soil and sediment were mentioned as the primary source of Ni. Nickel is used in a wide variety of processes including batteries and electroplating. Despite the low adsorption through gastrointestinal system, dietary exposure is the major route of exposure of general population [17, 22]. Ni is mainly excreted through urine by forming complexes with histidine [22].
Lead
Pb was measured in all of the samples of the studied animals. Two-way analysis of variance showed a significant interaction between animal and tissue (p < 0.004). The results of the present study showed that the sheep liver had the highest mean concentration of Pb (0.10341 mg/kg w/w) followed by cow kidney (0.07865 mg/kg w/w) (Table 1S). The least amount of Pb was detected in cow muscle (0.04276 mg/kg w/w). The comparison of mean level of Pb in the two animal and different tissues was done through independent t test and Tukey’s test, respectively. Results showed that only in the liver samples Pb concentration in cow was significantly lower than sheep (t test; p < 0.001). The muscle and kidney samples of these animals had equal concentration of Pb (t test; p > 0.05). In cow samples, mean amount of Pb in the kidney was significantly higher than in the liver (Tukey’s test; p = .05) and the muscle (Tukey’s test; p < 0.001). Also, the liver had a significantly higher concentration of Pb than the muscle (Tukey’s test; p < 0.001). Moreover, in sheep tissues, the liver had a significantly higher amount of Pb than the muscle (Tukey’s test; p < 0.001) and kidney (Tukey’s test; p < .03). The kidney and the muscle samples had similar amount of Pb (Tukey’s test; p < .088).
Generally, the low amount of Pb in the offal and muscle can be related to the tropism of lead for aggregation in bone and also more deposition in the aged animals [29]. Renal failure, liver damage, and neurologic disorders are some of the common health problems of lead. Humans are mainly exposed through diet. Children are at higher risk because of 50% adsorption of Pb in their diet in comparison to adults that absorb about 10–15% of ingested lead [30]. Upon absorption, distribution of Pb occurred in the blood, bone, and soft tissues including the liver, kidneys, brain, and muscle. After conjugation with glutathione, Pb is excreted mainly through urine [30]. Moreover, the intake of heavy metals can cause immunological dysfunction and retard the fetal growth (Cd and Pb) due to depletion of some essential micronutrients [3].
The results of the present study compared with the published data (Table 2S). Results of the current study in Cd concentration were found to be comparable with the other studies [1, 29, 31,32,33] that Cd was higher in the kidney than in the liver and muscle samples.
Cr amount in cow samples of the current study was similar to Bortey-Sam et al. [1] that the kidney had the highest and the liver had the lowest level. But, in the sheep samples, it was not comparable. The two other studies [3, 34] reported the higher level of Cu in the muscle (4.45 and 2.3 mg/kg in the sheep muscle, 1.8 mg/kg in the cow muscle) than present study. Also, in this study, Cu had the most level in the liver; the kidney had a higher amount than the muscle which was agreed with the other studies [1, 31]. Ni amount of cow muscle in this study is similar to SaifulIslam et al. [3] but in the sheep muscle SaifulIslam et al. [1] had the higher level of Ni (1.6 mg/kg) than the present study. Offal had higher level of Ni than the muscle in both animals in the present study (Table 1S) which was disagreed with Bortey-Sam et al. [1]. Lead concentration in the present study was according to Bortey-Sam et al. [1] and disagreed with Abou-Arab [31] and Caggiano et al. [29]. Some other studies reported the higher amount of Pb in samples [3, 33].
The average level of Cd and Pb were higher in the kidney and liver samples than in the muscle samples. Cd or Pb levels in the muscle was below the MRLs [29, 32], which was in agreement with the results of the present study.
Distribution of Metals
Comparison of the aggregation of metals in the organs of cow and sheep was performed through Tukey’s test of ANOVA. The pattern of distribution of the metal in the offal and muscle of cow and sheep were significantly different (p < 0.05). Clearance of heavy metals from the body mainly occurred through the liver and kidney. As a result, these two organs mostly select for surveillance of metal residues in food animals [31]. In fact, the kidney and liver of these two food animals had the higher concentration of all measured metals (Table 1). PCA was used to evaluate the scattering of the metals in the offal and muscle samples of cow and sheep. PCA results (Fig. 2) showed a dissociation between cow (C), and the sheep (S), clustered independently both in the kidney and liver. But in the muscle, they were not separated. Interestingly, in the liver and kidney, Cd, Cu, and Pb made one cluster and are positively correlated (Fig. 1). Also, Ni and Cr made another cluster (Fig. 2). The muscle samples showed different distribution pattern, with Cd, Cr, Cu, and Ni correlated together (Fig. 2). Pb and Cu were not correlated and with the decrease of Pb concentration, Ni concentration was increased. This could be related to the difference in the level of some conjugation protein such as metallothione in the sampled food animals [1].
Dietary Intake of Metals, Target Hazard Quotient, Hazard Index, and Carcinogenic Risk of Heavy Metals
The estimated daily intakes (EDI) of toxic metals for adults and children in this region with the consumption of offal and meat of cow and sheep are presented in Table 2. The numerically higher EDIs of Cd (0.0095 μg/kg bw/day) and Cr (0.34 μg/kg bw/day) were by eating of cow kidney and muscle, respectively (Table 2). Although, the numerically higher EDI of Ni (0.047 μg/kg bw/day) and Pb (0.026 μg/kg bw/day) was by use of sheep muscle, but the numerically higher EDI of Cu (1.207 μg/kg bw/day) was through consumption of sheep liver (Table 2).
The EDIs of Cd, Cr, Cu, Ni, and Pb of the two food animals were numerically smaller than TDI. Vulnerability of children is higher than adults to acute and chronic effects of intake of chemical pollutants, because of consuming twice the amount of food per unit of body weight [35]. Therefore, intake of toxic metals through food is higher for children in this region.
THQ value more than 1 shows the exposed population is likely to experience obvious adverse effects [25]. Meanwhile, all of the calculated THQ were below 1.0 (Fig. 3); numerically, higher THQ values in children should be noticeable. The THQs of Pb in the cow and sheep muscles which are a major part of the usual diet of people in this region are at the numerically higher level in children and adults. Similarly, the THQ for Cd in both adults and children had the numerically lower values. THQ of Cu and Ni had the similar level and was more than Cr.
The HI was calculated for each of tissues in both food animals in adults and children (Fig. 4). The numerically higher HI was measured for cow and sheep muscle in both adults and children, and the least was calculated for the sheep kidney (Fig. 4).
The CR values of Pb and Cd in adults from the consumption of the measured samples are presented in Table 3. In general, the values of CR less than E-6 are negligible, more than E-4 are unacceptable, and between E-6 to E-4 are acceptable [20]. In the current study, CR for Cd was numerically higher than the unacceptable range by use of the meat, liver, and kidney of cow and sheep (Table 3). CR of Cd was numerically higher for the cow kidney, sheep and cow muscle, 3.6E-3, 2.66E-3 and 2.66E-3, respectively. Also, consumption of sheep muscle was of concern due to unacceptable range of Pb (Table 3).
Consumption of these toxic metals through offal and meat in longer period can lead to toxicity due to accumulation nature of these metals in the body. As a result, there is a need for monitoring of these toxic metal residues in the meat, liver, and kidney of these food animals.
Conclusions
The results of this study showed the significant differences that existed in the mean concentrations of metals among both food animals and meat organs. Most concentration of metals is detected in the liver and kidney. Although the mean concentration of Cd and Pb was lower than MRLs, bioaccumulation of metals in the body can raise some toxicity in a longer period for national and international consumers. Furthermore, Cd concentration had CR above the permissible level. Moreover, Pb had higher THQs and HI in the beef and sheep muscle. They may be routinely controlled especially due to vulnerability of children.
References
Bortey-Sam N, Nakayama SMM, Ikenaka Y, Akoto O, Baidoo E, Yohannes YB, Mizukawa H, Ishizuka M (2015) Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol Environ Saf 111:160–167
Harmanescu M, Alda LM, Bordean DM, Gogoasa I, Gergen I (2011) Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem cent 5(64):10
SaifulIslam MD, KawserAhmed MD, Habibullah-Al-Mamun MD, Raknuzzaman M (2015) The concentration, source and potential human health risk of heavy metals in the commonly consumed foods in Bangladesh. Ecotoxicol Environ Saf 122:462–469
Atique Ullah AKM, Maksud MA, Khan SR, Lutfa LN, Quraishi SB (2017) Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol Rep 4:574–579
Zheng N, Wang QC, Zheng DM (2007) Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao zinc plant in China via consumption of vegetables. Sci Total Environ 383(1–3):81–89
John GF, Andrew BA (2011) Lead isotopic study of the human bioaccessibility of lead in urban soils from Glasgow, Scotland. Sci Total Environ 409:4958–4965
Jarup L, Akesson A (2009) Review: current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208
Ambushe AA, Hlongwane MM, McCrindle RI, McCrindle CME (2012) Assessment of levels of V, Cr, Mn, Sr, Cd, Pb and U in bovine meat. S Afr J Chem 65:159–164
Mokgalaka NS, Wondimu TA, McCrindle RI (2008) Slurry nebulization ICP-OES for the determination of Cu, Fe, Mg, Mn and Zn. Bull Chem Soc Ethiop 22:1–9. https://doi.org/10.4314/bcse.v22i3.61195
Shahryari T, Moashery BN, Sharifzadeh GR (2011) Concentrations of chromium and copper in the ground water and drinking water distribution network of Birjand, 2009-2010. J Birj uni med 17(4):62–67
Nriagu J, Boughanen M, Linder A, Howe A, Grant C, Rattray R, Vutchkov MA, Lalor G (2009) Levels of As, Cd, Pb, Cu, Se and Zn in bovine kidneys and livers in Jamaica. Ecotoxicol Environ Saf 72:564–571
Andrée S, Jira W, Schwind KH, Wagner H, Schwägele F (2010) Chemical safety of meat and meat products. Meat Sci 86:38–48. https://doi.org/10.1016/j.meatsci.2010.04.020
European Food Safety Authority (EFSA) (2009) Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on cadmium in food. EFSA J 980:1–139
European Commission (2006) COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OffJEurUnion L 364, 20.12.2006,:p. 5
JECFA (2009) Evaluations of the Joint FAO/WHO Expert Committee on Food Additives. <http://apps.who.int/food-additives-contaminants-jecfa-database/PrintPreview.aspx?chemID=5879>
European Food Safety Authority (EFSA) (2018) Reasoned opinion on the review of the existing maximum residue levels for copper compounds according to Article 12 of Regulation (EC) No 396/2005. EFSA J 16(3):135. https://doi.org/10.2903/j.efsa.2018.5212
EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel) (2015) Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J 13(2):202. https://doi.org/10.2903/j.efsa.2015.4002
Ihedioha JN, Okoye COB, Onyechi UA (2014) Health risk assessment of zinc, chromium, and nickel from cow meat consumption in an urban Nigerian population. Int J Occup Environ Health 20(4):281–288. https://doi.org/10.1179/2049396714Y.0000000075
Wikipedia (2018) South Khorasan Province. <https://en.wikipedia.org/wiki/South_Khorasan_Province>
US EPA (2010) Risk-based concentration table. <http://www.epa.gov/reg3hwmd/risk/human/index.htm>
ISIRI (2010) Food & feed-maximum limit of heavy metals. ISIRI 12968, 1st edn. Institute of Standards and Industrial Research of Iran, Tehran
US EPA (2007) Integrated Risk Information System-Database (IRIS). Philadelphia PA;. <https://www.epa.gov/iris>
Wang X, Sato T, Xing B, Tao S (2005) Health risk of heavy metals to the general public inTianjin,China via consumption of vegetables and fish. Sci Total Environ 350:28–37
Huang ML, Zhou SL, Sun B, Zhao QG (2008) Heavy metals in wheat grains: assessment of potential health risk for inhabitants in Khunshan, China. Sci Total Environ 405(1–3):54–61
US EPA (2000) Risk-based concentration table,. <https://www.epa.gov/iris>
Wang CL, Zhang FT, Cao WX, Wang JW (2013) The identification of apolipoprotein C-I in rare minnow (Gobiocypris rarus) and its expression following cadmium exposure. Environ Toxicol Pharmacol 35:419–426
Pompe-Gotal J, Crnic AP (2002) Cadmium in tissue of roe deer (Capreolus capreolus) in Croatia. VetArhiv 72:303–310
Agency for Toxic Substances and Disease Registry (ATSDR) (2004) U.S. Department of Health and Human Services. Atlanta, GA: Public Health Service. Toxicological profile for copper <http://www.atsdr.cdc.gov/toxprofiles/>
Caggiano R, Sabia S, D’Emilio M, Macchiato M, Anastasio A, Ragosta M, Paino S (2005) Metal levels in fodder, milk, dairy products, and tissues sampled in ovine farms of Southern Italy. Environ Res 99:48–57
JECFA (2000) Evaluation of certain food additives and contaminants. Report of the Fifty-Third of the Joint FAO/WHO Expert Committee on Food Additives. Technical Report Series No.896. Geneva
Abou-Arab AAK (2001) Heavy metal contents in Egyptian meat and the role of detergent washing on their levels. Food and chemical toxicology 39 (6):593–599
Pagan-Rodriguez D, O'Keefe M, Deyrup C, Zervos P, Walker H, Thaler A (2007) Cadmium and lead residue control in a hazard analysis and critical control point (HACCP) environment. J Agric Food Chem 55(4):1638–1642
Darwish WS, Hussein MA, El-Desoky K, Ikenaka Y, Nakayama S, Mizukawa HA, Ishizuka M (2015) Incidence and public health risk assessment of toxic metal residues (cadmium and lead) in Egyptian cattle and sheep meat. Int Food Res J 22(4):1719–1726
Alturiqi AS, Albedair LA (2012) Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. Egypt J Aquat Res 38:45–49
European Environment and Health Information System (EEHIS) (2007) Exposure of children to chemical hazards in food. Fact Sheet No.4.4,CODE:RPG4_Food_EX1
Funding
The authors acknowledge the research and technology deputy of Birjand University of Medical Sciences for support of this study (grant No 4273).
Author information
Authors and Affiliations
Contributions
TZ and KN contributed in the conception and design of the study. FS performed the statistical analysis and drafting of the manuscript. TZ conducted the experiments and wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
This study was carried out according to the international ethical guidelines and approved by the ethical committee of Birjand University of Medical Sciences.
Conflict of Interest
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeinali, T., Salmani, F. & Naseri, K. Dietary Intake of Cadmium, Chromium, Copper, Nickel, and Lead through the Consumption of Meat, Liver, and Kidney and Assessment of Human Health Risk in Birjand, Southeast of Iran. Biol Trace Elem Res 191, 338–347 (2019). https://doi.org/10.1007/s12011-019-1637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-1637-6