Abstract
Developmental lead (Pb) exposure involves various serious consequences, especially leading to neurotoxicity. In this study, we examined the possible role of monosialoganglioside (GM1) in lead-induced nervous impairment in the developing rat. Newborn male Sprague-Dawley rat pups were exposed to lead from birth for 30 days and then subjected to GM1 administration (0.4, 2, or 10 mg/kg; i.p.) or 0.9% saline. The results showed that developmental lead exposure significantly impaired spatial learning and memory in the Morris water maze test, reduced GM1 content, induced oxidative stress, and weakened the antioxidative systems in the hippocampus. However, co-treatment with GM1 reversed these effects. Moreover, GM1 counteracted lead-induced apoptosis by decreasing the expression of Bax, cleaved caspase-3, and by increasing the level of Bcl-2 in a dose-dependent manner. Furthermore, we found that GM1 upregulated the expression of SIRT1, CREB phosphorylation, and BDNF, which underlie learning and memory in the lead-treated developing rat hippocampus. In conclusion, our study demonstrated that GM1 exerts a protective effect on lead-induced cognitive deficits via antioxidant activity, preventing apoptosis, and activating SIRT1/CREB/BDNF in the developing rat hippocampus, implying a novel potential assistant therapy for lead poisoning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is an environmental neurotoxin; the presence of which is inevitable in air, soil, diet, and daily necessities [1]. In animals, it has been shown that lead has toxicological effects on multiple organs, including the liver, kidney, lung, bone, blood, testis, and especially on the developing central nervous system (CNS) [2,3,4,5,6,7]. Epidemiological researches have shown that developmental lead exposure is associated with intellectual deficits accompanied by abnormality of synapse function in children [8]. There were significant negative associations between school performance and intelligence quotient (IQ) with blood lead levels in children, even in the range of 5–50 μg/L [9]. In addition, developmental lead exposure also might be a risk factor for neurodegenerative disorder in late life by the animal test [10, 11]. Clinically, chelation therapy is typically adopted to exclude lead, which inevitably causes the loss of trace elements from the bodies of children. Thus, there is an urgent need to explore novel therapeutic strategies for lead poisoning.
Silent information regulator 2 homolog 1 (SIRT1), one of the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases, plays a pivotal role in DNA damage responses, glucose and lipid metabolism, and transcription through deacetylation of transcription factors and histones [12]. The activation of SIRT1 can inhibit the generation of reactive oxygen species (ROS) via modulation of p53 and the forkhead O (FOXO) family [13]. Recently, a novel pathway was proposed in which SIRT1 mediates the expression of cyclic AMP response element binding protein (CREB) and brain-derived neurotrophic factor (BDNF) by limiting the level of miR-134 through interacting with a repressor complex containing the transcription factor YY1 to modulate memory formation and synaptic plasticity [14]. Of note, CREB belongs to the family of CREB/activating transcription factors (ATF), which is targeted by non-coding small RNA and a large number of activity-inducible kinases, including protein kinase A, mitogen-activated protein kinase, and Ca2+/calmodulin-dependent protein kinases [15]. As an essential transcription factor, CREB is a molecular switch in learning and memory that regulates an array of brain-specific coding genes, including BDNF, c-fos, and activity-regulated cytoskeleton-associated protein (Arc), which are required for the process of memory formation and consolidation in the brain [16].
Monosialoganglioside (GM1), consisting of a sialic acid–containing oligosaccharide chain and a ceramide unit, is widely distributed in vertebrates with high expression in the central nervous system (CNS) [17]. GM1 was shown to exert significant benefits in stroke, hypoxic-ischaemic encephalopathy (HIE), Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) [18,19,20,21,22]. Supplementation with exogenous GM1 at nanomolar concentrations protected PC12 cells from ROS induced by H2O2 [23]. GM1 can partly inhibit benzo[a]pyrene-induced apoptosis and oxidative stress by modulation of iron transportation and intracellular acidification [24]. In addition, GM1 occurs in a high-affinity association with neurotrophic factor receptors, such as TrkA, TrkB, and Ret, mediating neurotrophin modulation and neuroprotection [25,26,27], which serves to preserve the viability of injured nerves. However, less has been reported about the effect of GM1 on neurotoxicity induced by lead, and it has been demonstrated that GM1 administration can alleviate the impairments of LTP/DP on synaptic plasticity and calcium overload induced by lead in rats [28]. However, whether GM1 improves lead-induced cognitive deficits in behavior research remains questionable, and the underlying molecular mechanisms of these effects are still not understood.
Considering the above mentioned findings, we hypothesized that GM1 may play a multi-faceted protective role in lead-induced neurotoxicity. Using a combination of behavior and molecular biology paradigms, we analyzed the effects of GM1 in the hippocampus of developing rats exposed to lead. This study provided a new perspective on GM1 and explored a new potential therapy for lead poisoning.
Materials and Methods
Animals and Ethics Statement
A total of 45 SPF grade Sprague-Dawley rats (30 females and 15 males, 8 weeks old) were purchased from the China National Laboratory Animal Resource Centre (Shanghai, China) and were housed under conditions of adequate temperature (22 ± 2 °C) and humidity (50 ± 5%) with a 12-h light/12-h dark cycle. Food and distilled water were available ad libitum. All procedures involving animal models and treatments were carried out in strict accordance with the international standards of animal care guidelines, and the experiments were approved by the Institutional Animal Care Committee at Shanghai Jiao tong University School of Medicine.
Experimental Design and Treatment
After 1 week of acclimatization, 15 male rats were randomly mated at a 1:2 ratio of male to female. Vaginal smears (or vaginal plugs) were taken to indicate the first day of pregnancy. Then, all 30 pregnant rats were divided into six groups by body weight randomly with five rats each group: (1) control group, (2) Pb group, (3) Pb + GM1 (0.4 mg/kg), (4) Pb + GM1 (2 mg/kg), (5) Pb + GM1 (10 mg/kg), (6) control + GM1 (10 mg/kg). All pregnant rats were housed individually and given distilled water during the gestation period. After delivery, the control group and control + GM1 (10 mg/kg) group were still served with distilled water, while the other four groups received 0.2% lead acetate (Sigma-Aldrich, USA) in drinking water ad libitum. After weaning, mother rats and female pups were euthanized, and male pups were randomly selected and grouped with 20 rats each group for further experiments (n = 20). Monosialoganglioside sodium for injection (TRB, AR) was intraperitoneally injected into male pups at three dosages (0.4 mg/kg, 2 mg/kg, and 10 mg/kg, respectively, PND 21) for 10 days, while the pups in the control group and the control + GM1 (10 mg/kg) received an equal volume of saline (0.9% NaCl) at the same time points. For biochemical and histochemical studies, the pups (n = 14) were randomly selected and anesthetized on PND 31 by injection of sodium pentobarbital (50 mg/kg, i.p.). Whole blood samples were collected into anticoagulant tubes for lead level determination. The brains were removed and fixed in 4% paraformaldehyde solution overnight at 4 °C. The hippocampus samples were quickly separated from brain samples in an ice bath and stored at − 80 °C for further use. Then, the remaining pups (n = 6) were subjected to behavioral tests, which were conducted at 08:00 for 6 days. After that, all pups were euthanized. The timeline about our experimental procedures can be seen in Fig. 1.
Timeline of experimental procedures. Newborn pups in the lead-treated group were exposed to lead from PND 0 to PND 31 via two different exposure pathways. After being weaned, GM1 or saline (i.p.) was administrated until PND 31. Then, 14 rats of each group were selected randomly and sacrificed for neurochemical analysis. The behavioral tests were performed on the remaining pups to evaluate cognitive ability, as described (n = 6). Whole blood was obtained on PND 21 and PND 31, and all tissue samples were collected from PND 31
Morris Water Maze Task
After 10 days of treatment, the Morris water maze was conducted to evaluate the spatial learning and memory of the developing rats in all groups according to a procedure from the literature [29]. The apparatus consists of a video camera and a circular pool with a diameter of 120 cm and a height of 50 cm. The procedure consisted of a navigation test with four trials per day for 5 consecutive days and one spatial probe test on the sixth day. The water was made opaque with edible melanin and was heated to 23 °C the night before trials. In the navigation test, rats were launched in different start points of four quadrants facing the wall of the pool and trained to reach the hidden platform within 60 s at an interval of 15 min between two consecutive tests. If the rat failed, the escape latency was recorded as 60 s, and the failed rat was guided to the platform to rest gently for 30 s the same as for the completed rats. After each trial, rats were towel dried and warmed using a heater. On the 6th day, trained rats were subjected to a 60-s spatial probe test in which the platform was removed from the target quadrant. The escape latency, time spent in the target quadrant, time to first platform crossing, and the number of platform crossings were recorded. All tests were recorded by a video camera and the data were generated by water maze software (XinRuan Information Technology Co., Ltd., China).
Determination of Lead Contents in the Blood and Hippocampus
On PND 21 and PND 31, venous blood from the tails was collected into tubes containing EDTA-2K for lead analysis. The blood lead level (μg dL−1) was measured at the wavelength of 283.3 nm by a graphite furnace atomic absorption spectrophotometer PinAAcle 900Z (PekinElmer Company, Waltham, MA). The method for the determination of blood lead was previously described [30]. Briefly, a quality control (QC) test (Contox, Kaulson Laboratories, Inc., NJ, USA) was conducted to check the accuracy of the calibration curve (r2 > 0.995) before the measurement of samples. After that, all samples were diluted with working solution containing 0.2% nitric acid and 0.5% triton and were analyzed twice to ensure repeatability and stability of the results. Only when the SD met the requirements was the mean of two repeated measurements recorded.
On PND 31, the pups were anesthetized, then the bilateral hippocampus was immediately separated after decollation. The hippocampus was precisely weighed and placed in microwave digestion vessels. To every specimen, we added 0.2 mL of nitric acid and 0.2 mL of hydrogen peroxide in the proper sequence, while the blank (deionized water) and the quality control were set up with the same processing method. A microwave digestion instrument (CEM, USA) was used to digest the hippocampus specimens with the abovementioned strong oxidation system. When digestion was completed, on cooling to room temperature, 2 mL 2% nitric acid was added to the solution and the mixture was transferred to a micro-centrifuge tube. All pretreated samples were stored at 4 °C prior to analysis. The lead content in the hippocampus was determined by a 7500CE ICP-MS (Agilent Technologies, USA).
Assay of Oxidative Stress Markers in the Hippocampus
The hippocampal tissue was precisely weighed and homogenized in ice-cold homogenizing buffer (pH 7.4, 0.01 mol/L Tris-HCl, 0.0001 mol/L EDTA-2Na, 0.01 mol/L sucrose 0.8% NaCl solution) by ninefold volume (w/v) using a motor-driven homogenizer. After centrifugation at 3000 rpm for 10 min, the supernatants were collected. The protein concentrations of extracts were quantified immediately using a BCA protein assay kit (Beyotime Institute of Biotechnology, China).
The activity of total superoxide dismutase (T-SOD), catalase (CAT), and the levels of glutathione (GSH), malondialdehyde (MDA) in hippocampal tissue were assayed by a superoxide dismutase (SOD) assay kit (WST-1 method), catalase activity (CAT) assay kit (visible light), reduced glutathione (GSH) assay kit, and malondialdehyde (MDA) assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively, according to the manufacturer’s instructions [31,32,33].
Protein Extraction and Immunoblotting
The hippocampal tissue was homogenized in ice-cold lyses buffer (RIPA) (Beyotime, Shanghai, China), and let stand for 30 min. After centrifugation at 1200g for 10 min at 4 °C, the supernatant was collected. The protein concentrations were measured immediately using the BCA protein assay kit (Beyotime, Shanghai, China). After denaturation, the samples were separated by 8–15% SDS/PAGE gel electrophoresis and then transferred to a 0.2-μm nitrocellulose membrane (GE Healthcare GmbH, Freiburg, Germany). Following blocking with 5% nonfat milk in Tris-buffered saline (TBST) for 1 h, membranes were incubated with primary antibody against Bax (1:1000; Beyotime, China), Bcl-2 (1:500; Absin, China), cleaved caspase-3 (1:1000; CST, USA), SIRT1 (1:500; Boster, China), CREB (1:1000; CST, USA), p-CREB (1:1000; CST, USA), and BDNF (1:1000; Abcam, UK) overnight at 4 °C. On the second day, the membranes were washed three times for 10 min in TBST before incubation with appropriate secondary horseradish peroxidase–linked (HRP) antibodies (1:2000) at room temperature for 2 h. Finally, the bands were detected using a ChemiDoc XRS+ Imaging System (Bio-Rad, USA) and the gray level of each band was measured using ImageJ software.
Nissl Staining
After being fixed in 4% paraformaldehyde solution (24 h), brains were preserved by paraffin embedding and cut into 4-μm sections. To assess the status of neurons, the brain slices were deparaffinized in xylene followed by rehydration with graded ethanol from 100 to 70%, and washed with dd H2O. The slides were immersed in cresyl violet stain for 5 min, rinsed in dd H2O, differentiated in 1% acetic acid, rinsed in dd H2O again, and dried. Finally, the slices were mounted with cover slips. Then, the slices were visualized and pictured using an imaging system (NIKON DS-U3, Japan).
Immunohistochemistry
To compare the effects of lead and GM1 administration on the expression of BDNF in the developing rat hippocampus, immunohistochemistry was conducted. The brain slides were deparaffinized and processed for antigen retrieval using citrate buffer (pH 6.0) in a microwave oven (8 min, 7 min). Once naturally cooled, slides were washed three times with PBS (pH 7.4) for 5 min each. Then, these slides were immersed in 3% hydrogen peroxide (H2O2) and washed with PBS (pH 7.4) for quenching of endogenous peroxidase activity. After being blocked with 3% BSA, these slides were incubated with specific primary antibodies (anti-BDNF) overnight at 4 °C. Subsequently, the slides were washed three times and incubated with horseradish peroxidase–conjugated secondary antibody. After that, they were visualized using DAB and counterstained with hematoxylin, dehydrated using gradient alcohol and xylene, then mounted in mounting medium. Images were obtained using a digital microscope (Leica, Microsystems, Berlin, Germany). Positive staining was defined as brown pigmentation and nuclei were stained blue.
Immunofluorescence
Immunofluorescence staining was used for the assessment of the changes of GM1. Paraffin sections were deparaffinized and processed for antigen retrieval using citrate buffer (pH 6.0). Once naturally cooled, the slides were washed in PBS (pH 7.4) three times before being blocked in BSA for 30 min and incubated in primary antibody against GM1 in a humid chamber overnight at 4 °C away from light. Then a fluorescent-labeled secondary antibody was incubated at room temperature. Following clearing, the slides were counterstained with DAPI for 10 min at room temperature away from light. Finally, the slides were mounted in mounting medium. Images were obtained using a fluorescence microscope (Leica, Microsystems, Berlin, Germany).
Statistical Analysis
All statistical analyses were processed using SPSS 22 statistical software (IBM, NY, USA) and all results are presented as the mean ± SD. The escape latency data from navigation tests were analyzed using two-way repeated measures ANOVA (days and treatment). The Spatial probe test and other data were analyzed using one-way ANOVA followed by Tukey’s post-hoc test. Differences were considered to be significant at P ≤ 0.05.
Results
The Effect of GM1 on the Lead Concentration in Whole Blood and Hippocampal Tissue
The blood samples were collected on PND 21 and PND 31 to address the efficacy of GM1 in lowing blood lead. Hippocampal lead concentrations were measured after euthanasia. The lead concentrations in whole blood and hippocampus in developing rats are shown in Fig. 2. The results showed a significant increase in blood lead levels in the lead-treated group compared to those of the control group on PND 21 (P < 0.001, Fig. 2a) and the blood lead levels in all groups were not significantly different before and after treatment (P > 0.05, Fig. 2a). Moreover, no significant difference was found in hippocampus lead levels among the Pb group and the Pb + GM1 groups (P > 0.05, Fig. 2b). These results indicated that lead exposure could significantly increase blood and hippocampal lead levels in developing rats, and GM1 did not exclude lead, reflecting the high levels of lead in whole blood and hippocampus.
Effects of GM1 on lead concentrations in whole blood and hippocampus. a Lead concentrations in blood were measured on PND 21 (pre-treatment) and PND 31 (post-treatment). b Lead concentrations in hippocampus were measured on PND 31. All values are expressed as the mean ± SD, n = 6, ***P < 0.001 compared to the control group
GM1 Ameliorated Spatial Learning and Memory Deficits in Lead-Exposed Rats
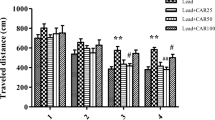
The effects of GM1 on lead-induced learning memory and cognitive deficits were evaluated by Morris water maze test on PND 31. The swimming path, escape latency, time to first platform crossing, time spent in target quadrant, crossing numbers, and speed were recorded for analysis. The results were summarized in Fig. 3. Two-way ANOVA indicated a significant day effect [F(4,120) = 561.407, P < 0.001] and a significant interaction between days and treatment [F(20,120) = 9.327, P < 0.05]. The analysis showed that lead exposure significantly delayed the escape latency in the Pb group and this spatial memory impairment was remarkedly reversed by GM1 after 10-day treatment. Of note, our study also discovered that GM1 administration led to an extra improvement in the control + GM1 (10 mg/kg) group which displayed shorter escape latencies from day 1 to day 3, compared with the control group (untreated) in Morris water maze test(Fig. 3a). On the 6th day of the probe trial, as illustrated in Fig. 3b, rats in the Pb + GM (10 mg/kg) group show increased crossing numbers compared with those in the Pb group (P < 0.05), but there was no statistical significance in crossing numbers between the control group and Pb group, although we noted a certain trend. Moreover, compared with the control group, the rats in the Pb group showed a remarkable prolongation in time to first platform crossing (P < 0.001, Fig. 3c), while they spent less time in the target quadrant (P < 0.001, Fig. 3d). Interestingly, these negative effects caused by lead were significantly ameliorated by GM1 treatment in a dose-dependent manner (Fig. 3c, d). No significant difference (P > 0.05) was found in the swimming speed among the all groups, reflecting that the treatments of Pb and GM1 did not affect athletic performance, and all the rats had similar locomotor activity (Fig. 3e). The representative trajectories for each group of developing rats in probe trials are shown in Fig. 3f. All these results indicated that lead exposure could cause severe impairments in spatial memory and memory retention of developing rats which was remarkably ameliorated by GM1 treatment.
Effects of GM1 on lead-induced spatial learning and memory deficits of developing rats in the Morris water maze. a Escape latency of the five acquisition training days for developing rats of each group. b Crossing numbers. c, d Time to first platform and the percentage of time spent in target quadrant of each group during the probe test. e Swimming speed on the first day of the acquisition training. f Representative trajectories of swimming rats during the probe test. All values are expressed as the mean ± SD, n = 6. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the Pb group
GM1 Attenuated Pb-Induced Neuropathological Changes and the Loss of Endogenous GM1 in the Developing Rat Hippocampus
The neuronal morphology changes in hippocampus after treatments were revealed by Nissl staining. As shown in Fig. 4a, compared with pyramidal neurons in the CA1 region of the control group, there were significant neuropathological changes, including Nissl bodies loss, unclear cell margins, and nucleus shrinkage in the Pb group. However, the status of pyramidal neurons was significantly improved in GM1-treated groups. In addition, to evaluate the effects of lead exposure on the expression of GM1, immunofluorescence staining was conducted. As shown in Fig. 4b, GM1 in the hippocampus content was remarkably decreased in the Pb group compared to the control group. As expected, GM1 administration prevented this reduction at a high dose (10 mg/kg). There was a significant elevation in the control + GM1 (10 mg/kg) group compared with the control group, suggesting that an exogenous supply of GM1 could improve the content in the brain.
Effects of GM1 on lead-induced neuropathological changes and the loss of endogenous GM1 in the hippocampus. Neuronal morphology changes in the developing rat hippocampal CA1 region in all groups were detected by Nissl staining. a Representative images for Nissl staining of hippocampus in the control group, Pb group, Pb + GM1 (0.4 mg/kg), Pb + GM1 (2 mg/kg), Pb + GM1 (10 mg/kg), and control + GM1 (10 mg/kg) groups (× 400, bar = 50 μm). b, c Representative and quantitative analysis of GM1 expression in the hippocampus (× 400, bar = 50 μm). All values are expressed as the mean ± SD, n = 5. *P < 0.05, **P < 0.01 compared to the control group; ##P < 0.01 compared to the Pb group
GM1 Inhibited Lead-Induced Oxidative Stress in Developing Rat Hippocampus
To evaluate the potentials of GM1 in ameliorating lead-induced antioxidant system impairments in the developing rats, various antioxidant enzyme activities, such as T-SOD, CAT, GSH, and MDA were measured. As shown in Fig. 5, between groups comparisons indicated that lead exposure significantly decreased the activities of T-SOD and CAT, as well as GSH levels in the Pb group compared to the control group (P < 0.001 for T-SOD and GSH, P < 0.01 for CAT, Fig. 3a–c), while MDA levels were remarkably increased after lead exposure (P < 0.001, Fig. 3d). Interestingly, GM1 treatment led to a reversal of low levels of antioxidant enzymes activities (T-SOD, CAT), GSH, and an increase in MDA levels induced by lead in a dose-dependent manner. There were no significant difference in MDA, GSH levels, and antioxidant enzyme activities (T-SOD, CAT) between the Pb + GM1 (10 mg/kg) group and the control group (P > 0.05). In summary, these results suggested that GM1 administration to lead-exposed rats abolished the negative changes to the antioxidant system induced by lead exposure.
Effects of GM1 on the levels of oxidative stress markers in the hippocampus of lead-treated rats. a T-SOD activity, b CAT activity, c GSH levels, and d MDA levels were detected in the hippocampus. All values are expressed as the mean ± SD, n = 6. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the Pb group
GM1 Reversed the Changes in Expression of Lead-Induced Apoptosis-Related Proteins in the Hippocampus
To analyze whether the amelioration effect of GM1 was related to inhibiting apoptosis caused by lead, the expression of apoptosis-related proteins Bax, Bcl-2, and cleaved caspase-3 were examined by Western blotting. As shown in Fig. 6, compared with the control group, the expression of the Bax and cleaved caspase-3 was significantly increased (Fig. 6b, d) (P < 0.001, P < 0.05, respectively), whereas the expression of Bcl-2 was decreased in the Pb group (Fig. 6c) (P < 0.001). However, a significant decline of Bax and elevation of Bcl-2 were shown in the group co-treated with lead and GM1 compared to the Pb group. In addition, ANOVA analysis showed that there was a significant difference in the levels of Bcl-2 expression between the control group and the control + GM1 (10 mg/kg) group (P < 0.05), which means that GM1 administration may induce Bcl-2 protein to be highly expressed. Together, these results confirmed that GM1 could significantly inhibit lead-induced apoptosis in the hippocampus of developing rats.
Effects of GM1 on the expression of apoptosis-related proteins in the hippocampus of lead-treated rats. a–d Representative Western blot and quantitative analysis of Bax, Bcl-2, and cleaved caspase-3 in the hippocampus. All values are expressed as the mean ± SD, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; ##P < 0.01, ###P < 0.001 compared to the Pb group
GM1 Elevated the Expression of SIRT1, CREB Phosphorylation, and BDNF in the Hippocampus
Considering the fact that SIRT1, CREB, and BDNF play a role in neural survival and synaptic plasticity, Western blotting was conducted to detect the expression of these proteins. As shown in Fig. 7, the expression of SIRT1, CREB phosphorylation, and BDNF in the hippocampus of the lead-treated group was markedly decreased compared with that of the control group (Fig. 7c–e) (P < 0.05, P < 0.01, and P < 0.05, respectively). However, the downregulation of these proteins was significantly inhibited by GM1 administration in a dose-dependent manner. Meanwhile, immunohistochemical staining of BDNF showed the same trend as Western blotting (Fig. 7f). Furthermore, ANOVA analysis showed that there was a significant difference in the levels of SIRT1 expression between the control group and the control + GM1 (10 mg/kg) group (P < 0.001), which suggested that GM1 administration may promote the SIRT1 protein to be highly expressed in the hippocampus of developing rats (Fig. 7c).
Effect of GM1 on the expression of SIRT1, CREB phosphorylation, and BDNF in the hippocampus of lead-treated rats. a, c, d, e Representative Western blot and quantitative analysis of SIRT1, CREB phosphorylation, and BDNF in the hippocampus. b The mRNA level of SIRT1 in the hippocampus by real-time PCR. f Representative immunohistochemistry staining images of BDNF expression in the rat hippocampal DG region (× 200, bar = 100 μm; × 400, bar = 50 μm). All values are expressed as the mean ± SD, n = 5. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the Pb group
Discussion
As a susceptible group, children are always the most vulnerable to lead exposure. In recent years, great attention has been paid to studying the correlation between developmental lead exposure and offspring health implications, especially for the nervous system in young ones. In this study, we demonstrated that GM1 exerts an ameliorative role in lead-induced neurotoxicity in the developing rat hippocampus. These beneficial effects of GM1, which are based on the elevation in GM1 expression, suppression of oxidative stress, anti-apoptosis, and neurotrophic action via activating the SIRT1/CREB/BDNF pathway, lead to a definite improvement in animal behavior.
Lead is a common environmental pollutant that can be absorbed into the blood stream quickly through the intestines, respiratory tract, and skin. Due to children’s unique exploratory behaviors (hand-to-mouth, object-to-mouth), stature, living zones, and immature physiological functions, the health effects of lead exposure in childhood are greater than at any other stage. To investigate the effects of lead exposure on early life, we established an animal model that exposed pups to lead after birth. In our study, we found that GM1 cannot lower the concentrations of lead in the blood and hippocampus of the developing rats, which was in accordance with a previous study [28]. However, the behavioral tests showed that GM1 administration significantly ameliorated impairment to rats’ spatial learning and memory induced by lead, with shorter escape latencies and longer time spent in the target quadrant. It is noteworthy that our study also found that extra GM1 administration to normal rats still leads to a significant improvement in spatial navigation in the Morris water maze, which suggests that GM1 may remain valid as a non-pathological target. Meng et al. also found similar results in animal behavior tests after ganglioside administration alone [34]. We suggest that GM1 may achieve cognitive improvement by promoting neural development to a more mature nervous system in advance in developing rats, but the specific mechanism requires further investigation. These results suggest that the cause of neuroprotective effects of GM1 is not by decreasing the amount of lead in the organism, but by acting on resistance to neurotoxic effects induced by lead.
Numerous experimental and epidemiological studies have demonstrated that oxidative stress by increasing ROS generation and damage to the antioxidant defense are essential mechanisms underlying lead neurotoxicity, probably triggering the apoptosis cascade [35]. T-SOD, CAT, GSH, and MDA, main biomarkers of oxidative stress, reflect the antioxidant capacity and oxidative damage degree of the body. A previous study substantiated that lead exposure causes maximum oxidative stress in the hippocampus at the lactation period, with significantly increased levels of TBARS and glutathione peroxidase [36]. Moreover, Yang et al. found that GM1 was able to ameliorate oxidative stress through inhibiting the levels of MDA and HNE in the hippocampus of Alzheimer disease mice [37]. Moreover, Gong et al. also found that exogenous supplementation of GM1 protected rats suffering from high-altitude cerebral edema through suppressing oxidative stress and inflammation via the PI3K/AKT-Nrf2 pathway [38]. Concordant with previous studies, our study demonstrated that lead exposure caused severe oxidative stress in the hippocampus. However, GM1 administration significantly attenuated lead-induced oxidative stress, as reflected by the decreased level of MDA, the restored activity of SOD, CAT, and level of GSH in the hippocampus. Increasing of SOD and CAT activity is considered to be a marker for an elevated antioxidation system.
It is well-documented that Bcl-2 family proteins, including both pro-survival (e.g., Bcl-2) and pro-death (e.g., Bax) proteins, regulate the mitochondria-dependent apoptotic pathway by modulation of mitochondrial outer membrane (MOM) permeabilization and the release of cytochrome c to the cytoplasm, which can lead to caspase activation [39, 40]. Developmental lead exposure can trigger neuronal apoptosis in the neonatal mouse brain, reflecting an activation of caspase-3, a pivotal executor in the process of the caspase cascade [41]. In line with the previous studies, our study shows that lead significantly increases the expression of Bax, decreases in Bcl-2, and activates of cleaved caspase-3. However, GM1 administration reversed these effects in the hippocampus of developing rats. Furthermore, a previous study showed that Bcl-2 overexpression could protect against neuronal apoptosis through restraining cytochrome c release and caspase-3 activation [42]. In our study, a slight overexpression of the Bcl-2 protein was found after GM1 administration alone in the control + GM1 (10 mg/kg) group, which may imply that GM1 could improve the survival of nerve cells against apoptotic stimuli through upregulating the Bcl-2 family to resist apoptosis.
SIRT1 has been well-studied in metabolism, aging, gene silencing, inflammation, cancer, and neurodegenerative diseases [43,44,45]. However, recent studies linked SIRT1 to brain function and demonstrated that it is also involved in synaptic plasticity, memory formation, neurogenesis, and neuroprotection. Convincing evidence indicates that deficiencies of SIRT1 are associated with abnormal dendrite architecture and disordered expression of genes involved in myelination and synaptic function [46]. In contrast, SIRT1 overexpression can improve cell viability and promote neuron survival through mTOR signaling [47]. In addition, the suppression of SIRT1 can also inhibit CREB activity via a miR-134-mediated post-transcriptional mechanism, decreasing the binding of CREB to BDNF promoters, which induces a reduction of BDNF at both mRNA and protein levels [14]. In line with the results of Feng et al., our study found that the expression of SIRT1 was significantly decreased, accompanied by decreased expressions of CREB phosphorylation and the downstream protein of BDNF, in the hippocampus of lead-treated developing rats [48]. Here, GM1 administration upregulated the expression of SIRT1, CREB phosphorylation, and BDNF in a dose-dependent manner. Of note, there was a slight increase in the expression of SIRT1 in the hippocampus of the rats administrated GM1 alone. Accordingly, we surmised that GM1 may be a potential activator of SIRT1 which protects neuron from the neurotoxicity of lead through pleiotropic downstream regulation, but the molecular mechanisms of how GM1 upregulates the expression of SIRT1 and other potential pathways involved are not yet known.
Conclusion
In conclusion, we demonstrated that monosialoganglioside GM1 possesses protective effects on lead-induced impairment of learning and memory through recovering the antioxidant system, anti-apoptosis, and activating the SIRT1/CREB/BDNF pathway in the developing hippocampus. Our study provides new evidence for the mechanisms of GM1 on lead-induced neural damage and proposes a novel potential assistant therapy for lead poisoning.
References
Health CE (2016) Prevention of childhood lead toxicity. Pediatrics 138:e20161493–e20161493. https://doi.org/10.1542/peds.2016-1493
Abdou HM, Hassan MA (2014) Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. Biomed Res Int 2014:435857. https://doi.org/10.1155/2014/435857
Boskabady MH, Tabatabai SA, Farkhondeh T (2016) Inhaled lead affects lung pathology and inflammation in sensitized and control Guinea pigs. Environ Toxicol 31:452–460. https://doi.org/10.1002/tox.22058
Luo W, Ruan D, Yan C, Yin S, Chen J (2012) Effects of chronic lead exposure on functions of nervous system in Chinese children and developmental rats. Neurotoxicology 33:862–871. https://doi.org/10.1016/j.neuro.2012.03.008
Mabrouk A, Ben Cheikh H (2016) Thymoquinone supplementation ameliorates lead-induced testis function impairment in adult rats. Toxicol Ind Health 32:1114–1121. https://doi.org/10.1177/0748233714548474
Senut M-C, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, Suhr ST, Ruden D (2012) Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 4:665–674. https://doi.org/10.2217/epi.12.58
Sharifi AM, Ghazanfari R, Tekiyehmaroof N, Sharifi MA (2011) Investigating the effect of lead acetate on rat bone marrow-derived mesenchymal stem cells toxicity: role of apoptosis. Toxicol Mech Methods 21:225–230. https://doi.org/10.3109/15376516.2010.543943
Canfield RL, Henderson CRJ, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348:1517–1526. https://doi.org/10.1056/NEJMoa022848
Skerfving S, Lofmark L, Lundh T, Mikoczy Z, Stromberg U (2015) Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology 49:114–120. https://doi.org/10.1016/j.neuro.2015.05.009
Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH (2008) Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28:3–9. https://doi.org/10.1523/JNEUROSCI.4405-07.2008
Zhou C-C, Gao Z-Y, Wang J, Wu M-Q, Hu S, Chen F, Liu J-X, Pan H, Yan C-H (2018) Lead exposure induces Alzheimers's disease (AD)-like pathology and disturbes cholesterol metabolism in the young rat brain. Toxicol Lett 296:173–183. https://doi.org/10.1016/j.toxlet.2018.06.1065
Horio Y, Hayashi T, Kuno A, Kunimoto R (2011) Cellular and molecular effects of sirtuins in health and disease. Clin Sci (Lond) 121(5):191–203. https://doi.org/10.1042/cs20100587
Hori YS, Kuno A, Hosoda R, Horio Y (2013) Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One 8(9):e73875. https://doi.org/10.1371/journal.pone.0073875
Gao J, Wang W-Y, Mao Y-W, Graff J, Guan J-S, Pan L, Mak G, Kim D, Su SC, Tsai L-H (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109. https://doi.org/10.1038/nature09271
Kida S, Serita T (2014) Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res Bull 105:17–24. https://doi.org/10.1016/j.brainresbull.2014.04.011
Alberini CM, Kandel ER (2014) The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol 7:a021741. https://doi.org/10.1101/cshperspect.a021741
Ledeen RW (1978) Ganglioside structures and distribution: are they localized at the nerve ending? J Supramol Struct 8:1–17. https://doi.org/10.1002/jss.400080102
Ba X-h (2016) Therapeutic effects of GM1 on Parkinson's disease in rats and its mechanism. Int J Neurosci 126:163–167. https://doi.org/10.3109/00207454.2014.996640
Di Pardo A, Maglione V, Alpaugh M, Horkey M, Atwal RS, Sassone J, Ciammola A, Steffan JS, Fouad K, Truant R, Sipione S (2012) Ganglioside GM1 induces phosphorylation of mutant huntingtin and restores normal motor behavior in Huntington disease mice. Proc Natl Acad Sci U S A 109:3528–3533. https://doi.org/10.1073/pnas.1114502109
Kreutz F, Frozza RL, Breier AC, De Oliveira VA, Horn AP, Pettenuzzo LF, Netto CA, Salbego CG, Trindade VMT (2011) Amyloid-β induced toxicity involves ganglioside expression and is sensitive to GM1 neuroprotective action. Neurochem Int 59:648–655. https://doi.org/10.1016/j.neuint.2011.06.007
Li L, Tian J, Long MK-W, Chen Y, Lu J, Zhou C, Wang T (2016) Protection against experimental stroke by ganglioside GM1 is associated with the inhibition of autophagy. PLoS One 11:e0144219. https://doi.org/10.1371/journal.pone.0144219
Zhu X-Y, Ye M-Y, Zhang A-M, Wang W-D, Zeng F, Li J-L, Fang F (2015) Influence of one-year neurologic outcome of treatment on newborns with moderate and severe hypoxic-ischemic encephalopathy by rhuEP0 combined with ganglioside (GM1). Eur Rev Med Pharmacol Sci 19:3955–3960
Vlasova IA, Zakharova IO, Sokolova TV, Avrova NF (2013) Metabolic effects of ganglioside GM1 on PC12 cells at oxidative stress depend on modulation of activity of tyrosine kinase of trk receptor. Zh Evol Biokhim Fiziol 49:15–23
Gorria M, Huc L, Sergent O, Rebillard A, Gaboriau F, Dimanche-Boitrel M-T, Lagadic-Gossmann D (2006) Protective effect of monosialoganglioside GM1 against chemically induced apoptosis through targeting of mitochondrial function and iron transport. Biochem Pharmacol 72:1343–1353. https://doi.org/10.1016/j.bcp.2006.07.014
Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N (1995) Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci U S A 92:5087–5091
Rabin SJ, Bachis A, Mocchetti I (2002) Gangliosides activate Trk receptors by inducing the release of neurotrophins. J Biol Chem 277:49466–49472. https://doi.org/10.1074/jbc.M203240200
Zakharova IO, Sokolova TV, Vlasova YA, Furaev VV, Rychkova MP, Avrova NF (2014) GM1 ganglioside activates ERK1/2 and Akt downstream of Trk tyrosine kinase and protects PC12 cells against hydrogen peroxide toxicity. Neurochem Res 39:2262–2275. https://doi.org/10.1007/s11064-014-1428-6
She J-Q, Wang M, Zhu D-M, Sun L-G, Ruan D-Y (2005) Effect of ganglioside on synaptic plasticity of hippocampus in lead-exposed rats in vivo. Brain Res 1060:162–169. https://doi.org/10.1016/j.brainres.2005.08.044
Deniz BF, Confortim HD, Deckmann I, Miguel PM, Bronauth L, de Oliveira BC, Barbosa S, Cechinel LR, Siqueira IR, Pereira LO (2018) Folic acid supplementation during pregnancy prevents cognitive impairments and BDNF imbalance in the hippocampus of the offspring after neonatal hypoxia-ischemia. J Nutr Biochem 60:35–46. https://doi.org/10.1016/j.jnutbio.2018.06.008
Wang J, Gao Z-Y, Yan J, Ying X-L, Tong S-L, Yan C-H (2017) Sex differences in the effects of prenatal lead exposure on birth outcomes. Environ Pollut 225:193–200. https://doi.org/10.1016/j.envpol.2017.03.031
Wu J, Pan X, Fu H, Zheng Y, Dai Y, Yin Y, Chen Q, Hao Q, Bao D, Hou D (2017) Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci Rep 7(1):10114. https://doi.org/10.1038/s41598-017-10693-4
Chen L, Liu P, Feng X, Ma C (2017) Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med 21(12):3178–3189. https://doi.org/10.1111/jcmm.12871
Zhang L, Tu R, Wang Y, Hu Y, Li X, Cheng X, Yin Y, Li W, Huang H (2017) Early-life exposure to lead induces cognitive impairment in elder mice targeting SIRT1 phosphorylation and oxidative alterations. Front Physiol 8
Meng H, Wang L, He J, Wang Z (2016) The protective effect of gangliosides on lead (Pb)-induced neurotoxicity is mediated by autophagic pathways. Int J Environ Res Public Health 13:365. https://doi.org/10.3390/ijerph13040365
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158. https://doi.org/10.1016/j.neuro.2016.07.013
Barkur RR, Bairy LK (2015) Assessment of oxidative stress in hippocampus, cerebellum and frontal cortex in rat pups exposed to lead (Pb) during specific periods of initial brain development. Biol Trace Elem Res 164:212–218. https://doi.org/10.1007/s12011-014-0221-3
Yang R, Wang Q, Min L, Sui R, Li J, Liu X (2013) Monosialoanglioside improves memory deficits and relieves oxidative stress in the hippocampus of rat model of Alzheimer's disease. Neurol Sci 34:1447–1451. https://doi.org/10.1007/s10072-012-1263-y
Gong G, Yin L, Yuan L, Sui D, Sun Y, Fu H, Chen L, Wang X (2018) Ganglioside GM1 protects against high altitude cerebral edema in rats by suppressing the oxidative stress and inflammatory response via the PI3K/AKT-Nrf2 pathway. Mol Immunol 95:91–98. https://doi.org/10.1016/j.molimm.2018.02.001
Renault TT, Manon S (2011) Bax: addressed to kill. Biochimie 93:1379–1391. https://doi.org/10.1016/j.biochi.2011.05.013
Volkmann N, Marassi FM, Newmeyer DD, Hanein D (2014) The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ 21:206–215. https://doi.org/10.1038/cdd.2013.153
Dribben WH, Creeley CE, Farber N (2011) Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol 33:473–480. https://doi.org/10.1016/j.ntt.2011.05.006
Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK (2003) Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem 85:1026–1036
Chang H-C, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25:138–145. https://doi.org/10.1016/j.tem.2013.12.001
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344–352. https://doi.org/10.1002/emmm.201302451
Hwang J-w, Yao H, Caito S, Sundar IK, Rahman I (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110. https://doi.org/10.1016/j.freeradbiomed.2013.03.015
Michan S, Li Y, Chou MM-H, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LEH, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30:9695–9707. https://doi.org/10.1523/JNEUROSCI.0027-10.2010
Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J (2011) Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res 89:1723–1736. https://doi.org/10.1002/jnr.22725
Feng C, Gu J, Zhou F, Li J, Zhu G, Guan L, Liu H, Du G, Feng J, Liu D, Zhang S, Fan G (2016) The effect of lead exposure on expression of SIRT1 in the rat hippocampus. Environ Toxicol Pharmacol 44:84–92. https://doi.org/10.1016/j.etap.2016.04.008
Acknowledgements
Fei Chen especially wants to thank the support from his senior CanCan Zhou.
Funding
This work was supported by the National Basic Research Program of China (973 Program, 2012CB525001), the National Natural Science Foundation of China (Grant No. 81472993), and National Key R&D Program of China (2017YFC1600500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was carried out in strict accordance with the international standards of animal care guidelines, and the experiments were approved by Institutional Animal Care Committee at Shanghai Jiao tong University School of Medicine.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, F., Zhou, CC., Yang, Y. et al. GM1 Ameliorates Lead-Induced Cognitive Deficits and Brain Damage Through Activating the SIRT1/CREB/BDNF Pathway in the Developing Male Rat Hippocampus. Biol Trace Elem Res 190, 425–436 (2019). https://doi.org/10.1007/s12011-018-1569-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1569-6