Abstract
Ectomycorrhizal fungi (EcMF) can mobilize mineral elements directly from insoluble mineral sources and accumulate various metallic elements and metalloids from soils to their fruiting bodies. Mushrooms from genus Boletus and its related genus are one of the most important EcMF which are consumed worldwide as wild edible mushrooms. Yunnan province (China) is a high biodiversity of genus Boletus mushrooms but is also an area with potential elevated contents of toxic elements in soil. Total contents of As, Ag, Ba, Cd, Co, Cr, Cs, Cu, Li, Mn, Ni, Pb, Rb, Sb, Sr, Tl, U, V, and Zn in three edible EcMF species collected from five sites of Yunnan were analyzed by inductively coupled plasma mass spectrometer. The highest contents for As, Cd, and Pb were 7.8 mg kg−1 dry weight (dw) in the caps of Butyriboletus roseoflavus, 3.4 mg kg−1 dw in the caps of B. roseoflavus, and 6.4 mg kg−1 dw in the stipes of Hemileccinum impolitum. Health risk assessment of As, Cd, and Pb indicated that the estimated exposure due to intakes of some mushroom samples from the sites were above the limits recommended by the Joint FAO/WHO Expert Committee on Food Additives. Since EcMF were considered as bioexclusors of Cr, higher Cr contents in the mushroom samples, compared with previous studies, indicated high geochemical background value of Cr in the sampling sites. Relatively higher V contents in mushrooms from family Boletaceae could also associate with the high V contents in Yunnan soil. Further work is needed to identify the places in Yunnan with geochemical anomalies resulting in high levels of toxic elements in EcMF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ectomycorrhizal fungi (EcMF) that have occurred since the Cretaceous are now associated with the plant species such as families Betulaceae, Fagaceae, and Pinaceae comprising the dominant trees in boreal and temperate forests [1]. They can mobilize mineral elements directly from insoluble mineral sources through excretion of organic acids [2]. Furthermore, many EcMF have variable and specific ability to take up various metallic elements and metalloids from soils and accumulate them in their fruiting bodies [3]. The trace element (such as Co, Cd, Cu, Pb, Mn, Ni, and Zn) contents in mushrooms were found to depend on the natural factors, environmental pollution, microbial communities, and the physiology of the mushroom species (particularly on its trophic pattern) [3,4,5]. Amongst EcMF, mushrooms from genus Boletus are one of the most important groups which are consumed worldwide as wild edible mushrooms [6]. Notably high contents of Ag and Au in Boletus edulis indicated its specific ability of taking up the trace elements from the soils with high geochemical background or contaminated by the metal ore smelters [7, 8].
Arsenic is a natural environmental contaminant that can be taken up and accumulated in mushrooms [9, 10]. Mleczek et al. [11] found that the As content was 450 mg/kg dw in Imleria badia (previously Boletus badius) collected from sludge deposits contaminated with As at level 490 mg/kg dw. Several Boletus mushroom species can be considered as an As accumulator, since the bioconcentration factor (the quotient of the As content in mushrooms to the As content in soil, calculated on dry weight basis) was above 1 in those species [12, 13]. Cadmium is one of the most detrimental elements of mushrooms and can be also found at elevated concentrations in wild-grown mushrooms [3, 14]. Lead toxicity is well known [15]. Lead content in edible wild mushrooms can be considered as indicator of environmental contamination [16].
Yunnan province of China has high biodiversity of mushrooms from family Boletaceae [17]. These species can be usually foraged from June to September. The toxic element contamination of these species in the region increased concerns due to elevated soil toxic element contents discovered in recent years [18]. For example, the mean soil As content in Yunnan is relatively very high (18–20 mg kg−1 dry weight, dw) compared with many other provinces in China [19]. The highest median content (120 mg kg−1 dw; 6.7–1500 mg kg−1 dw) of V in soils in China was also observed in Yunnan province [20]. Moreover, serious Cr slag contamination accident occurred in Qujing in Yunnan on August 13, 2011 [21]. Previous studies on wild growing mushrooms have shown that variations of elements between caps and stipes of Boletus fungi are mainly related to different bedrock soil geochemistry, enrichment capability for various elements as well as mushroom species [22,23,24]. To get a valid panorama and understand the elemental composition, contents and health risk assessment of elements in edible mushrooms from vast areas with polymetallic soils are sufficiently large database of species and results from different geological regions. Therefore, there is a need to investigate biodiverse mushrooms from polymetallic soils, especially using advanced and fully validated instrumental methodologies and the edible species that are lacking of reliable data on elemental composition.
The aims of this study were to (1) determine the contents of As, Cd, Pb, and other sixteen elements in three edible EcMF from Yunnan, (2) compare our data with those of other studies from Yunnan and European countries, and (3) evaluate the potential health risk of As, Cd, and Pb exposure of the studied mushrooms for the Chinese population.
Materials and Methods
Mushroom Samples
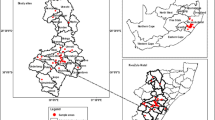
Hemileccinum impolitum (Fr.) Šutara (previously named ‘Boletus impolitus’) (20 specimens), Butyriboletus roseoflavus (M. Zang & H.B. Li) D. Arora & J.L. Frank (previously named ‘Boletus speciosus’) (30 specimens), and Boletus umbriniporus Hongo (24 specimens) from family Boletaceae were collected from five distantly distributed sites in Yunnan province, China in 2011 and 2012 (Fig. 1). Identification of those specimens was performed by taxonomic keys of Mao [25] and Wang [26] and was further confirmed by professional mycologists. Fungal nomenclature followed the Index Fungorum (www.indexfungorum.org).
Fresh mushrooms were cleaned to remove any visible plant and soil debris with a plastic knife, and the bottom part of the stipe was cut off. The fruiting bodies were then separated into caps and stipes and prepared and pooled respectively. Thereafter, the mushroom samples were placed into plastic trays of an electrically heated dehydrator (Ultra FD1000, Ezidri, Australia) and dried at 65 °C to constant mass. Dried mushrooms were pulverized in a porcelain mortar and finally kept in brand new sealed polyethylene bags under dry conditions.
Elemental Analysis
Directly before chemical analysis, the samples were kept at 65 °C for 12 h using an electrically heated laboratory oven before digestion. The subsamples of dried and powdered mushrooms (ca. 0.5 g) were digested with 5 mL of 65% HNO3 (Suprapure, Merck, Germany) under pressure in a microwave oven (Ethos One, Milestone Srl, Italy) [27]. The heating program was performed in one step: the power of the process was 1500 W, ramp time 20 min, temperature 200 °C, and hold time 30 min. Reagent blank solutions were prepared in the same way. Two blank samples were run for every set of 10 mushroom samples digested. The digest was diluted to 10 mL using deionized water (TKA Smart2Pure, Niederelbert, Germany) [28, 29].
Instrumentation
As, Ag, Ba, Cd, Co, Cr, Cs, Cu, Li, Mn, Ni, Pb, Rb, Sb, Sr, Tl, U, V, and Zn were determined using the ELAN DRC II ICP-MS inductively coupled plasma mass spectrometer (PerkinElmer, SCIEX, Canada). The spectrometer was equipped with a Meinhard concentric nebulizer, cyclonic spray chamber, dynamic reaction cell, Pt cones, and a quadruple mass analyzer. DRC was employed to remove spectral interferences. Typical spectrometer operating conditions for the ICP-MS spectrometer were as follows: RF power, 1100 W; plasma Ar flow rate, 15 L min−1; nebulizer Ar flow rate, 0.87 L min−1; and auxiliary Ar flow rate, 1.2 L min−1 and lens voltage, (7.5–9.0) V. The performance of the analytical method applied was under rigorous control using the quality control/quality assurance protocol as was described in detail in previous reports [28, 29]. Certified reference materials SRM NIST 1570a Spinach Leaves (National Institute of Standards and Technology, Department of Commerce, USA), CRM CS-M-4 Boletus edulis mushroom powder (Institute of Nuclear Chemistry and Technology, Poland), and CRM CS-M-4 Leccinum scabrum mushroom powder (Institute of Nuclear Chemistry and Technology, Poland) were used to verify the element determinations.
Health Risk Assessment of Arsenic, Cadmium, and Lead
In Yunnan, wild edible mushrooms belonging to family Boletaceae can be foraged from June to September and eaten by the population for at least one time each week during the mushroom fruiting season. The estimated As, Cd, and Pb intakes were calculated by consumption from a single meal containing 300-g fresh mushrooms (ca. 30-g dry mushrooms) for a 60-kg body weight individual. We compared the estimated As intake with the inorganic arsenic lower limit on the benchmark dose for a 0.5% increased incidence of lung cancer (BMDL0.5), which was 3.0 μg kg−1 bw per day [30]. The dietary exposure of Pb corresponding to an increase in systolic blood pressure of 1 mmHg (0.1333 kPa) was estimated to be 1.2 μg/kg bw per day [31]. The corresponding dietary exposure for Cd is 0.8 μg/kg bw per day which would result in a urinary Cd content at the breakpoint of 5.24 μg of Cd per gram creatinine [31].
Results and Discussions
Arsenic
The range of As contents was 0.88–1.2 mg kg−1 dw in H. impolitum, 0.24–7.8 mg kg−1 dw in Butyriboletus roseoflavus, and 0.61–1.3 mg kg−1 dw in Boletus umbriniporus (Table 1). The highest As contents (7.8 mg kg−1 dw in the caps and 4.5 mg kg−1 dw in the stipes) were found in Butyriboletus roseoflavus from Yuanmou County, Yunnan.
In previous studies, the As contents in composite samples for different Boletus species from different locations in Yunnan were at the range of 0.090–53 mg kg−1 dw [22, 29, 32, 33]. Elevated As contents have been found in other Boletus mushrooms, such as B. edulis (7.0 mg kg−1 dw) and B. bicolor (5.6 mg kg−1 dw) [34, 35]. However, low As contents can be also found in B. tomentipes (0.10–0.24 mg kg−1 dw) and Boletus sp. (0.26 mg kg−1 dw) from Yunnan [32, 36]. Moreover, relatively low As contents were also detected in Boletus spp. mushrooms from Italy (0.10–0.41 mg kg−1 dw) and Serbia (0.32–1.66 mg kg−1 dw) [37, 38]. Considering intense mining activities in Yunnan [39], further work is needed to understand whether the elevated As contents in Boletus mushrooms is due to the high As content in soil or the bioaccumulation process.
In this study, the estimated As intakes from most of the samples were below the limit recommended by JECFA (Table 1). The only exception was the caps of Butyriboletus roseoflavus from Yuanmou County, in which the estimated As intake was 3.9 μg kg−1 bw (Table 1). If the As in B. roseoflavus from this site is dominated by the inorganic species, it should not be eaten. Unfortunately, as far as we know, few studies have been carried out on As speciation in Boletaceae mushrooms [40, 41]. Chen et al. [40] reported that As (V) (0.20 mg kg−1 dw) was the dominant As species in a sample named as “yellow bolete,” while monomethylarsonic acid (200 mg kg−1 dw) was accounted for 94% of the total arsenic in a sample named as “black bolete.” Komorowicz et al. [41] recently found in some bolete species from Yunnan the arsenobetaine, dimethylarsenic acid, As(III), monomethylarsenic acid, and As(V) at very broad range of contents. Braeuer et al. [42] found that no inorganic arsenic was detected in Cyanoboletus pulverulentus (family Boletaceae) mushroom samples from the European countries and the USA, and the arsenic speciation in the samples consisted solely of dimethylarsinic acid which is a probable human carcinogen [43].
Cadmium
Cadmium content was 0.22–3.4 mg kg−1 dw in the caps and 0.18–1.4 mg kg−1 dw in the stipes (Table 2). In previous studies, nd.− 19 mg kg−1 dw in caps and 0.24–12 mg kg−1 dw in stipes of Boletus spp. from Yunnan [29, 44]. Other studies on the whole fruiting bodies of Boletus spp. from Yunnan showed Cd content was 0.16–1.1 mg kg−1 dw [32, 45]. In European countries, 0.46–15.6 mg kg−1 dw in Boletus spp. [37, 38, 46,47,48]. However, up to 52 mg kg−1 dw Cd was detected in the cap of a specimen of B. edulis from Poland [49], and 120 ± 65 mg kg−1 dw) in specimens (n = 24) grew in area impacted by zinc smelter [50]. In this study, the estimated Cd intake was 1.7 μg/kg bw in the caps of Butyriboletus roseoflavus from Qilin, 1.1 μg/kg bw in the caps of Butyriboletus roseoflavus from Yuanmou, and 1.0 μg/kg bw in the caps of Boletus umbriniporus from Longyang. These values were above the limit recommended by JECFA.
Lead
When sampling from the same area, EcMF presented lower Pb levels than saprophite species [16]. In this study, Pb content was 0.56–1.5 mg kg−1 dw in the caps and 0.78–6.4 mg kg−1 dw in the stipes (Table 3). The results were in agreement with previous studies in Boletus spp. from Yunnan (0.16–2.9 mg kg−1 dw in caps and 0.15–3.2 mg kg−1 dw in stipes) [29, 44]. For the whole fruiting bodies of Boletus spp. from Yunnan, the reported value was 1.4–6.2 mg kg−1 dw [32, 36, 45]. In European countries, Pb content was 0.22–1.5 in fruiting bodies of most studied Boletus spp. [37, 38, 48, 51, 52], but 11 mg kg−1 Pb was found in one sample of B. edulis from Serbia [38]. For B. edulis in Poland, the value was 0.18–3.6 mg kg−1 dw in caps and 0.35–1.7 mg kg−1 dw in stipes [49]. In terms of health risk assessment, our data showed that the stipes of H. impolitum from Wuhua (3.2 μg/kg bw) and Yimen (1.9 μg/kg bw) might cause excessive intake of Pb.
Silver
In general, the Ag contents in saprobic fungi are higher than those in EcMF, but Boletus mushrooms can be considered as one of the most efficient Ag accumulators [8]. 242 mg kg−1 dw Ag has been detected in B. edulis in the polluted area in Czech Republic [8]. In Imleria badia from smelter-polluted area, Ag content can be up to 385 mg kg−1 dw [53]. In pristine area of European continent, Ag contents in Boletus spp. were in the range of 0.41–41 mg kg−1 dw [38, 49, 51, 54, 55]. In this study, 0.63–5.0 mg kg−1 dw in caps and 0.13–1.2 mg kg−1 dw in stipes were found in the studied fungal species (Table 4), which were relatively similar to a previous study (1.2–4.7 mg kg−1 dw in caps and 0.23–11 mg kg−1 dw in stipes) on Boletus mushrooms from Yunnan [29].
Barium
Barium content was 0.06–2.9 mg kg−1 dw in caps and 0.13–2.1 mg kg−1 dw in stipes of B. edulis in Poland [49, 56,57,58], while it was 0.07–0.92 mg kg−1 dw in fruiting bodies of B. edulis from Croatia [58]. Higher levels of Ba (1.3–32 mg kg−1 dw in caps and 1.2–100 mg kg−1 dw) in stipes have been identified in Boletus spp. from Yunnan [29]. Our Ba data were in the range of those reported from Yunnan (Table 4).
Cobalt
Cobalt content was 0.28–2.5 mg kg−1 dw in the caps and 0.58–2.6 mg kg−1 dw in the stipes (Table 4). The range of the values was slightly higher than the Co content (1.0–1.7 mg kg−1 dw) found in B. griseus and Butyriboletus roseoflavus from Yunnan [59]. However, 5.2 mg kg−1 Co was detected in the stipes of B. tomentipes from Panzhihua, Sichuan province [29]. In Europe, Co content was 0.01–2.2 in Boletus spp. from different countries [38, 49, 60].
Chromium
Mushrooms were considered as bioexclusors of Cr and Cr content in EcMF usually lower than that in saprophytic fungi [61]. Previous works showed that Cr content was 0.060–0.60 mg kg−1 dw in Boletus spp. in Europe [38, 49], whereas elevated Cr contents (0.45–15 mg kg−1 dw) were detected in Boletus spp. from Yunnan [29, 32, 59]. In this work, the highest Cr content was 24 mg kg−1 dw in the caps of Butyriboletus roseoflavus from Yuanmou county in Yunnan and 16 mg kg−1 dw in the stipes of Boletus umbriniporus from Yimen county in Yunnan (Table 4). It indicated high geochemical background value of Cr in these locations.
Cesium
Cesium content was 0.81–32 mg kg−1 dw in the caps and 0.46–9.5 mg kg−1 dw in the stipes (Table 4), which was similar or relatively lower than those (0.24–160 mg kg−1 dw in caps and 0.29–38 mg kg−1 dw in stipes) in previous studies on Boletus spp. [29, 49].
Copper
Copper contents in caps of Boletaceae species were higher than those in stipes [62]. Copper content was 6.9–110 mg kg−1 dw in caps, stipes, or whole fruiting bodies of Boletus spp. from Yunnan or Europe [22, 29, 32, 38, 46, 49, 59]. Our results (18–77 mg kg−1 dw in the caps and 8.3–57 mg kg−1 dw in the stipes) were in the range of the data reported from literature (Table 4).
Lithium
In Hungary, Li content was nd. to 0.61 mg kg−1 dw in 38 common, edible wild grown mushrooms [63]. For Boletus spp. in Yunnan, Li content was 0.072–8.7 mg kg−1 dw in caps and 0.06–4.1 mg kg−1 dw in stipes [23, 29]. In this study, relatively low level of Li was found in the caps (0.18–0.38 mg kg−1 dw) and in the stipes (0.13–1.3 mg kg−1 dw) (Table 4).
Manganese
Manganese content was 2.0–118 in Boletus spp. from Europe and China and the stipes accumulated more Mn than the caps [29, 32, 38, 46, 49, 59, 64, 65]. In this study, Mn content was 8.8–22 mg kg−1 dw in the caps and 9.3–38 mg kg−1 dw in the stipes (Table 4).
Nickel
Nickel content was 0.10–1.2 mg kg−1 dw in Boletus spp. from Europe [38, 66]. Elevated Ni content was 0.39–10 mg kg−1 dw in caps and 0.28–11 mg kg−1 dw in stipes in Boletus spp. from Yunnan in previous work [29, 32]. In this study, Ni content was 0.81–7.3 mg kg−1 dw in the caps and 0.86–5.2 mg kg−1 dw in the stipes (Table 4).
Rubidium
Fungal species from family Boletaceae are considered as the Rb accumulators [67]. In this study, Rb content was 95–950 mg kg−1 dw in the caps and 50–430 mg kg−1 dw in the stipes (Table 4). Similar results (65–850 mg kg−1 dw in caps and 50–300 mg kg−1 dw in stipes) were obtained from other Boletus species in Yunnan [29]. In Europe, Rb content was 24–1000 mg kg−1 dw in caps, stipes, or whole fruiting bodies of Boletus spp. [38, 49].
Antimony
In general, Sb contents in EcMF from clean areas were mostly below 0.1 mg kg−1 dw [49, 68]. In this study, Sb content was 0.017–0.11 mg kg−1 dw in the caps and < 0.001–0.11 mg kg−1 dw in the stipes (Table 4), which indicated that the sampling sites did not contain high level of Sb. However, in another study, Sb content was 0.34 mg kg−1 dw in stipes of B. luridus from Mindu county in Yunnan [29].
Strontium
Strontium content was 0.44–1.6 mg kg−1 dw in the caps and 0.66–1.7 mg kg−1 dw in the stipes (Table 4). Higher Sr contents (0.24–5.3 mg kg−1 dw in caps and 0.21–26 mg kg−1 dw) in stipes were reported from other Boletus species from Yunnan [29]. In Europe, Sr content was 0.050–2.1 mg kg−1 dw in caps, stipes, or whole fruiting bodies of Boletus spp. [38, 49, 57].
Thallium
Thallium content was 0.0092–0.063 mg kg−1 dw in the caps and 0.027–0.30 mg kg−1 dw in the stipes (Table 4), which was similar to the study by Falandysz et al. (0.010–0.095 mg kg−1 dw in caps and 0.0070–0.27 mg kg−1 dw in stipes) [29]. Compared with those data from Yunnan, B. edulis from European countries had a wider range of Tl content (nd. − 0.74 mg kg−1 dw in caps, stipes, or whole fruiting bodies) [49, 56, 69, 70].
Uranium
EcMF can interact with, and transform certain U species [71], but EcMF from unpolluted areas usually did not accumulate high levels of U [51]. In this study, U content was 0.017–0.052 mg kg−1 dw in the caps and 0.023–0.24 mg kg−1 dw in the stipes (Table 4). Similar results were reported in previous work (0.014–0.29 mg kg−1 dw in caps and 0.011–0.21 mg kg−1 dw in stipes of Boletus spp.) [29, 49].
Vanadium
Vanadium content was 0.76–2.5 mg kg−1 dw in the caps and 0.60–8.7 mg kg−1 dw in the stipes of the studied mushroom species (Table 4). Similar results (0.18–4.4 mg kg−1 dw in caps and 0.24–10 mg kg−1 dw in stipes) were obtained by Falandysz et al. [29]. However, much lower V contents (0.004–0.61 mg kg−1 dw) have been reported in Boletus spp. from Poland and Czech Republic [49, 70].
Zinc
Zinc in Boletus spp. was generally accumulated more in the caps than in stipes [3]. Our results confirmed this (99–290 mg kg−1 dw in the caps and 43–120 mg kg−1 dw in the stipes) (Table 4). In Yunnan, Zn in Boletus spp. was found 14–240 in caps and 33–140 in stipes [29, 65]. For whole fruiting bodies of Boletus spp., Zn content was 19–97 mg kg−1 dw [32, 45, 59]. Slightly higher Zn contents (76–360 mg kg−1 dw in caps, 24–210 mg kg−1 dw in the stipes, and 65–350 mg kg−1 dw in whole fruiting bodies) have been found in Boletus spp. from Europe [38, 46, 49, 64].
Conclusion
Three EcMF species, H. impolitum, Butyriboletus roseoflavus, and Boletus umbriniporus, collected from different sites in Yunnan are relatively rich in numerous mineral constituents. The estimated As intake from consuming the sample of Butyriboletus roseoflavus from Yuanmou was above the limit recommended by JECFA, if the As in this sample is dominated by the inorganic As species. However, form under which As was present remains unknown. The estimated Cd intakes from some caps of Butyriboletus roseoflavus and Boletus umbriniporus were above the Cd limit. The stipes of H. impolitum might cause excessive intake of Pb. Relatively high contents of Cr and V in some samples indicated high geochemical background levels of the two elements in the sampling sites. The places in Yunnan with geochemical anomalies resulting in high-level toxic elements in mushrooms from family Boletaceae need to be further identified.
References
Taylor LL, Leake JR, Quirk J, Hardy K, Banwart SA, Beerling DJ (2009) Biological weathering and the long-term carbon cycle: integrating mycorrhizal evolution and function into the current paradigm. Geobiology 7:171–191
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breemen N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Falandysz J, Borovička J (2013) Macro and trace mineral constituents and radionuclides in mushrooms: health benefits and risks. Appl Microbiol Biotechnol 97:477–501
Jing XB, He N, Zhang Y, Cao YR, Xu H (2011) Isolation and characterization of heavy-metal-mobilizing bacteria from contaminated soils and their potential in promoting Pb, Cu, and Cd accumulation by Coprinus comatus. Can J Microbiol 58:45–53
Lepšová A, Mejstřík V (1988) Accumulation of trace elements in the fruiting bodies of macrofungi in the Krušné hory mountains, Czechoslovakia. Sci Total Environ 76:117–128
Cui YY, Feng B, Wu G, Xu J, Yang ZL (2016) Porcini mushrooms (Boletus sect. Boletus) from China. Fungal Divers 81:189–212
Borovička J, Dunn CE, Gryndler M, Mihaljevič M, Jelínek E, Rohovec J, Rohošková M, Řanda Z (2010a) Bioaccumulation of gold in macrofungi and ectomycorrhizae from the vicinity of the Mokrsko gold deposit, Czech Republic. Soil Biol Biochem 42:83–91
Borovička J, Kotrba P, Gryndler M, Mihaljevič M, Řanda Z, Rohovec J, Cajthaml T, Stijve T, Dunn CE (2010b) Bioaccumulation of silver in ectomycorrhizal and saprobic macrofungi from pristine and polluted areas. Sci Total Environ 408:2733–2744
Falandysz J, Rizal LM (2016) Arsenic and its compounds in mushrooms: a review. J Environ Sci Health C 34:217–232
Mleczek M, Niedzielski P, Siwulski M, Rzymski P, Gąsecka M, Goliński P, Kozak L, Kozubik T (2016b) Importance of low substrate arsenic content in mushroom cultivation and safety of final food product. Eur Food Res Technol 242:355–362
Mleczek M, Niedzielski P, Rzymski P, Siwulski M, Gąsecka M, Kozak L (2016a) Variations of arsenic species content in edible Boletus badius growing at polluted sites over four years. J Environ Sci Health B 51:469–476
Liu B, Huang Q, Cai H, Guo X, Wang T, Gui M (2015) Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem 188:294–300
Liu Y, Chen D, You Y, Zeng S, Li Y, Tang Q, Han G, Liu A, Feng C, Li C, Su Y, Su Z, Chen D (2016) Nutritional composition of Boletus mushrooms from Southwest China and their antihyperglycemic and antioxidant activities. Food Chem 211:83–91
Kalač P (2010) Trace element contents in European species of wild growing edible mushrooms: a review for the period 2000–2009. Food Chem 122:2–15
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Garcia MA, Alonso J, Fernández MI, Melgar MJ (1998) Lead content in edible wild mushrooms in northwest Spain as indicator of environmental contamination. Arch Environ Contam Toxicol 34:330–335
Wu G, Li YC, Zhu XT, Zhao K, Han LH, Cui YY, Li F, Xu JP, Yang ZL (2016) One hundred noteworthy boletes from China. Fungal Divers 81:25–188
Falandysz J, Zhang J, Wang YZ, Saba M, Krasińska G, Wiejak A, Li T (2015) Evaluation of mercury contamination in fungi boletus species from latosols, lateritic red earths, and red and yellow earths in the circum-Pacific mercuriferous belt of southwestern China. PLoS One 10:e0143608
Weng H, Liu Y, Chen H (1997) Environmental geochemical features of arsenic in soil in China. J Environ Sci 9:385–395
Yang J, Teng Y, Wu J, Chen H, Wang G, Song L, Yue W, Zuo R, Zhai Y (2017) Current status and associated human health risk of vanadium in soil in China. Chemosphere 171:635–643
Gao Y, Xia J (2011) Chromium contamination accident in China: viewing environment policy of China. Environ Sci Technol 45:8605–8606
Wang XM, Zhang J, Li T, Li JQ, Wang YZ, Liu HG (2015) ICP-AES determination of mineral content in Boletus tomentipes collected from different sites of China. Spectrosc Spectr Anal 35:1398–1403
Wang XM, Zhang J, Li T, Li JQ, Wang YZ, Liu HG (2015) Variations in element levels accumulated in different parts of Boletus edulis collected from Central Yunnan Province, China. J Chem 2015:372152
Wang XM, Liu HG, Zhang J, Li T, Wang YZ (2017) Evaluation of heavy metal concentrations of edible wild-grown mushrooms from China. J Environ Sci Heal B 52:178–183
Mao XL (2000) The macrofungi of China. Henan Science and Technology Press, Zhengzhou
Wang XH, Liu PG, Yu FQ (2004) Color atlas of wild commercial mushrooms in Yunnan. Yunnan Science and Technology Press, Kunming
Mędyk M, Chudzińska M, Barałkiewicz D, Falandysz J (2017) Specific accumulation of cadmium and other trace elements in Sarcodon imbricatus using ICP-MS with a chemometric approach. J Environ Sci Health B 52:361–366
Falandysz J, Chudzińska M, Barałkiewicz D, Saba M, Wang Y, Zhang J (2017) Occurrence, variability and associations of trace metallic elements and arsenic in sclerotia of medicinal Wolfiporia extensa from polymetallic soils in Yunnan, China. Acta Poloniae Pharmaceutica, Drug Res 74:1379–1387
Falandysz J, Zhang J, Wiejak A, Barałkiewicz D, Hanć A (2017b) Metallic elements and metalloids in Boletus luridus, B. magnificus and B. tomentipes mushrooms from polymetallic soils from SW China. Ecotox Environ Safe 142:497–502
JECFA (2010a) Joint FAO/WHO Expert Committee on Food Additives seventy-second meeting Rome, 16–25 February 2010, Food and Agriculture Organization of the United Nations World Health Organization
JECFA (2010b) Joint FAO/WHO eExpert Committee on Food Additives seventy-third meeting Geneva, 8–17 June 2010 Food and Agriculture Organization of the United Nations World Health Organization
Li T, Wang Y, Zhang J, Zhao Y, Liu H (2011) Trace element content of Boletus tomentipes mushroom collected from Yunnan, China. Food Chem 127:1828–1830
Zhang J, Liu H, Li SJ, Li JQ, Wang Y, Li T (2015) Arsenic in edible and medicinal mushrooms from Southwest China. Int J Med Mushrooms 17:601–605
Xing B, Zhang J, Li J, Wang Y, Liu H (2016) Determination of mineral elements contents in eight wild Boletus species from Yunnan by ICP-MS. Food Sci 37:89–94 (in Chinese with English abstract)
Yang TW, Zhang J, Liu HG, Wang YZ (2016) Determination and food safety assessment of arsenic in wild-grown bolete mushrooms from Yunnan province. Asian J Ecotoxicol 11:755–761 (in Chinese with English abstract)
Zheng GQ, Wang L, Li J (2014) Assessment of lead, arsenic, and mercury in wild-grown mushrooms from Baoshan, Yunnan. Strait J Prev Med 20:58–59 (in Chinese)
Cocchi L, Vescovi L, Petrini LE, Petrini O (2006) Heavy metals in edible mushrooms in Italy. Food Chem 98:277–284
Dimitrijevic MV, Mitic VD, Cvetkovic JS, Jovanovic VPS, Mutic JJ, Mandic SDN (2016) Update on element content profiles in eleven wild edible mushrooms from family Boletaceae. Eur Food Res Technol 242:1–10
Xiao XY, Chen TB, Liao XY, Wu B, Yan XL, Zhai LM, Xie H, Wang LX (2008) Regional distribution of arsenic contained minerals and arsenic pollution in China. Geogr Res 27:201–212
Chen S, Guo Q, Liu L (2017) Determination of arsenic species in edible mushrooms by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry. Food Anal Methods 10:740–748
Komorowicz I, Hanć A, Lorenc W, Barałkiewicz D, Falandysz J, Wang Y (2019) Arsenic speciation in mushrooms using dimensional chromatography coupled to ICP-MS detector. Chemosphere 233:223–233
Braeuer S, Goessler W, Kameník J, Konvalinková T, Žigová A, Borovička J (2018) Arsenic hyperaccumulation and speciation in the edible ink stain bolete (Cyanoboletus pulverulentus). Food Chem 242:225–231
Kenyon EM, Hughes MF (2001) A concise review of the toxicity and carcinogenicity of dimethylarsinic acid. Toxicology 160:227–236
Ma P, Zhang D, Yang LB, Zeng XD (2012) Bioaccumulation of heavy metal in wild edible Boletus fruiting body. Environ Sci Technol 35:5–8 (in Chinese with English abstract)
Zhao B (2007) Study on the safety evaluation and control methods of four species of Boletus. Master’s Degree Thesis. Southwest University, Chongqing. (in Chinese with English abstract)
Blanuša M, Kučak A, Varnai VM, Sarić MM (2001) Uptake of cadmium, copper, iron, manganese, and zinc in mushrooms (Boletaceae) from Croatian forest soil. J AOAC Int 84:1964–1971
Vetter J (1994) Data on arsenic and cadmium contents of some common mushrooms. Toxicon 32:11–15
Širić I, Kasap A, Bedeković D, Falandysz J (2017) Lead, cadmium and mercury contents and bioaccumulation potential of wild edible saprophytic and ectomycorrhizal mushrooms, Croatia. J Environ Sci Health B 52:156–165
Falandysz J, Kunito T, Kubota R, Bielawski L, Frankowska A, Falandysz JJ, Tanabe S (2008) Multivariate characterization of elements accumulated in King Bolete Boletus edulis mushroom at lowland and high mountain regions. J Environ Sci Health A 43:1692–1699
Collin-Hansen C, Andersen RA, Steinnes E (2005) Molecular defense systems are expressed in the king bolete (Boletus edulis) growing near metal smelters. Mycologia 97:973–983
Borovička J, Kubrová J, Rohovec J, Řanda Z, Dunn CE (2011) Uranium, thorium and rare earth elements in macrofungi: what are the genuine concentrations? Biometals 24:837–845
García MÁ, Alonso J, Melgar MJ (2009) Lead in edible mushrooms: levels and bioaccumulation factors. J Hazard Mater 167:777–783
Cejpková J, Gryndler M, Hršelová H, Kotrba P, Řanda Z, Synková I, Borovička J (2016) Bioaccumulation of heavy metals, metalloids, and chlorine in ectomycorrhizae from smelter-polluted area. Environ Pollut 218:176–185
Byrne AR, Dermelj M, Vakselj T (1979) Silver accumulation by fungi. Chemosphere 8:815–821
Falandysz J, Bona H, Danisiewicz D (1994) Silver content of wild-grown mushrooms from Northern Poland. Z Lebensm Unters Forsch 199:222–224
Falandysz J, Frankowska A, Jarzyńska G, Dryżałowska A, Kojta AK, Zhang D (2011) Survey on composition and bioconcentration potential of 12 metallic elements in King Bolete (Boletus edulis) mushroom that emerged at 11 spatially distant sites. J Environ Sci Health B 46:231–246
Frankowska A, Ziółkowska J, Bielawski L, Falandysz J (2010) Profile and bioconcentration of minerals by King Bolete (Boletus edulis) from the Płocka Dale in Poland. Food Addit Contam B 3:1–6
Zhang D, Frankowska A, Jarzyńska G, Kojta AK, Drewnowska M, Wydmańska D, Bielawski L, Wang J, Falandysz J (2010) Metals of King Bolete (Boletus edulis) Bull.: Fr. collected at the same site over two years. Afr J Agric Res 5:3050–3055
Liu H, Zhang J, Li T, Shi Y, Wang Y (2012) Mineral element levels in wild edible mushrooms from Yunnan, China. Biol Trace Elem Res 147:341–345
Borovička J, Řanda Z (2007) Distribution of iron, cobalt, zinc and selenium in macrofungi. Mycol Prog 6:249–259
Garcia MA, Alonso J, Melgar MJ (2013) Bioconcentration of chromium in edible mushrooms: influence of environmental and genetic factors. Food Chem Toxicol 58:249–254
Svoboda L, Zimmermannová K, Kalač P (2000) Concentrations of mercury, cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area of a copper smelter and a mercury smelter. Sci Total Environ 246:61–67
Vetter J (2005) Lithium content of some common edible wild-growing mushrooms. Food Chem 90:31–37
Kojta AK, Falandysz J (2016) Metallic elements (Ca, Hg, Fe, K, Mg, Mn, Na, Zn) in the fruiting bodies of Boletus badius. Food Chem 200:206–214
Wang XM, Zhang J, Li T, Wang YZ, Liu HG (2015c) Content and bioaccumulation of nine mineral elements in ten mushroom species of the genus Boletus. J Anal Methods Chem 2015:165412
Reczyński W, Muszyńska B, Opoka W, Smalec A, Sułkowska-Ziaja K, Malec M (2013) Comparative study of metals accumulation in cultured in vitro mycelium and naturally grown fruiting bodies of Boletus badius and Cantharellus cibarius. Biol Trace Elem Res 153:355–362
Kalač P (2013) A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J Sci Food Agric 93:209–218
Borovička J, Řanda Z, Jelínek E (2006) Antimony content of macrofungi from clean and polluted areas. Chemosphere 64:1837–1844
Širić I, Žurga P, Barkić D, Malenica Staver M (2016) Trace element contents in the edible mushroom Boletus edulis Bull. ex Fries. Agric Conspec Sci 80:223–227
Svoboda L, Chrastný V (2008) Levels of eight trace elements in edible mushrooms from a rural area. Food Addit Contam 25:51–58
Gadd GM, Fomina M (2011) Uranium and fungi. Geomicrobiol J 28:471–482
Funding
This work was financially supported by the National Natural Science Foundation of China (grant number 31660591).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Barałkiewicz, D., Hanć, A. et al. Contents and Health Risk Assessment of Elements in Three Edible Ectomycorrhizal Fungi (Boletaceae) from Polymetallic Soils in Yunnan Province, SW China. Biol Trace Elem Res 195, 250–259 (2020). https://doi.org/10.1007/s12011-019-01843-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01843-y