Abstract
This study investigated the effects of chronic aluminum exposure on apoptosis of hippocampal neurons, and synaptic plasticity in the hippocampus in rats. Rats were divided into the control, low-dose (L-Al), mid-dose (M-Al), and high-dose (H-Al) groups. After chronic exposure of aluminum, the Morris water maze (MWM) and open-field (OF) tests were performed to assess the behavioral performance. Electrophysiological measurements were conducted. Flow cytometry was used to assess the apoptotic processes. Quantitative real-time PCR and ELISA were performed to measure mRNA and protein expression levels of caspases. After 90 days of aluminum exposure, the aluminum contents in the brain of the rats were increased, with the increasing exposure dose. The MWM and OF tests showed that chronic exposure of aluminum significantly impaired the neurobehavior of rats. Moreover, after high-frequency stimulation (HFS), the average amplitudes of field excitatory postsynaptic potentials (fEPSPs) for the M-Al and H-Al groups were lower than the control group at 10, 20, 30, 40, 50, and 60 min. Furthermore, the apoptotic rates in the M-Al and H-Al groups were significantly higher than the control group. The qRT-PCR and ELISA showed that, compared with the control group, the mRNA and protein expression levels of caspases-3, -8, and -9 were significantly increased in the aluminum-treated groups compared with the control group. Long-term exposure to aluminum could induce the apoptosis of hippocampal neurons, damage the synaptic plasticity, and impair the learning and memory functions in rats. There might be a close relationship between the neuronal apoptosis and synaptic plasticity damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum is one of the most abundant elements in the Earth’s crust, ranking the third only after oxygen and silicon. The reserves of aluminum deposits account for more than 8% of the crustal constituents by mass [1]. At present, the production and consumption of aluminum are second only to the steel, which has become the second largest metal in application. Aluminum is characterized by high melting and boiling points, low density, and corrosion resistance, and therefore, it has been widely used in the aerospace, automobile, and some construction industries. Moreover, various compounds of aluminum have been commonly used to produce the cooking utensils, cosmetics, drinking containers, food additives, and pharmaceutical products [2, 3]. However, it has been shown that excessive intake of aluminum would cause a series of adverse reactions, impairing the normal physiology of the nervous [4,5,6], skeletal [7,8,9], immune [6, 10], and reproductive [11] systems. In recent years, the neurotoxicity of aluminum has attracted more and more attention in research field.
After entering into the human body, aluminum can interfere with the metabolism of many trace elements. Higher plasma aluminum concentrations may interfere with both the zinc (Zn) and selenium (Se) homeostasis, which would consequently lead to low plasma Zn and Se contents and high oxidative stress in patients with the long-term dialysis [12]. Studies have shown that Al3+ can substitute Ca2+ in the hydroxyapatite crystals in patients with exostosis [13], and aluminum deposition in the bone could decrease the Ca, Mg, and P levels, inhibiting the bone mineralization process [14]. On the other hand, aluminum would alter the function of the blood-brain barrier, and therefore accumulate in the cortex, cingulate bundles, corpus callosum, and hippocampus, to exert neurotoxic effects [5, 15]. Studies have shown that aluminum would act as an important risk factor for the neurodegenerative diseases with learning and memory impairment, including Alzheimer’s disease (AD) [4], amyotrophic lateral sclerosis (ALS) [16, 17], and Parkinson’s syndrome (PD) [18]. Moreover, epidemiological studies have also shown that the long-term exposure to aluminum might lead to neurological dysfunctions, such as the learning/memory and cognitive impairments [19, 20]. Nevertheless, although a large quantity of data have been documented concerning the relationship between the aluminum exposure and neurodegenerative effects, the underlying mechanisms of the disease development have not yet been fully elucidated [21]. At present, the researches of aluminum neurotoxicity mainly focus on its effects on lipid peroxidation, neuronal apoptosis, Aβ deposition, and Tau protein phosphorylation.
Learning and memory function results from the interaction and coordination between numerous neurons in the brain, involving the synaptic plasticity (electrophysiologically expressed as long-term potentiation (LTP)) [22]. Caspases, as a family of conserved cysteine proteases, play essential roles in cellular apoptosis. Mammalian caspases could be divided into the initiator (caspases 2, 8, 9, and 10) and executioner (caspases 3, 6, and 7) caspases. The initiator caspases initiate the apoptotic signals, while the executioner caspases carry out the mass proteolysis, leading to apoptosis [23]. In this study, the effects of sub-chronic aluminum exposure on the learning and memory functions in rats were investigated. Moreover, the neuronal apoptosis, caspase activity, and synaptic plasticity in the hippocampus, as well as the aluminum content in the cerebral cortex were also analyzed.
Materials and Methods
Study Animals and Grouping
Male Sprague-Dawley (SD) rats (6–7 weeks old), weighing 160–170 g, were purchased from the Experiment Animal Center of Academy of Military Medical Science (Beijing, China). These animals were housed in cages in a temperature-controlled room, with a 12-h light/dark cycle. The rats were fed with standard commercial rodent diet (Beijing Keao Xieli Feed Co, Ltd., Beijing, China) and drinking water ad libitum. These rats were randomly divided into the following four groups (n = 10/group): (1) the control (control) group, in which the rats received no treatment; (2) the low-dose (L-Al) group, in which the rats were treated with aluminum lactate at 10 mg/kg body weight once a day for 90 days; (3) the mid-dose (M-Al) group, in which the rats were treated with aluminum lactate at 30 mg/kg body weight once a day for 90 days; and (4) the high-dose (H-Al) group, in which the rats were treated with aluminum lactate at 90 mg/kg body weight once a day for 90 days.
Aluminum lactate (C9H15AlO9; CAS: 18917-91-4) was purchased from Sigma-Aldrich (St. Louis, MO, USA), which was dissolved in distilled water. Rats in the aluminum groups were exposed to aluminum lactate by gavage for a period of 90 days, at indicated concentrations [24]. The animal weight was weighed once a week, and the drug dosage was adjusted accordingly. Rats in the control group received an equivalent amount of distilled water. At 24 h after the last dosing, behavioral test was carried out. Thereafter, five rats in each group were subjected to the electrophysiological detection. On the other hand, the remaining five rats were sacrificed by exsanguination from the abdominal aorta after being anesthetized with pentobarbital sodium (50 mg/kg body weight). The brains were removed and rinsed with ice-cold saline.
Behavioral Tests
Cognitive abilities of rats were assessed by the Morris water maze (MWM) test and the open-field (OF) test. In the MWM test, a circular tank (120 cm in diameter and 50 cm in height) was filled with water (22 ± 1 °C) to the depth of 25 cm. An escape platform (12 cm in diameter) was placed at the center of one quadrant of the tank, with the top 1.5 cm beneath the water surface. The test was performed for five consecutive days (four sessions per day, with interval of at least 1 h). Each rat was released into the water facing the wall at one of the four standard starting locations (N, S, W or E). When the rat succeeded in locating the platform, it was allowed to remain on the platform for 10 s. If the rat failed to find the platform within 2 min, it would be placed on the platform and allowed to remain on the platform for 2 min. On the day after this task, the 1-min probe trial was carried out, without the platform, and the starting point was the quadrant opposite the target one. Rat performance was monitored with the SMART video-tracking system 3.0 (Panlab).
Animal behavior was assessed in the OF apparatus to evaluate the locomotor and exploratory activities. Rats were individually placed in an open-field apparatus (L × W × H of 90 cm × 90 cm × 45 cm). The animal was allowed to explore freely for 10 min, and the movement was recorded by a video camera (Sony). Time spent in the center and periphery was recorded. Data were collected and analyzed using the SMART v3.0 software (Panlab).
Electrophysiological Measurement
Surgical procedure was applied as previously described, with minor modifications [25, 26]. Briefly, five rats from each group were anesthetized and fixed in a stereotaxic device (Narishige, Japan). Two small holes were drilled on the right side of the skull. Then, the bipolar stimulating electrode (FHC, Bowdoin, ME, USA) was inserted into the Schaffer collateral-commissural pathway (4.2 mm posterior to bregma, 3.8 mm lateral to the midline), and the monopolar recording electrode (FHC) was positioned in the stratum radiatum of area CA1 (3.8 mm posterior to bregma, 2.9 mm lateral to the midline). Stimulating and recording electrodes were inserted into the hippocampus by a micro-propulsion unit (Narishige, Japan). The electrodes were slowly lowered into the CA1 region of the hippocampus until the field excitatory postsynaptic potentials (fEPSPs) appeared. Baseline fEPSPs were elicited by the test stimuli at an interval of 30 s (with an intensity eliciting 50% of the maximal response), which were monitored for 30 min. LTP was induced by a train of high-frequency stimulation (HFS) consisting of 20 pulses at 200 Hz. After measuring HFS, the test stimuli were applied again for 60 min, and the changes in the fEPSP amplitudes were detected.
Flow Cytometry
After trypsinization, cells were harvested from the hippocampal tissue. To detect the apoptosis, these cells were subjected to the double staining of Annexin V-FITC and propidium iodide (PI) (KeyGEN BioTECH, Nanjing, China), according to the manufacturer’s instructions. Then, the cells were washed with ice-cold D-Hanks and centrifuged at 4 °C at 500×g for 5 min. The supernatant was discarded and the pellets were re-suspended. The cells were stained with 5 μl Annexin-V and 5 μl PI. After gentle mixing, the suspension was incubated in the dark for 15 min, and then the fluorescence was detected with a flow cytometer (Beckman Coulter, Miami, FL, USA). Under the excitation of 488-nm excitation light, the emission light waves of FITC and PI were 525 nm and 630 nm, which would be received by the FL1 and FL3 channels, respectively. For the gating strategy, through the FSC and SSC Scatter plot, the gate was set to exclude cell debris or sticky cells, and the FL1 and FL3 channel scatter plots were used to set the cross gate position. Percentage statistics of cells in each quadrant were analyzed and compared. No less than 1 × 104 cells were collected, and the data were analyzed with the EXP032 software. The early apoptotic cells were positive for Annexin V staining and negative for PI staining, while the late apoptotic cells were positive for Annexin V staining and positive for PI staining. The total apoptotic rates included both the early apoptotic cells and late apoptotic cells.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA in hippocampus was extracted with the RNA pure Tissue & Cell Kit (DNase I) (Beijing CoWin Biotech, Beijing, China), according to the manufacturer’s instructions. Complementary DNA synthesis of mRNA was performed using the Prime Script™ RT Master Mix (Takara Bio, Dalian, Liaoning, China). The qRT-PCR was performed with the SYBR® Premix Ex Tap™ (Takara Bio). Primer sequences were as follows: caspase-3, forward 5′-GAGACAGACAGTGGAACTGACGATG-3′ and reverse 5′-GGCGCAAAGTGACTGGATGA-3′; caspase-8, forward 5′-TGGTATATCCAGTCACTTTGCCAGA-3′ and reverse 5′-CTCACATCATAGTTCACGCCAGTC-3′; caspase-9, forward 5′-CTGAGCCAGATGCTGTCCCATA-3′ and reverse 5′-GACACCATCCAAGGTCTCGATGTA-3′; and GAPDH, forward 5′-GGCACAGTCAAGGCTGAGAATG-3′ and reverse 5′-ATGGTGGTGAAGACGCCAGTC-3′. The PCR conditions were set as follows: 95 °C for 30 s; 95 °C for 5 s, and 60 °C for 45 s, for totally 40 cycles. The expression levels of target genes were calculated using the 2−ΔΔCt method. GAPDH was used as internal reference.
Enzyme-Linked Immunosorbent Assay
Rat hippocampus (50 mg) was homogenized in 500 μl ice-cold Tissue Protein Extraction Kit (Beijing CoWin Biotech). The homogenates were centrifuged at 10,000×g at 4 °C for 20 min. Total protein concentration was determined using the BCA Kit (Beijing CoWin Biotech). The concentrations of caspases-3, -8, and -9 were determined using the ELISA Kits for caspase 3 (No. SEA626Ra), caspase 8 (No. SEA853Ra), and caspase 9 (No. SEA627Ra) (Uscn Life Science Inc., Wuhan, Hubei, China), respectively. In these kits, the microtiter plate was pre-coated with the antibody specific to caspase-3, -8, or -9. Standards or samples were then added to the wells with a biotin-conjugated polyclonal. The standard curve was obtained, and the target protein concentration was calculated accordingly.
Aluminum Detection
Concentrations of aluminum in the brain tissues were determined with the iCE™ 3500 AAS Atomic Absorption Spectrometer (Thermo Fisher Scientific, Somerset, NJ, USA). The instrument was adjusted to a wavelength of 309.3 nm, with a slit of 0.5 nm, and used a hollow cathode lamp. Rat cortex (50 mg) was digested in 5 ml nitric acid with the Microwave Accelerated Reaction System (CEM Corp), which was then diluted with the Mili-Q water. Briefly, the prefrontal cortex samples were removed and collected from the five rats in each group. Totally, 50 mg (wet weight) cortical sample was put into the microwave digestion tube, and 5 ml nitric acid was added, followed by the microwave digestion. The heating program was as follows: 6 min to 120 °C, 120 °C for 3 min, 6 min to 150 °C, 150 °C for 6 min, 6 min to 180 °C, 180 °C for 20 min. The solution was filtered with a 0.22-mm micropore filter into a 10-ml centrifuging tube. The volume was adjusted to 5 ml with nitric acid and diluted with ultrapure water. Detection was performed with the graphite furnace atomic absorption spectrometer. The aluminum content was calculated based on the following formulation: aluminum content (μg/mg) = (A − A0) × V × X/m, where A (μg/L) was the mass concentration of aluminum in the digestive solution, A0 was the mass concentration of aluminum in the blank solution, V (L) was the total volume of sample digestive solution, X was the sample dilution factor, and m (mg) was the sample mass. For quality control, the blank sample (Mili-Q water) and the aluminum standard solution (10 μg/L) were measured in each assay with the same method and instrument condition as the measurement of aluminum content in rat brain tissue.
Statistical Analysis
Data were expressed as mean ± SD. Statistical analysis were performed using the SPSS 25.0 package software. One-way ANOVA was used for group comparison, followed by the Dunnett t test. P < 0.05 was considered statistically significant.
Results
Effects of Aluminum Lactate Exposure on Neurobehavior
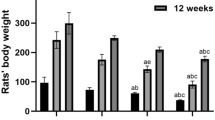
To investigate the effects of aluminum lactate on the behavioral performance of these animals, behavioral tests were performed. Our results from the MWM test showed that, with the increase of training days, the escape latency of each group was gradually shortened. Compared with the control group, the escape latencies in the M-Al and H-Al groups were significantly prolonged (P < 0.05) (Fig. 1a). On the sixth day of the test, in the M-Al and H-Al groups, the number of rats crossing the platform were significantly reduced (P < 0.05) (Fig. 1b), and the residence time in the target quadrant was significantly shortened (P < 0.05) (Fig. 1c), compared with the control group. On the other hand, our results from the OF test showed that, compared with the control group, the central residence time was significantly longer for the M-Al and H-Al groups (P < 0.05) (Fig. 1d). However, there was no significant difference in the number of rearing between the Al-exposed and control groups (P > 0.05 (Fig. 1e). These results suggest that the cognitive function of rats in the M-Al and H-Al groups are impaired versus the control group.
Effects of aluminum lactate exposure on neurobehavior. After the treatments, the behavior performance of the rats from the control (control), low-dose aluminum (L-Al), mid-dose aluminum (M-Al), and high-dose aluminum (H-Al) groups was assessed with the MWM and OF tests. a–c Analysis of escape latencies (a), number of crossing the platform (b), and residence time in the target quadrant (c) in the MWM test. d, e Analysis of central residence time (d) and number of rearing (e) in the OF test. Compared with the control group, *P < 0.05, **P < 0.01
Effects of Aluminum Lactate Exposure on Electrophysiological Properties of Hippocampal Neurons
Animal behavior is controlled by the hippocampal zone of the brain. Therefore, the electrophysiological changes in the hippocampal CA1 region of these rats were detected. As shown in Fig. 2, after HFS at 10, 20, 30, 40, 50, and 60 min, the average amplitudes of fEPSPs for the M-Al and H-Al groups were lower than the control group (P < 0.05). These results suggest that the synaptic plasticity of rats in the M-Al and H-Al groups are also changed.
Effects of aluminum lactate exposure on the electrophysiological properties of hippocampal neurons. After the treatments, the electrophysiological properties of hippocampal neurons from the control (control), low-dose aluminum (L-Al), mid-dose aluminum (M-Al), and high-dose aluminum (H-Al) groups, were assessed. a Calibrated fEPSPs amplitudes at different time points before and after high-frequency stimulation. b Comparison of the amplitude of fEPSP in rats exposed to aluminum lactate. Compared with the control group, *P < 0.05, **P < 0.01
Effects of Aluminum Lactate Exposure on Neuronal Apoptosis
To detect the effects of aluminum lactate exposure on the neuronal apoptosis, flow cytometry was performed. Our results showed that, the total apoptotic rate of hippocampal neurons in rats exposed to Al was dose-dependent. Moreover, the apoptotic rates in the M-Al and H-Al groups were significantly higher than the control group (P < 0.05) (Fig. 3). These results suggest that the neuronal apoptotic rate is increased in the M-Al and H-Al groups.
Effects of aluminum lactate on apoptosis of hippocampal neurons. After the treatments, the apoptosis of hippocampal neurons from the control (control), low-dose aluminum (L-Al), mid-dose aluminum (M-Al), and high-dose aluminum (H-Al) groups, was assessed with the flow cytometry. a Representative figures from flow cytometry for these groups. b Statistical analysis. Compared with the Control group, *P < 0.05, **P < 0.01
Effects of Aluminum Lactate Exposure on Caspases-3, -8, and -9 Gene Expression
To investigate the effects of aluminum lactate exposure on the expression levels of caspases-3, -8, and -9, these caspases were detected with qRT-PCR and ELISA, respectively. Our results from the qRT-PCR showed that, compared with the control group, the mRNA expression levels of all caspases-3, -8, and -9 were significantly increased in the M-Al and H-Al groups (P < 0.05) (Fig. 4a). Caspase-8 mRNA was also increased in the L-Al group compared with the control group (P < 0.05) (Fig. 4). Moreover, similar results were observed for ELISA. As shown in Fig. 4b, our results showed that, compared with the control group, the concentrations of caspases-8 and -9 were significantly elevated in all Al-treated groups (P < 0.05), while the caspase-3 concentration was significantly increased in M-Al and H-Al groups (P < 0.05) (Fig. 4b). Taken together, these results suggest that the mRNA and protein expression levels of caspases-3, -8, and -9 in rats exposed to aluminum are significantly increased, especially for the M-Al and H-Al groups.
Effects of aluminum lactate exposure on caspases-3, 8, and 9 expression. a, b After the treatments, the expression levels of caspases-3, 8, and 9 from the control (control), low-dose aluminum (L-Al), mid-dose aluminum (M-Al), and high-dose aluminum (H-Al) groups, were assessed with the quantitative real-time PCR (a) and ELISA (b), respectively. Compared with the control group, *P < 0.05, **P < 0.01
Contents of Al in Brain
After 90 days of Al exposure, the Al contents in the brain of rats were detected. Our results showed that the Al contents in the brain of the rats from the experimental groups were increased with the increasing exposure dose. Moreover, the Al contents in the brain of rats from the M-Al and the H-Al groups were significantly higher than the control group (P < 0.05) (Fig. 5).
Discussion
In the present study, our results showed that chronic exposure to aluminum lactate could lead to the aluminum accumulation in the brain and alter the cognitive function of rats. General population is primarily exposed to aluminum through the food consumption, taking antacids, drinking water, and ambient air inhalation [27]. The increased biological availability of aluminum has been linked to various acute and chronic diseases in humans [28]. After entering the brain, aluminum could accumulate in the medial striatum, corpus callosum, and cingulate bundle, causing learning/memory impairments [29]. Our results showed that, after 90 days of exposure, there was obvious aluminum accumulation in the brain in the M-Al and H-Al groups. Rats exposed to aluminum, especially in the M-Al and H-Al groups, had a slower learning curve and performed poorly in the MWM and OF tests, in a dose-dependent manner, in line with our previous findings [25, 26].
Apoptosis plays important roles in various biological processes, including the cell turnover, immune system development and function, hormone-dependent atrophy, embryonic development, and chemical-induced cell death. However, inappropriate apoptosis (either too little or too much) has been observed in many conditions in human beings, including the neurodegenerative diseases, ischemic damages, autoimmune disorders, and cancers [30]. In the present study, the total apoptotic rates (early and late apoptotic rates) of hippocampal neurons in the M-Al and H-Al groups were significantly higher than the control group, in a dose-dependent manner. In response to death-inducing signals, pro-caspases, a pre-existing group of pro-enzymes, would be activated to caspases within the cells [31]. As the executors of apoptosis, caspases are responsible for the cleavage of cellular substrates, leading to morphological changes [32]. Based on the action mechanisms in apoptosis, caspases could be classified into either initiators (caspases-8 and -9) or executors (caspases-3, -6, and -7) [23]. Therefore, the apoptotic pathways can be distinguished by the adapter and initiator caspases involved, falling into either the extrinsic or intrinsic category. Caspases-8 and -9 are key initiators in both the extrinsic and intrinsic pathways. In this study, our results showed that the mRNA and protein expression levels of caspases-3, -8, and -9 in rats exposed to aluminum were significantly increased, especially for the M-Al and H-Al groups. These findings suggest that the long-term aluminum exposure, especially at high concentrations, can cause neuronal apoptosis through both the extrinsic and intrinsic pathways.
LTP is a well-characterized form of synaptic plasticity. There is a strong body of evidence demonstrating the underlying molecular mechanisms in LTP and memory [33]. It has been well recognized that the synaptic plasticity, which can be assessed by LTP, is the essential condition for the learning and memory functions [34]. In this study, our results showed that the aluminum lactate suppressed the fEPSP amplitude of LTP, in a dose-dependent manner. Moreover, after the administration of aluminum lactate for 90 days, the rats exhibited significant impairments in the LTP of the hippocampal CA1 area, and in the learning and memory function, in line with our previous reports [26, 35]. Signals between neurons are transduced primarily by the receptors, second messengers, and kinase cascades, which are located in the pre- and post-synaptic terminals. Many signaling pathways have been shown to be able to affect the survival of neurons by promoting or preventing apoptosis, including the cAMP-PKA-CREB, L-arginine-NO, and Akt/GSK-3b pathways [26, 36,37,38]. In addition, the non-apoptotic roles of caspases in the neuronal development, plasticity, and disease development have attracted more and more attention in recent years [39].
In conclusion, our results showed that long-term exposure to aluminum induced apoptosis of hippocampal neurons, damaged the synaptic plasticity, and impaired the learning and memory functions in rats. There might be a close relationship between the neuronal apoptosis and synaptic plasticity damage. The advantage of this study is that we parallelly measured aluminum-induced behavior changes, brain aluminum contents, apoptotic rates of neuronal cells, apoptosis essential caspases, and LTP in rats, and found a strong positive relationship between them. The deficiency of this study is that we have not observed the aluminum-induced effects under the condition of blocking caspases. Of course, the underlying mechanisms of aluminum-induced synaptic plasticity impairment still need to be elucidated in the future, especially concerning the roles of the caspase family which we will study by blocking the caspases and to compare the results between blocking them and without blocking them.
References
Wesdock JC, Arnold IM (2014) Occupational and environmental health in the aluminum industry: key points for health practitioners. J Occup Environ Med 56(5 Suppl):S5–S11. https://doi.org/10.1097/JOM.0000000000000071
Stahl T, Falk S, Rohrbeck A, Georgii S, Herzog C, Wiegand A, Hotz S, Boschek B, Zorn H, Brunn H (2017) Migration of aluminum from food contact materials to food—a health risk for consumers? Part I of III: exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environ Sci Eur 29(1):19. https://doi.org/10.1186/s12302-017-0116-y
Stahl T, Taschan H, Brunn H (2011) Aluminium content of selected foods and food products. Environ Sci Eur 23(1):1–11. https://doi.org/10.1186/2190-4715-23-37
Colomina MT, Peris-Sampedro F (2017) Aluminum and Alzheimer’s disease. Adv Neurobiol 7(6):183–197. https://doi.org/10.1007/978-3-319-60189-2_9
Kumar V, Gill KD (2009) Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol 83(11):965–978. https://doi.org/10.1007/s00204-009-0455-6
Shaw CA (2018) Aluminum as a CNS and immune system toxin across the life span. Adv Exp Med Biol 1091:53–83. https://doi.org/10.1007/978-981-13-1370-7_4
Klein GL (2019) Aluminum toxicity to bone: a multisystem effect? Osteoporos Sarcopenia 5(1):2–5. https://doi.org/10.1016/j.afos.2019.01.001
Li X, Hu C, Zhu Y, Sun H (2011a) Effects of aluminum exposure on bone mineral density , mineral , and trace elements in rats. Biol Trace Elem Res 143:378–385. https://doi.org/10.1007/s12011-010-8861-4
Mjöberg B, Hellquist E, Mallmin H, Lindh U (1997) Aluminum, Alzheimer’s disease and bone fragility. Acta Orthop Scand 68(6):511–514. https://doi.org/10.3109/17453679708999016
Zhu YZ, Liu DW, Liu ZY, Li YF (2013) Impact of aluminum exposure on the immune system: a mini review. Environ Toxicol Pharmacol 35(1):82–87. https://doi.org/10.1016/j.etap.2012.11.009
Mouro VGS, Menezes TP, Lima GDA, Domingues RR, Souza ACF, Oliveira JA, Matta SLP, Machado-Neves M (2017) How bad is aluminum exposure to reproductive parameters in rats? Biol Trace Elem Res 183(2):1–11. https://doi.org/10.1007/s12011-017-1139-3
Guo C, Chen P, Hsu GW, Wang C (2013) Zinc supplementation alters plasma aluminum and selenium status of patients undergoing dialysis: a pilot study. Nutrients 5:1456–1470. https://doi.org/10.3390/nu5041456
Chappard D, Mabilleau G, Moukoko D et al (2015) Aluminum and iron can be deposited in the calcified matrix of bone exostoses. J Inorg Biochem 152:6–11. https://doi.org/10.1016/j.jinorgbio.2015.09.008
Li X, Zhang L, Zhu Y, Li Y (2011b) Dynamic analysis of exposure to aluminum and an acidic condition on bone formation in young growing rats. Environ Toxicol Pharmacol 31(2):295–301. https://doi.org/10.1016/j.etap.2010.11.007
Kim YS, Lee MH, Wisniewski HM (1986) Aluminum induced reversible change in permeability of the blood-brain barrier to [14C]sucrose. Brain Res 377(2):286–291. https://doi.org/10.1016/0006-8993(86)90870-x
He BP, Strong MJ (2010) Motor neuronal death in sporadic amyotrophic lateral sclerosis (ALS) is not apoptotic. A comparative study of ALS and chronic aluminium chloride neurotoxicity in New Zealand white rabbits. Neuropathol Appl Neurobiol 26(2):150–160. https://doi.org/10.1046/j.1365-2990.2000.026002150.x
Yasui M, Yase Y, Ota K, Garruto RM (1991) Aluminum deposition in the central nervous system of patients with amyotrophic lateral sclerosis from the Kii Peninsula of Japan. Neurotoxicology 12(3):615–620
Altschuler E (1999) Aluminum-containing antacids as a cause of idiopathic Parkinson’s disease. Med Hypotheses 53(1):22–23. https://doi.org/10.1054/mehy.1997.0701
Giorgianni C, Faranda M, Brecciaroli R, Beninato G, Saffioti G, Muraca G et al (2003) Cognitive disorders among welders exposed to aluminum. G Ital Med Lav Ergon 25(Suppl(3)):102
Rifat SL, Eastwood MR, Mclachlan DRC, Corey PN (1990) Effect of exposure of miners to aluminium powder. Lancet 336(8724):1162–1165. https://doi.org/10.1016/0140-6736(90)92775-D
Mirza A, King A, Troakes C, Exley C (2017) Aluminium in brain tissue in familial Alzheimer’s disease. J Trace Elem Med Biol 40:30–36. https://doi.org/10.1016/j.jtemb.2016.12.001
Stuchlik A (2014) Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Front Behav Neurosci 8:106. https://doi.org/10.3389/fnbeh.2014.00106
Mcilwain DR, Thorsten B, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5(4):a008656. https://doi.org/10.1101/cshperspect.a008656
Kaur A, Gill KD (2010) Disruption of neuronal calcium homeostasis after chronic aluminium toxicity in rats. Basic Clin Pharmacol Toxicol 96(2):118–122. https://doi.org/10.1111/j.1742-7843.2005.pto960205.x
Song J, Liu Y, Zhang HF, Zhang QL, Niu Q (2014) Effects of exposure to aluminum on long-term potentiation and AMPA receptor subunits in rats in vivo. Biomed Environ Sci 27(2):77–84. https://doi.org/10.3967/bes2014.006
Zhang HF, Yang XJ, Qin XJ, Niu Q (2016) Caspase-3 is involved in aluminum-induced impairment of long-term potentiation in rats through the Akt/GSK-3β pathway. Neurotox Res 29(4):484–494. https://doi.org/10.1007/s12640-016-9597-5
Niu Q (2018) Overview of the relationship between aluminum exposure and health of human being. Adv Exp Med Biol 1091(Chapter 1):1–31. https://doi.org/10.1007/978-981-13-1370-7_1
Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41:154–166. https://doi.org/10.1016/j.neuro.2014.02.004
Platt B, Fiddler G, Riedel G, Henderson Z (2001) Aluminium toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res Bull 55(2):257–267. https://doi.org/10.1016/s0361-9230(01)00511-1
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Zhang Q, Li N, Jiao X, Qin X, Kaur R, Lu X et al (2014b) Caspase-3 short hairpin RNAs: a potential therapeutic agent in neurodegeneration of aluminum-exposed animal model. Curr Alzheimer Res 11(10):961–970. https://doi.org/10.2174/1567205011666141107150938
Yuan JY, Lipinski M, Degterev A (2003) Diversity in the mechanisms of neuronal cell death. Neuron 40(2):401–413. https://doi.org/10.1016/s0896-6273(03)00601-9
Cooke SF, Bliss TVP (2006) Plasticity in the human central nervous system. Brain 129(Pt 7):1659–1673. https://doi.org/10.1093/brain/awl082
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44(1):5–21. https://doi.org/10.1016/j.neuron.2004.09.012
Liang RF, Li WQ, Wang XH, Zhang HF, Wang H, Wang JX, Zhang Y, Wan MT, Pan BL, Niu Q (2012) Aluminium-maltolate-induced impairment of learning, memory and hippocampal long-term potentiation in rats. Ind Health 50(5):428–436. https://doi.org/10.2486/indhealth.MS1330
Gilman CP, Mattson MP (2002) Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromolecular Med 2(2):197–214. https://doi.org/10.1385/NMM:2:2:197
Zhang LF, Jin CH, Lu XB, Yang JH, Wu SW, Liu QF et al (2014a) Aluminium chloride impairs long-term memory and downregulates cAMP-PKA-CREB signalling in rats. Toxicology 323:95–108. https://doi.org/10.1016/j.tox.2014.06.011
Zou BD, Zhang ZD, Xiao HM, Li A (1998) Effect of aluminum on long-term potentiation and its relation to L-arg-no-pathway in hippocampal CA3 area of rats. J Tongji Med Univ 18(4):193–196. https://doi.org/10.1007/BF02886470
Mukherjee A, Williams DW (2017) More alive than dead: non-apoptotic roles for caspases in neuronal development, plasticity and disease. Cell Death Differ 24(8):1411–1421. https://doi.org/10.1038/cdd.2017.64
Funding
This work was supported by NSFC grant (No. 81430078).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were approved by the Bioethics Committee of China Institute for Radiation Protection and were performed in strict accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals (Publication No. 85–23; revised in 1996) and the UK Animals (Scientific Procedures) Act 1986.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, X., Li, L., Nie, X. et al. Effects of Chronic Aluminum Lactate Exposure on Neuronal Apoptosis and Hippocampal Synaptic Plasticity in Rats. Biol Trace Elem Res 197, 571–579 (2020). https://doi.org/10.1007/s12011-019-02007-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-02007-8