Abstract
Lead and di-2-ethylhexyl phthalate (DEHP) are widely distributed in the environment, and their neurotoxicity has caused a widespread concern. The complexity of environmental exposure provides the possibility of their combined exposure. The present study aims to describe a joint neurotoxicity and clarify the potential mechanism after combined exposure to lead and DEHP. A 2 × 3 factorial design was used to analyze either single effects or their interaction by a subchronic lead and DEHP exposure model of the male weaning rats. Similar to the previous study, lead or DEHP single exposure showed an increased neurotoxicity. Interestingly, our neurobehavioral test showed the rats in the combined exposure groups had a better ability of learning and memory compared with the single-exposure ones. It seemed to reflect an antagonism joint action in neurotoxicity after combined exposure. The content of dehydroepiandrosterone (DHEA) in serum and the mRNA level of brain-derived neurotrophic factor (Bdnf) in the hippocampus showed a similar trend to the ability of learning and memory. However, there was insufficient evidence to support the joint action on some indexes of oxidative stress such as malondialdehyde (MDA), the ratio of reduced glutathione(GSH) to oxidized glutathione(GSSG), γglutamylcysteine synthetase (γ-GCS), glutathione-s transferase (GST), and nuclear factor E2-related factor 2 (Nrf2) mRNA expression in the hippocampus. In a word, our current study reminded a unique antagonism joint action of neurotoxicity after combined exposure to lead and DEHP, which may contribute to understanding some shallow mechanism of the joint toxicity due to the complexity of environmental pollutant exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead and its compounds widely exist in the environment, absorbed through the digestive tract and respiratory tract. The amount of lead consumption worldwide is about 4 million tons per year, only 1/4 of which is recycled. Food contamination and atmospheric pollution are the primary exposure sources of lead [1]. Irrigation of farmland with the sewage discharged from factories can increase lead content in grains, vegetables, and aquatic products. In our daily life, it is universal exposure to lead-containing toys, school supplies, paints, cosmetics, solders, glazes, etc [2, 3].
The red blood cell, liver, kidneys, and brain have been considered as the main target organs of chronic lead exposure [4,5,6]. It has long been recognized to cause neurological alterations in children. Lead gets into the brain tissue through the immature blood-brain barrier, interfering with the function of cells. Animal experiments show that lead exposure can bring about the retardation of the development of the central nervous system in offspring rats and disturb the ability of learning and memory [7]. Epidemiological surveys remind that lead exposure can affect infants’ neurobehavioral and intellectual development [8, 9]. Extensive studies on the mechanism of lead neurotoxicity suggest that lead can induce the increase of oxygen free radicals and produce oxidative damage [10]; competitively antagonize Ca2+, simulating the entry of Ca2+ channels into the cells and destroying the blood-brain barrier [11]; and interact with N-methyl-D-aspartate receptors (NMDARs), interfering with the inhibition of long-term potentiation (LTP) induction and impairing hippocampal memory formation [12].
Di-2-ethylhexyl phthalate (DEHP), the most common member of the class of phthalates, is the main plasticizer and its global output can reach 3 ~ 4 million tons [13]. Phthalates are used in the manufacture of rubber clogs, rubber boots, soap packaging, products made from polyvinyl chloride (PVC), bath mats, and soft toys [14]. As we have known, DEHP, as one of the most common pollutants, cannot polymerize to the carbon chain of the PVC polymer and is continuously released from the plastic film with the passage of time [15]. DEHP in the air, soil, and water can enrich in vegetables, algae, fish, and finally cause damage to multiple organisms, which shows reproductive toxicity, liver and kidney toxicity, and carcinogenicity [16,17,18]. As DEHP contamination gradually increases in the environment, its risk assessment and safety monitoring caused a widespread concern [14].
DEHP neurotoxicity is widely recognized, especially the effects on learning and memory. Epidemiological surveys show that DEHP metabolite mono-2-ethylhexyl phthalate (MEHP) concentration in urine is related to neurobehavioral impairment [19]; PAEs metabolite content in the urine of school-age children is inversely proportional to their intelligence quotient (IQ) [20], and positively correlates with attention deficit hyperactivity disorder [21]. Animal studies also have demonstrated that DEHP could change neurosteroid level and affect the development and cognitive function of the nervous system by modulatingγ-aminobutyric acid (GABA) receptors and NMDARs [22, 23]; DEHP could cause oxidative damage to the neuronal membranes, interfering with nerve signal transmission and affecting learning and memory functions [24]. However, some related mechanism is uncertain, and further exploration is also necessary.
There is an increasing variety of pollutants in the environment with the development of industry and agriculture. Although the toxic effect of a single chemical has been explored in depth, it is more necessary and critical to evaluate the impact on living organisms and clarify the potential mechanism of the combined exposure. As we all know, the joint action of chemicals mainly includes enhancement, additive effect, independent effect, and antagonism (inhibition). Lead is an old poison, while DEHP is a modern plasticizer. However, the neurotoxicity of their combined exposure has not been reported, and how to evaluate their joint action is not yet fully understood.
The present study aims to describe the joint neurotoxicity and clarify the possible mechanism of combined exposure of lead and DEHP. Therefore, we performed a 2 × 3 factorial design to establish a subchronic model of male weaning rats after lead and DEHP exposure. Firstly, a Morris water maze test was used to assess neurobehavioral impairment, especially the ability of learning and memory. Furthermore, we measured the neurosteroid content in serum, the level of malondialdehyde (MDA), reduced glutathione(GSH), oxidized glutathione(GSSG), the activity of glutathione-s transferase (GST), and γglutamylcysteine synthetase (γ-GCS) in the hippocampus. In addition, the mRNA level of the brain-derived neurotrophic factor (Bdnf), cAMP-response element-binding protein (Creb), and nuclear factor E2-related factor 2 (Nrf2) in the hippocampus was also measured to clarify possible neurotoxic mechanisms of their combined exposure. Hopefully, our data will contribute to providing some scientific clues for the safety assessment and risk evaluation of combined exposure to lead and DEHP in the environment.
Materials and Methods
Animal Grouping and Drug Administration
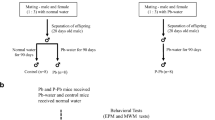
Sixty male Wistar rats (just weaning, 80-100 g), purchased from the Experimental Animal Centre of China Medical University (SPF grade, license number SYXK-2013-0001), were housed in a standard laboratory environment (temperature, 22 ± 1 °C; humidity, 55 ± 5%). Experiments and surgical procedures were conducted in conformity with the Animal Care and Use Guidelines of China Medical University, which confirmed to the National Institute of Health Guide for the Care and Use of Laboratory Animals. After being observed for 1 week, rats were randomly divided into six groups: the control group was given corn oil by gavage and fed with distilled water for 3 months. The concentration of DEHP dissolved in corn oil was 2 and 10%; the concentration of lead acetate (PbAc2) solution dissolved in distilled water was 0.5%. The detailed grouping and administration are shown in Table 1.
Morris Water Maze
According to the previous experimental method [25], a black circular pool (diameter, 150 cm; height, 60 cm), divided into four quadrants and filled with water (20 ± 1 °C), was used to perform the test. A platform (diameter, 10 cm) was positioned in the middle of the second quadrant, submerged 1.0 cm below the water surface. The rats entered the water facing the same point on the wall from each quadrant and were trained four times per day for 5 days. To assess memory at the end of learning, the trained rats were given two subsequent tests after 5-day resting. Firstly, the hidden platform was placed in the same position as before in the place navigation test, and the rats’ spatial memory abilities were assessed according to the escape latency time (the time spent to find the platform). We removed the submerged platform and began the spatial probe trial. Each rat was put in the pool to swim for 60 s. The rats’ spatial memory abilities were evaluated according to the time spent in the target quadrant and the number of target quadrant crossings (the second quadrant). Their search strategies were recorded and analyzed using the maze video tracking software (Ethovision XT, Noldus, Netherlands). We monthly performed Morris water maze experiment after administration to dynamically assess the ability of rats’ memory and learning.
Neurosteroid Levels in Serum
ELISA kits, purchased from Nanjing Jiancheng Bioengineering Institute, were used to measure the content of testosterone, pregnenolone (PREG), and dehydroepiandrosterone (DHEA) in serum. The experiment procedure and formulas for the calculation strictly followed the kit instructions.
Levels of MDA, GSH/GSSG, and Activities of GST, γ-GCS in the Hippocampus
Make hippocampal homogenates based on the different instructions of assay kits (purchased from Nanjing Jiancheng, China) for the further experiments. The microplate reader (BioTek, USA) read optical density (OD) values on the corresponding wavelengths. Then, calculate the respective concentrations according to the manufacturer’s instructions.
RNA Extraction and Quantitative Real-Time PCR
QPCR was conducted to detect the mRNA expression of Bdnf, Creb, and Nrf2. Homogenize 30-mg hippocampus tissue in 1 mL of Trizol reagent (Invitrogen Inc., Burlington, ON, Canada). After chloroform separation and isopropanol precipitation, total RNA was extracted according to the manufacturer’s instructions. One microgram of total RNA was converted to cDNA and then was amplified in duplicate using SYBR Premix Ex Taq II (Takara, Dalian, China) and a Light Cycler 480 II (Roche, Germany) PCR detection system. The primer showed in Table 2. Gapdh, was used as an internal reference. The relative quantification of mRNA levels was calculated by the 2−ΔΔCt method [26].
Statistical Analysis
Experimental data were described by mean ± standard deviation (SD) and analyzed by factorial design analysis of variance (ANOVA) with SPSS 20.0 software. LSD method was used for multiple comparisons between groups. Data transformation or nonparametric test was performed when the data did not satisfy the homogeneity of the variance. P < 0.05 was considered statistically significant.
Results
Effects on Learning and Memory after Exposure to Lead and DEHP
All rats could find the hidden platform after 5-day training in Morris water maze. Interestingly, the rats in the combined exposed groups (group 5, 2% DEHP + 0.5% lead and group 6, 10% DEHP + 0.5% lead), as well as the control, spent a shorter time finding the platform than other groups and showed a stronger ability of learning.
Firstly, the rats in the 10% DEHP group spent significantly less time in the target quadrant and crossed the target quadrant significantly less than the control in the spatial probe trial of the first Morris water maze (Table 3). A month later, we performed the second Morris water maze and found that 0.5% lead significantly reduced the rats’ retention time in the target quadrant of the spatial probe trial (lead, Ftime = 6.533, P = 0.013); and the rats’ number crossing target quadrant, treated by 10% DEHP, is still significantly less than the control group (Table 4). Finally, the third water maze test indicated the rats exposed to 10% DEHP or 0.5% lead spent significantly less time in the target quadrant (Table 5 and Fig. 1c); rats exposed to 2 or 10% DEHP or 0.5% lead had less number of target quadrant crossing than control animals (Tabl 5 and Fig. 1e); in addition, the rats in groups 2 to 5 spent longer time (escape latency) to reach the platform location than the control. However, the animals exposed to 10% DEHP + 0.5% lead showed similar results to those animals in the control group (Table 5 and Fig. 1g).
Effects of lead and DEHP on learning and memory in rats (n = 10 rats per group): a represented for swimming route of looking for the platform; b represented for search strategy in the spatial probe trial; c, e, and g represented for time spent in the target quadrant, number of crossing target quadrant, and escape latency of the spatial probe trial in the third Morris water maze; d, f, and h, originated from SPSS20.0, were the interaction profile plots. ∗P < 0.05
The swimming routes of finding the platform showed in Fig. 1a. Compared with 0.5% lead and 10% DEHP groups, the rats of the control and combined exposure group (0.5% lead + 10% DEHP) found the platform more quickly and directly; Fig. 1b showed the search strategy of rats after removing the hidden platform. The rats of 0.5% lead and 10% DEHP groups performed a disorganized and purposeless search strategy, hardly crossing the platform area and the target quadrant compared with the control. Interestingly, the rats, exposed to 0.5% lead and 10% DEHP, performed an explicit search strategy, frequently crossing the platform area in the target quadrant.
The interaction of lead and DEHP on the indicators of the spatial probe trial was found according to the factorial design analysis (lead × DEHP: Ftime = 4.653, P = 0.017; Fnumber = 6.814, P = 0.003; Flatency = 9.548, P = 0.001). A crossing point in the right interaction diagram indicated the existence of the interaction (Fig. 1d, f, h). Besides, the retention time in the target quadrant was gradually shortened with increasing concentration of DEHP, while the retention time was gradually increased after simultaneous exposure to 0.5% lead (Fig. 1c). Similarly, lead altered the single effect of DEHP on the number of crossing target quadrant (Fig. 1e) and escape latency (Fig. 1g). The above results indicated that lead and DEHP damaged the spatial learning and memory abilities of rats, whereas their combined exposure was not significantly different from the control, namely, there was an antagonism joint action in neurotoxicity.
Contents of Testosterone, PREG, and DHEA in Serum
Testosterone content was significantly reduced by DEHP (Ftestosterone = 3.614, P = 0.044) (Table 6 and Fig. 2a). 0.5% lead exposure significantly reduced the level of testosterone (P < 0.05) (Table 6 and Fig. 2a). However, no statistical significance was found in PREG content exposed to lead and DEHP (Table 6 and Fig. 2b). The higher the DEHP concentration, the lower the DHEA level (FDHEA = 3.512, P = 0.046). However, this trend had been significantly reversed with the addition of 0.5% lead (lead × DEHP: FDHEA = 5.850, P = 0.009) (Table 6 and Fig. 2c, d).
Levels of MDA, GSH, GSSG, and Activities of γ-GCS, GST in the Hippocampus
The present experiment showed that lead significantly increased the level of MDA, reduced the GSH content, and increased GSSG content (FMDA = 17.044, P = 0.001; FGSH = 11.348, P = 0.006; FGSSG = 14.283, P = 0.003; FGSH/GSSG = 18.170, P = 0.001) (Table 7 and Fig. 3a and b). However, the activity of γ-GCS in the hippocampus significantly increased after exposure to DEHP (Fγ-GCS = 16.040, P < 0.05) (Table 7 and Fig. 3c). No significant effects on GST and the interaction between lead and DEHP were observed (Table 7).
Bdnf, Creb, and Nrf2 mRNA Expression in the Hippocampus
According to factorial design ANOVA, a statistical significance of the main effect of lead and their interaction on the mRNA expression of Bdnf were shown (lead, FBdnf = 13.603, P = 0.001; lead × DEHP: FBdnf = 19.440, P < 0.05) (Table 8 and Fig. 4a, b). Compared to the control, 10% DEHP or 0.5% lead exposure remarkably reduced the expression of Bdnf mRNA (Table 8 and Fig. 4a). However, no statistical significance of the mRNA expression of Creb and Nrf2 were found (Table 8 and Fig. 4c, d). Interestingly, there was a dose-response relationship of DEHP exposure on Nrf2 mRNA expression, which reminded us of an inhibition of DEHP on Nrf2 mRNA expression (Fig. 4c).
Discussion
Lead, with apparent neurotoxicity, has adverse effects on children’s nervous system [27]. DEHP, a common plasticizer, shows neurotoxicity and reproductive and developmental toxicity [28, 29]. It is definitely worth exploring the joint neurotoxicity after combined exposure to them. In our current study, Morris water maze experiment was monthly performed to evaluate rats’ ability of memory and learning dynamically. Similar to the previous research, lead or DEHP single exposure shows increasing neurotoxicity [30, 31]. Interestingly, our neurobehavioral test suggested that rats in the combined exposure groups had a better ability of learning and memory compared with the single exposure ones. Namely, there seems to be an antagonism joint action after exposure to lead and DEHP, and it means that their combined neurotoxicity is lower than the single effect of lead or DEHP. To further explain the results of the above neurobehavioral test and provide evidence of their antagonism joint action, we separately analyzed neurosteroid contents, oxidative stress levels, and CREB-BDNF signaling pathways’ changes after exposure to lead and DEHP.
Neurosteroids mainly include testosterone, PREG, DHEA, and its sulfate derivatives (PS, DHEAS). Our data showed that DEHP-treated rats had a lower content of testosterone and DHEA after a 3-month exposure. In addition, there was an antagonism joint action between lead and DEHP in terms of the effects on DHEA. DHEA has been found to directly act as a positive allosteric modulator of the NMDARs, which is essential for controlling synaptic plasticity and memory function [32, 33]. Ca2+ flux through NMDARs is thought to be critical in synaptic plasticity, a cellular mechanism for learning and memory. In brief, DEHP could reduce the content of DHEA via targeting NMDARs as a result of impairing hippocampal learning and memory functions.
BDNF, the brain-derived neurotrophic factor, also showed the same trend. Bdnf mRNA level of all administration groups was far less than the control, while the expression of combined exposure groups was slightly increased compared with the single ones. Neal AP’s [34] research suggests that presynaptic deficits, resulting from lead exposure, are mediated by the disruption of NMDAR-dependent BDNF signaling during synaptogenesis. In the light of Zhao Q’s [11] and Guilarte TR’s [12] research indicates that lead can competitively antagonize Ca2+ and act as a potent NMDAR antagonist. In our study, lead inhibited the activity of NMDA receptors and significantly reduced the mRNA expression level of BDNF, thus might lead to a decline in learning and memory abilities. However, there was no significant difference of its transcriptional enhancer CREB in the present study. Therefore, we speculated that other pathway-related transcription factors may be involved in transcriptional regulation of BDNF.
Oxidative stress is one of the indicators of early changes in the damage of the central nervous system. The brain tissue is rich in unsaturated fatty acids and is particularly sensitive to oxidative damage. Our study confirmed that DEHP reduced the expression of Nrf2 mRNA in spite of no significance while its downstream enzyme activity of γ-GCS was improved. Lead significantly increased MDA level and reduced GSH/GSSG value, which was consistent with the results from Lu [10]. However, there was an insufficient evidence to support the joint action of lead and DEHP on oxidative stress.
Taken all results into consideration, we speculated that the antagonistic joint action might be due to receptor antagonism, which means that two compounds competitively bind to the same receptor in vivo. We further concluded that the antagonistic effect of lead and DEHP on neurobehavior originated from their competitively binding to NMDARs [32, 35], which play crucial roles in both the development of the nervous system and the formation of neuronal circuits. In addition, another antagonism joint action, configuration antagonism, should be considered. It means that a chemical can affect the absorption, distribution, excretion, and metabolism of another chemical, making it less likely to reach the target organ. Lead can cross the blood-brain barrier by mimicking Ca2+and degrade the myelin sheaths of neurons, reduce their numbers, interfere with neurotransmission routes, and decrease neuronal growth [11]. Similarly, DEHP can also exert the toxicity through the blood-brain barrier [36]. Therefore, an antagonistic effect on learning and memory can happen after exposure to both lead and DEHP due to the configuration antagonism as well.
In a word, although there were some limitations such as no data about daily water consumption and lead intake, the present study reminded that the joint neurotoxicity of lead and DEHP may be an interesting antagonistic effect initially. In subsequent experiments, we will be in an effort to make the research conclusion more persuasive and scientific. Although as a preliminary work of a battery of profound studies, deep functional research is necessary; our current study may contribute to understanding the unique joint neurotoxicity and some shallow mechanism after combined exposure to both lead and DEHP.
References
Aksu D, Didin M, Kayikci F (2012) The protective role of polyphenols on blood cells in rats exposed to lead Rolul protectiv al polifenolilor asupra celulelor sanguine la şobolanii expuşi la plumb
Murata K, Iwata T, Dakeishi M, Karita K (2009) Lead toxicity: does the critical level of lead resulting in adverse effects differ between adults and children? J Occup Health 51(1):1–12
Rosner D, Markowitz G (2007) The politics of lead toxicology and the devastating consequences for children. Am J Ind Med 50(10):740–756. https://doi.org/10.1002/ajim.20435
Saripinar Aksu D, Saglam YS, Aksu T (2016) The investigation of neuroprotective effects of pomegranate juice on oxidative damage in brain caused by lead in rats. Eurasian J Vet Sci 32(4):255–255. https://doi.org/10.15312/EurasianJVetSci.2016422397
Aksu DS, Saglam YS, Yildirim S, Aksu T (2017) Effect of pomegranate (Punica granatum L.) juice on kidney, liver, heart and testis histopathological changes, and the tissues lipid peroxidation and antioxidant status in lead acetate-treated rats. Cell Mol Biol (Noisy-le-grand) 63(10):33–42. https://doi.org/10.14715/cmb/2017.63.10.5
Ozkaya A, Sahin Z, Kuzu M, Saglam YS, Ozkaraca M, Uckun M, Yologlu E, Comakli V, Demirdag R, Yologlu S (2018) Role of geraniol against lead acetate-mediated hepatic damage and their interaction with liver carboxylesterase activity in rats. Arch Physiol Biochem 124(1):80–87. https://doi.org/10.1080/13813455.2017.1364772
Krigman MR, Hogan EL (1974) Effect of lead intoxication on the postnatal growth of the rat nervous system. Environ Health Perspect 7:187–199
Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ (2005) IQ and blood lead from 2 to 7 years of age: are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect 113(5):597–601
Ordemann JM, Austin RN (2016) Lead neurotoxicity: exploring the potential impact of lead substitution in zinc-finger proteins on mental health. Metallomics 8(6):579–588. https://doi.org/10.1039/c5mt00300h
Lu X, Jin C, Yang J, Liu Q, Wu S, Li D, Guan Y, Cai Y (2013) Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats’ hippocampus. Biol Trace Elem Res 151(1):75–84. https://doi.org/10.1007/s12011-012-9531-5
Zhao Q, Slavkovich V, Zheng W (1998) Lead exposure promotes translocation of protein kinase C activities in rat choroid plexus in vitro but not in vivo. Toxicol Appl Pharmacol 149(1):99–106
Guilarte TR, McGlothan JL (1998) Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res 790(1–2):98–107
Kamrin MA (2009) Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev 12(2):157–174. https://doi.org/10.1080/10937400902729226
Joffe M (2001) Are problems with male reproductive health caused by endocrine disruption? Occup Environ Med 58(4):281–287 quiz 287–288, 260
Gartner S, Balski M, Koch M, Nehls I (2009) Analysis and migration of phthalates in infant food packed in recycled paperboard. J Agric Food Chem 57(22):10675–10681. https://doi.org/10.1021/jf902683m
Beliles R, Salinas JA, Kluwe WM (1989) A review of di(2-ethylhexyl)phthalate (DEHP) risk assessments. Drug Metab Rev 21(1):3–12. https://doi.org/10.3109/03602538909029952
Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, Chu I (1997) Subchronic oral toxicity of di-n-octyl phthalate and di(2-ethylhexyl) phthalate in the rat. Food Chem Toxicol 35(2):225–239
Zarean M, Keikha M, Poursafa P, Khalighinejad P, Amin M, Kelishadi R (2016) A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ Sci Pollut Res Int 23(24):24642–24693. https://doi.org/10.1007/s11356-016-7648-3
Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, Wolff MS (2009) Prenatal phthalate exposure and performance on the neonatal behavioral assessment scale in a multiethnic birth cohort. Neurotoxicology 30(4):522–528. https://doi.org/10.1016/j.neuro.2009.04.001
Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, Yoo HJ, Cho IH, Kim HW (2010) Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect 118(7):1027–1032
Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW, Yang YH, Kim HW, Bhang SY, Hong YC (2009) Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol Psychiatry 66(10):958–963. https://doi.org/10.1016/j.biopsych.2009.07.034
Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I (1999) Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1(3):165–170. https://doi.org/10.1038/11086
McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA (1996) Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87(7):1339–1349
Dzhekova-Stojkova S, Bogdanska J, Stojkova Z (2001) Peroxisome proliferators: their biological and toxicological effects. Clin Chem Lab Med 39(6):468–474. https://doi.org/10.1515/cclm.2001.076
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. https://doi.org/10.1373/clinchem.2008.112797
Jett DA, Kuhlmann AC, Farmer SJ, Guilarte TR (1997) Age-dependent effects of developmental lead exposure on performance in the Morris water maze. Pharmacol Biochem Behav 57(1–2):271–279
Arcadi FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, Trimarchi GR, Costa G (1998) Oral toxicity of bis(2-ethylhexyl) phthalate during pregnancy and suckling in the long-Evans rat. Food Chem Toxicol 36(11):963–970
Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V (2015) In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod Toxicol 51:47–56. https://doi.org/10.1016/j.reprotox.2014.12.005
Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, Yolton K, Lanphear BP (2017) Prenatal phthalate, triclosan, and bisphenol a exposures and child visual-spatial abilities. Neurotoxicology 58:75–83. https://doi.org/10.1016/j.neuro.2016.11.009
Smith CA, Holahan MR (2014) Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to di(2-ethylhexyl) phthalate in male long Evans rats. PLoS One 9(10):e109522. https://doi.org/10.1371/journal.pone.0109522
Dai Y, Yang Y, Xu X, Hu Y (2015) Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Horm Behav 71:41–48. https://doi.org/10.1016/j.yhbeh.2015.03.008
Fu H, Chen Z, Josephson L, Li Z, Liang SH (2018) Positron emission tomography (pet) ligand development for ionotropic glutamate receptors: challenges and opportunities for radiotracer targeting N-methyl-d-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors. J Med Chem. https://doi.org/10.1021/acs.jmedchem.8b00714
Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR (2010) Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci 116(1):249–263. https://doi.org/10.1093/toxsci/kfq111
Cao XJ, Huang SH, Wang M, Chen JT, Ruan DY (2008) S-adenosyl-L-methionine improves impaired hippocampal long-term potentiation and water maze performance induced by developmental lead exposure in rats. Eur J Pharmacol 595(1–3):30–34. https://doi.org/10.1016/j.ejphar.2008.07.061
Wu Y, Li K, Zuo H, Yuan Y, Sun Y, Yang X (2014) Primary neuronal-astrocytic co-culture platform for neurotoxicity assessment of di-(2-ethylhexyl) phthalate. J Environ Sci 26(5):1145–1153. https://doi.org/10.1016/s1001-0742(13)60504-5
Funding
This study was supported by the National Natural Science Foundation of China 81773470 and the Provincial Natural Science Foundation of Liaoning 20170540991.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval for all animals involved in this study was from the Institutional Animal Care and Use Committee of China Medical University.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Li, H., Qu, P. et al. An Antagonism Joint Action of Lead and Di-2-Ethylhexyl Phthalate Explains an Improved Ability of Learning and Memory after Combined Exposure in Weaning Rats. Biol Trace Elem Res 191, 126–134 (2019). https://doi.org/10.1007/s12011-018-1586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1586-5