Abstract
Selenium (Se), an essential trace element and potent nutritional antioxidant, exerts its biological effects through incorporation into selenoproteins like glutathione peroxidase (GPx). Modest decrement in the levels of GPx could be partly responsible for peroxidation of RBCs, which results into hemolytic anemia. Therefore, it is hypothesized that dietary Se, as selenoproteins (GPx), can maintain the homeostasis in RBCs and regulate the erythropoiesis by preventing oxidative stress-mediated hemolysis. Se-deficient (0.01 ppm), Se-adequate (0.1 ppm sodium selenite), and Se-supplemented (0.5 ppm sodium selenite) status were created in Balb/c mice by feeding yeast-based diets for 8 weeks and established by measuring Se levels in plasma and activities, expressions of Se-dependent selenoproteins. Fifty percent of mice from each differential Se group were treated with phenylhydrazine (PHZ, 20 mg/kg, i.p.) to induce hemolytic anemia. Results indicated that PHZ-treated Se-deficient animals demonstrated increased hemolysis, abnormal RBC morphology, increase in Heinz bodies and reticulocytes, and denaturation of hemoglobin to globin precipitates and methemoglobin. Se supplementation protected against these hemolytic changes and makes RBCs less fragile. These findings were consistent with dietary Se concentration-dependent changes in activity and expression of GPx indicating that ROS-mediated oxidative stress is integral to hemolysis. Protective effects of Se supplementation against increased levels of ROS, protein carbonyls, and peroxide damage to membrane lipids and enzymatic antioxidants validated these observations. In conclusion, dietary Se supplementation protected the RBCs against hemolysis by mitigating ROS-mediated oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) as an essential trace element, micronutrient, and antioxidant [1] has important structural and enzymatic functions. Diet is the major Se source in the body, and about 80% of Se gets absorbed in the body depending on the type of food consumed. Therefore, the factors such as geographical location, protein content, seasonal changes, and food processing detrimentally affect the quantity of Se in food [2] and thus in our body. On the basis of data from animal studies, the recommended dose of Se in adult human beings is 55 μg/day [3], which correspond to approximately 0.8 μg/kg/day [4] and favorably complements the animal studies. Based on the epidemiological studies, the pathophysiological functions of Se can be divided into two different areas, which involve the importance of Se nutritional essentiality and toxicity [5]. Se deficiency is associated with variety of oxidative damage-mediated pathological conditions such as Keshan’s disease, Kashin-Beck disease, and variety of cancers and inflammatory conditions [6]. On the other hand, at adequate levels, it contributes in many biochemical and physiological processes, including regulation of ion fluxes across membranes, biosynthesis of coenzyme Q, and stimulation of antibody synthesis, besides its role as antioxidant enzymes including glutathione peroxidase (GPx) and thioredoxin reductase (TR).
In relation to anemia, epidemiological data is suggestive of an inverse relationship between serum Se levels and increased risk as well as incidence for anemia [7, 8]. Although there is a little direct evidence that serum Se is related to erythrocyte and hemoglobin stability in humans; increased erythrocyte damage has been shown due to increased oxidative stress in animals [9]. Reactive oxygen species (ROS)-mediated oxidative stress is not only implicated as one of the principal causative factors in the pathogenesis of various disorders of erythrocytes including various anemias [10] but can also exacerbate the symptoms of these diseases due to ineffective erythropoiesis in the bone marrow and shortened survival of red blood cells (RBC) in the circulation inducing hemolysis [11]. Further, the fact that RBCs lack the cellular and molecular machinery necessary for the renewal of oxidized biomolecules makes them more susceptible to oxidative insults [12]. For example, Fe (II)-heme in oxygenated (Hb-O2) form is prone to autoxidation leading to formation of Fe (III)-heme that is unable to bind oxygen and releases superoxide radicals, which actively participate in the pathogenesis of anemia [13].
Therefore, the supplementation of nutritional antioxidants such as Se holds great potential to protect against anemia. The lack of GPx (a selenoprotein), an integral and primary antioxidant enzyme in RBCs, may render them prone to oxidative damage, and thus, hemolysis [14] indicates the critical role of Se in maintaining the low levels of peroxides in cells and, thereby, ablates the damaging effects of oxidative stress as seen in anemia [15]. However, in view of lack of experimental and mechanistic studies on protective effects of Se, it was hypothesized that Se as an antioxidant selenoprotein may protect from phenylhydrazine (PHZ)-induced hemolytic anemia via redox modulation and maintaining the redox homeostasis in RBCs.
Materials and Methods
Experimental Design
Balb/c mice of 3–4 weeks of age were procured from the Central Animal House, Panjab University, Chandigarh. They were fed on yeast-based diets containing different concentrations of Se (added as sodium selenite), viz. 0.01 ppm (Se-deficient), 0.1 ppm (Se-adequate), or 0.5 ppm (Se-supplemented) for 8 weeks as reported earlier [16]. Following 8 weeks of diet feeding schedule, each group was divided into two subgroups, i.e., with or without the treatment of phenylhydrazine (PHZ). The mice were 12 weeks old and weighed ~ 25 g at this time. Table 1 gives a detailed account of experimental design.
Establishment of Se Status
After diet feeding schedule, Se status was established by measuring the levels of Se in the blood plasma using 2,3-diaminonaphthalene (DAN) as a fluorophore as described previously [17]. The principle of this method involves conversion of Se present in the samples to selenous acid on acid digestion, which on reaction with aromatic-o-diamines such as DAN, leads to the formation of 4,5-benzopiazselenol, which displays brilliant lime-green fluorescence. Suitable Se standards and blanks were run simultaneously. Further, these results were validated by measuring the activities and expression of dietary Se-dependent selenoprotein GPx and TR-1 in liver and plasma according to the method of Paglia and Valentine [18] and Holmgren and Bjornstedt [19], respectively. The total protein estimation was done by the method of Lowry et al. [20].
Thereafter, animals from each of above mentioned groups were divided into two subgroups leading to the formation of a total of six groups (n = 4 per subgroup). Three subgroups received PHZ at dose of 20 mg/kg body weight, intraperitoneally (IP). PHZ was dissolved in sterile PBS in a glass vial at a concentration of 2 mg/mL. For each injection, fresh PHZ was prepared and 250 μL of stock was injected into each animal of weight 25 g for 1 week to induce hemolytic anemia [21]. PHZ was handled wearing gloves, apron, and face mask inside a hood. After the PHZ dosing schedule, 1.0 mL blood was obtained through the retro-orbital plexus. Two hundred microliters of fresh blood was used, only for hematological estimations, and from remaining the blood, plasma was prepared to be used for biochemical estimations of oxidative stress markers and antioxidant enzymes. Following blood withdrawal, the mice were put under deep anesthesia with ether followed by cervical dislocation. Liver samples were collected from the animals, and a 10% homogenate was made in RIPA buffer, pH = 7.4 for further use. The details regarding the volume blood/plasma samples used for various estimations have been included in supplementary Fig 1.
The protein estimation was done by Lowry method [20] and used for further analyses.
Hematological Parameters
Blood smears (4–5 μL blood) were prepared to study the morphological changes in erythrocytes. For analyses of reticulocytes and Heinz bodies, the RBCs were stained with new methylene blue and observed under a Leica microscope (DM 3000). Total RBC cell count and hemoglobin (Hb) content were measured using hemocytometer and Sahli’s hemoglobinometer, respectively, using the standard protocol [22].
Levels of MetHb
MetHb content was determined by the method of Sakata et al. [23]. Sodium cyanide solution (10%) was added to heparinized blood (100 μL). Disappearance of the characteristic absorption at 635 nm signifying the formation of cyanmethemoglobin was monitored spectrophotometrically.
Osmotic Fragility Test
Heparinized venous blood (10 μL) was added into tubes with increasing concentration (0.3%, 0.5%, 0.7, and 0.9%) of NaCl (pH = 7.4). The tubes were gently mixed and incubated at 25 °C for 30 min. Samples were centrifuged at 1500g for 10 min, and hemolysis was evaluated spectrophotometrically at 540 nm.
Analysis of Globin Precipitates
For globin chain analysis in membrane skeleton, freshly drawn blood (75 μL) was lysed and membranes were washed extensively in phosphate buffered saline (0.05%) followed by extraction of membrane lipids with sodium borate (56 mM, pH 8.0). Precipitated globins were dissolved in 8 M urea containing 10% acetic acid, 10% β-mercaptoethanol, and 0.04% pyronin. The samples were then electrophoresed on triton-acetate-urea (TAU) gels and stained with Coomassie brilliant blue as described [24].
Measurement of ROS-Mediated Oxidative Stress
Total ROS Levels
ROS levels in 50 μL of plasma sample was estimated using the fluorescent probe 2,7 dichlorofluorescein diacetate (DCFH-DA) as described by Best et al. [25]. This method is based on deacetylation of DCFH-DA by ROS-mediated intercellular esterases to reduce dichlorofluorescein (DCFH), which on reaction with ROS, gets oxidized to fluorescent 2,7-dichlorofluorescein (DCF).
Determination of Protein Carbonyl Content
Protein carbonyl content was measured in 100 μL of plasma by using 2,4-dinitrophenylhyrdazine (DNPH) as described by Levine et al. [26]. It is based on the reaction of DNPH and protein carbonyls. DNPH reacts with protein carbonyl by forming a Schiff base to produce the corresponding hydrazone, which can be analyzed spectrophotometrically at 366 nm. The results are expressed as nanomoles of carbonyl groups per milligrams of protein, using the extinction coefficient of 22.0 mM−1 cm−1 for aliphatic hydrazones.
Lipid Peroxidation Assay
The levels of lipid peroxidation (LPO) were assayed in 50 μL of the blood plasma by the method of Wills [27] using 1,1,3,3-tetraethoxypropane as standard. This assay is based on reaction of malondialdehyde (MDA), a degradation product of peroxidized lipids, with thiobarbituric acid (TBA) making a pink-colored TBA-MDA chromophore with the absorption characteristics (absorption maxima at 532 nm).
Activities of Antioxidant Enzymes
Catalase activity assay was done in 10 μL plasma by the method of Luck, 1963 [28]. The activity was calculated using an extinction coefficient of 0.0394 mM−1 cm−1 at 240 nm and expressed as milliMolars of H2O2 decomposed/min/mg protein.
The activity of the dietary Se-dependent selenoprotein GPx in liver and plasma (25 μL) was measured according to the method of Paglia and Valentine [18]. The activity was expressed as micromoles of nicotinamide adenine dinucleotide phosphate (reduced) (NADPH) oxidized/min/mg protein. TR-1 activity was measured in 25 μL of blood by a modification of the method described by Holmgren and Bjornstedt [19] and expressed as units per milligram of protein.
The activity of superoxide dismutase (SOD) was estimated in both liver and plasma (50 μL) according to the method of Kono [29]. This method was based on the principle of the inhibitory effect of SOD on reduction of nitro blue tetrazolium (NBT) dye by superoxide anions, which were generated by the photo-oxidation of hydroxylamine hydrochloride (NH2OH.HCl). The activity of SOD was expressed as international units per milligram of protein, where 1 IU was defined as the amount of enzyme inhibiting the increase in OD by 50%.
Glutathione reductase (GR) activity was measured in 10 μL of blood plasma by the method of Massey and Williams [30]. The consumption of NADPH is directly related to the activity of GR and was expressed as micromoles of NADPH oxidized/min/mg protein.
Gene Expression Studies
Gene Expression Studies by Real-Time PCR
The changes in gene expression of selenium-dependent selenoproteins which includes GPx-1, GPx-2, and TR-1 were analyzed by real-time PCR. RNA was isolated from blood and liver tissues using TRIzol Reagent, and Real-time PCR was carried out on Applied Biosystems, Step one Real-Time PCR system. cDNA was prepared by using Prime Script™ 1st strand cDNA kit (TaKaRa Clontec, USA). Gapdh was used as an internal control to normalize the data. Real-time PCR was performed by using the SYBR® Green PCR Mastermix. The sequences of the primers used are given below: mouse Gapdh sense 5′-AAGGTCATCCCAGAGCTGAA-3′ and antisense 5′-CTGCTTCACCACCTTCTTGA-3′; mouse GPx-1 sense 5′-GGTTCGAGCCCAATTTTACA-3′ and antisense 5′-CATTCCGCAGGAAGGTAAAG-3′; mouse GPx-2 sense 5′-ACCGATCCCAAGCTCATCAT-3′ and antisense 5′-CAAAGTTCCAGGACACGTCTGA-3′; mouse TR-1 sense 5′-CGTGGTGGACTTCTCTGCTACGTGGTG-3′ and antisense 5′-GGTCGGCATGCATTTGACTTCACAGTC-3′.
Protein Expression by Western Blot Analysis
Protein samples (50 μg) from each treatment group were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. The blots were probed using primary antibodies against GPx (Santa Cruz Biotechnology Inc.) at a dilution of 1:1000 and alkaline phosphatase-conjugated secondary antibody at a dilution of 1:10,000 (Genei, Bangalore) followed by development using BCIP-NBT detection system. Bands obtained were densitometrically analyzed using Image J software (NIH, Bethesda, MD, USA), and normalized densitometric units w.r.t β-actin (served as protein loading control) were plotted.
Enzyme-Linked Immunosorbent Assay
Twenty micrograms of protein sample was loaded on to enzyme-linked immunosorbent assay (ELISA) strip wells in carbonate buffer (100 μL) and kept overnight at 4 °C. Blocking was done using 1% BSA (200 μL) for 1 h at 37 °C. After washing with PBS-Tween 20 (0.05%), the wells were incubated with GPx/β-actin antibodies (1:1000) overnight (100 μL). After washing with PBS-Tween 20 (0.05%), the wells were incubated with alkaline phosphatase-labeled secondary antibody (1:2500) for 2 h at 37 °C (100 μL). After washing with PBS-Tween 20 (0.05%), plates were developed using BCIP/NBT kit and quantitated at 405 nm using ELISA strip reader. The normalization was done using β-actin protein as internal control. Protein concentrations for all the methods were determined by the method of Lowry et al. [20].
Statistical Analysis
All data were expressed as mean ± SD of at least four independent observations. The data was analyzed using one-way ANOVA (Tukey’s multiple comparison method) to compare various treatment groups using GraphPad Prism 5.0 program (GraphPad Software, San Diego, CA).
Results
Establishment of Differential Se Status
In the present study, the differential Se status in mice was established by measuring the levels of Se in the blood and the dietary Se-dependent changes in the expression of selenoproteins GPx-1, GPx-2, and TR-1. Currently, a statistically significant change in the levels of Se in the plasma was found in different groups fed on differential Se diets. Consistent with these changes, Se concentration-dependent increases in the activity and expression of GPx (1/2), and TR-1 in blood plasma and liver was noticed from Se-deficient (group I) to Se-adequate (group II) and Se-supplemented group of animals (group III) (Fig. 1a–e) indicating that differential physiological Se status has been achieved in these animals.
Dietary Se-dependent changes in markers of Se status: a Se concentration in blood plasma; b changes in the enzymatic activity of GPx; c changes in GPx expression by ELISA (inset shows Western Immunoblot of GPx); and d and e shows the mRNA expression of GPx-1, GPx-2, and TR-1 in the blood plasma and liver samples. Data is expressed as mean ± SD of at least four independent observations and analyzed using one-way ANOVA (Tukey’s multiple comparison method). (a) Represents p < 0.05 when compared between Se D vs Se A, Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (b) represents p < 0.05 when compared between Se A vs Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ
Se Protects Against Phenylhydrazine-Induced Hemolytic Anemia
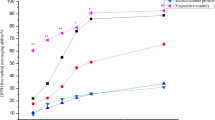
Increased hemolysis was observed in the blood smears of Se-deficient (group I) and Se-Def + PHZ (group IV). Further, the loss of normal biconcave morphology, polychromatophils and fragmented red cells, and frequent presence of star-shaped echinocytes and spherocytosis was seen in Se-deficient + PHZ-treated group (group IV) (Fig. 2a, d). On the contrary, the blood smears of Se-supplemented-treated (group III) and Se-supplemented + PHZ (group VI) mice showed normal biconcave morphology and looked normocytic (Fig. 2c, f). Noteworthy was the exacerbation of these changes on PHZ administration to Se-deficient mice, whereas Se supplementation posed some protective effect against PHZ-induced hemolytic damage. These changes were consistent with the changes in the total RBC count and standard osmotic fragility tests, which revealed that the erythrocytes of Se-deficient (group I) and Se-deficient + PHZ (group IV) mice were more prone to hemolysis as compared to Se-adequate + PHZ (group V), Se-supplemented + PHZ (group VI), and respective controls (groups II and III) (Fig. 2g, h).
Blood smears indicating the morphology and shape of RBCs in peripheral blood of mice in different treatment groups. Increased number of fragmented RBCs, echinocytes (star-shaped erythrocytes), and spherocytes in the Se-deficient and Se-deficient + PHZ groups indicate oxidative stress-mediated damage to erythrocytes to a much greater extent when compared to the Se-supplemented diet-fed group (a–f). Representative of n = 4 mice per group. Effect of dietary Se shows changes in total RBC count. These findings are consistent with changes in number of reticulocytes seen in panel g. h Dietary Se deficiency makes the RBCs osmotic fragile and PHZ treatment exacerbates these changes. Erythrocytes were subjected to buffered hypo-osmolar salt solutions (0.3–0.9%, w = v), following which cell lysis was monitored by measuring optical density at 540 nm
On the similar lines, a significant increase in the reticulocytes and Heinz bodies in the blood smears of Se-deficient + PHZ (group IV) mice compared to Se-adequate + PHZ (group V) and Se-supplemented + PHZ (group VI) mice and their respective controls (groups II and III) (Fig. 3a–g) were observed. Although we saw some improvement in Se Sup + PHZ (group VI) compared to Se-deficient (group I) and Se-deficient + PHZ (group IV) mice, these differences were relatively milder when compared to Se Sup (group III) mice (Fig. 3a–g).
New methylene blue staining of the blood smears of different Se-treated groups for reticulocyte counts and Heinz bodies. Increased number of granulated spots are observed in Se-deficient and Se-deficient + PHZ groups (a–f). These findings are consistent with changes in number of reticulocytes seen in panel g. Data is expressed as mean ± SD of at least four independent observations and analyzed using one-way ANOVA (Tukey’s multiple comparison method). (a) Represents p < 0.05 when compared between Se D vs Se A, Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (b) represents p < 0.05 when compared between Se A vs Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (c) represents p < 0.05 when compared to Se S vs Se D + PHZ, Se A + PHZ, Se S + PHZ; (#) represents p < 0.05 when compared to Se D + PHZ vs Se A + P, Se S + PHZ; (@) represents p < 0.05 when compared to Se A + PHZ vs Se S + PHZ
An increase in the levels of MetHb and decrease in hemoglobin (Hb) (Fig. 4a, b) indicated increased oxidation of ferrous ions in Hb in the erythrocytes of Se-deficient + PHZ group (group IV) of animals, while the improvement in the levels of Hb and reduced MetHb levels upon Se supplementation (Fig. 4a, b) was observed. Corroborating with these findings, analysis of the membrane ghosts for precipitated globin chains by TAU gel electrophoresis demonstrated a higher intensity of bands corresponding to α- and β-chains in the Se-deficient (group I) and Se-deficient + PHZ (group IV) mice, whereas those in the Se-adequate + PHZ (group V), Se-supplemented + PHZ (group VI), and respective controls were substantially reduced (Fig. 4c). Consistently, additional high intensity molecular-mass band of denatured β-globin chain (represented by an asterisk) was observed only in the ghosts of Se-deficient and Se-deficient + PHZ (groups I and IV) mice, which is suggestive of ROS-mediated oxidative damage. These observations are in consistence with the increased number of echinocytes present in Se-deficient and Se-deficient + PHZ groups suggesting potential fragmentation and spherocytosis, signifying enhanced susceptibility to lysis during circulation.
a, b Effect of dietary Se shows changes in the levels of Hb and MetHb in different treatment groups. Increased levels of methemoglobin in the mature erythrocytes of Se-deficient and Se-deficient + PHZ groups suggest oxidation of ferrous ions in HGB to ferric form. c Effects of Se variation-induced oxidative stress on Hb subunits. Triton-acid-urea gel demonstrates the precipitation of α- and β-chains of Hb after oxidative denaturation. The asterisk represents an additional high intensity molecular-mass bands of denatured β-globin chain in TAU-Gel, which is suggestive of ROS-mediated oxidative damage. These oxidized and denatured globins as seen in Se-deficient and PHZ groups have been proposed as possible mechanisms of variety of hematological disorders. Data is expressed as mean ± SD of at least four independent observations and analyzed using one-way ANOVA (Tukey’s multiple comparison method). (a) Represents p < 0.05 when compared between Se D vs Se A, Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (b) represents p < 0.05 when compared between Se A vs Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (c) represents p < 0.05 when compared to Se S vs Se D + PHZ, Se A + PHZ, Se S + PHZ; (#) represents p < 0.05 when compared to Se D + PHZ vs Se A + P, Se S + PHZ; (@) represents p < 0.05 when compared to Se A + PHZ vs Se S + PHZ
Protective Effects of Se Against PHZ-Induced Hemolytic Anemia Are Mediated by Redox Modulation
A statistically significant increase in the levels of free radicals (measured by fluorescence of DCF) in the blood of Se-deficient (group I) was observed compared to Se-adequate (group II) and Se-supplemented (group III) mice (Fig. 5a, b, c). Administration of PHZ significantly increased the ROS levels, lipid peroxidation, and protein carbonyls in Se-deficient animals (group IV) compared to Se-adequate + PHZ (group V) and Se-supplemented + PHZ (group VI) groups indicating the exacerbation of oxidative stress (Fig. 5a, b, c). Currently, although the levels of protein carbonyls were elevated in Se-deficient (group I) animals compared to Se-adequate (group II) and Se-Supplemented (group III), these differences were not found to be statistically significant (Fig. 5b).
Induction of oxidative stress in erythrocytes following dietary Se variation and PHZ treatments. a Alterations in the level of total ROS as measured by changes in the fluorescence of DCF in plasma b levels of protein carbonyls in plasma and c malondialdehyde levels in Se-deficient and Se-deficient + PHZ groups indicate induction of oxidative stress conditions in these mice as compared to Se-supplemented groups. Data is expressed as mean ± SD of at least four independent observations and analyzed using one-way ANOVA (Tukey’s multiple comparison method). (a) Represents p < 0.05 when compared between Se D vs Se A, Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (b) represents p < 0.05 when compared between Se A vs Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (c) represents p < 0.05 when compared to Se S vs Se D + PHZ, Se A + PHZ, Se S + PHZ; (#) represents p < 0.05 when compared to Se D + PHZ vs Se A + P, Se S + PHZ; (@) represents p < 0.05 when compared to Se A + PHZ vs Se S + PHZ
Se concentration-dependent change in the GPx and TR-1 expression and activity was seen in the liver and blood of mice. GPx and TR-1 were significantly reduced in Se-deficient (group I) as compared to Se-supplemented group (III) (Fig. 6a, b, c). Administration of PHZ in these mice however exacerbated these differences. It was found that Se-deficient mice when given PHZ (group IV), the GPx levels reduced even more when compared to Se-deficient (group I) mice alone (Fig. 6a, b, c), indicating increased production of peroxides. Further, no change in the catalase activity was observed in the plasma of Se-deficient (group I), Se-adequate (group II), and Se-supplemented (group III) mice when compared amongst each other (Fig. 6d). However, on the administration of PHZ to Se-deficient animals (group IV), the catalase expression was found to be significantly elevated in plasma as compared to Se-adequate (group II), Se-supplemented (group III), and respective PHZ-treated groups (groups V and VI) (Fig. 6d). On the other hand, in the liver homogenates, PHZ administration to Se-deficient animals (group IV) significantly reduced the catalase expression when compared to Se-deficient (group I), Se-adequate (group II), Se-supplemented (group III), and respective PHZ-treated groups (groups II and V) except Se-supplemented + PHZ group (VI), where an increase is seen (Fig. 6d).
Alterations in the antioxidant enzymatic activities. a, b, c Changes in GPx and TR-1 activity and GPx expression in the plasma and liver. d, e, f Depicts changes in activities of antioxidant enzymes catalase, SOD, and GR respectively in the plasma and liver. Data is expressed as mean ± SD of at least four independent observations and analyzed using one-way ANOVA (Tukey’s multiple comparison method). (a) Represents p < 0.05 when compared between Se D vs Se A, Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (b) represents p < 0.05 when compared between Se A vs Se S, Se D + PHZ, Se A + PHZ, Se S + PHZ; (c) represents p < 0.05 when compared to Se S vs Se D + PHZ, Se A + PHZ, Se S + PHZ; (#) represents p < 0.05 when compared to Se D + PHZ vs Se A + P, Se S + PHZ; (@) represents p < 0.05 when compared to Se A + PHZ vs Se S + PHZ
In the present study, a significant decrease in SOD activity was observed in Se-deficient (group1), Se-deficient + PHZ (group IV), and in Se-adequate + PHZ (group V) animals, whereas its activity was increased in Se-adequate (group II), Se-supplemented group (group III), and Se supplemented + PHZ (group VI). The SOD activity in the liver samples also follow the same pattern (Fig. 6e). Also, a significant decrease was observed in the activity of GR in Se-deficient (group I) when compared to Se-adequate (group II) and Se-supplemented (group III). After PHZ administration, also, a significant decrease was observed in Se-deficient + PHZ (group IV) and Se-adequate + PHZ when compared with Se-supplemented + PHZ (Fig. 6f). In case of liver homogenate also, the GR activity pattern was the same.
Discussion
Recent past has witnessed a substantial progress in our knowledge and understanding of the beneficial roles of variety of essential and non-essential trace elements acting as biologically active antioxidants in disease and functions. In this context, their protective role against experimental hemolytic anemia has been extensively studied [31]. It is a well-known fact that oxidative stress is a key player in blood-related disorders including anemia, and GPx, a well-known selenoprotein, is of prime importance in erythrocytes [32]. These studies prompted us to investigate the potential role of dietary Se in regulating red cell homeostasis and protecting against PHZ-induced hemolytic anemia by mitigating oxidative stress-dependent debilitating effects in RBCs.
More than four decades ago, Rotruck et al. [33] proposed that Se, which functions as an antioxidant through selenoproteins, prevented the erythrocyte lysis (autohemolysis) in vitro. Since then, the plethora of studies have shown an inverse relationship between serum Se levels and increased risk and incidence for anemia [7, 8]. However, none of these follow-ups address the underlying molecular mechanisms and lack of direct evidence between serum Se levels and erythrocyte and/or hemoglobin stability. Since increased oxidative stress has been implicated as one of the key factors in inducing erythrocyte damage [34], it was hypothesized that Se, as an essential dietary antioxidant, holds the potential to protect these changes.
Since dietary Se, a metalloid reportedly, preferentially incorporates into selenoproteins including GPx; therefore, activity and expression of the enzyme GPx are considered as physiological indicators of Se concentrations [35]. In the present study, the differential Se status in mice was established by measuring the Se concentration-dependent changes in the expression of selenoproteins GPx-1, GPx-2, and TR-1. Currently, a dietary Se concentration-dependent change in the GPx (1 and 2) and TR-1 activity and expression reflects the achievement of differential Se status in these animals. These findings are in corroboration with the studies suggesting regulation of selenoproteins is dietary Se-mediated [36].
In the present study, widely used PHZ-induced experimental hemolytic anemia was established and characterized using the classical histopathological markers such as changes in RBC morphology, reticulocyte counts, and presence of Heinz bodies. Significantly increased reticulocyte counts and Heinz bodies in Se-deficient + PHZ (group IV) demonstrate that Se deficiency and PHZ-mediated structural-functional RBC abnormalities making Se-deficient RBCs susceptible to hemolysis [16]. Studies in the past have also suggested Heinz bodies as markers to depict oxidative stress-mediated changes in the red blood cell metabolism and hemoglobinopathies [37]. On the similar lines, presence of Heinz bodies as the inclusions in red blood cells represent the accumulation of denatured hemoglobin and the formation of MetHb [38]. Results suggest that Se deficiency and PHZ as pro-oxidants can cause oxidation of hemoglobin and its precipitation to produce Heinz bodies, which become antigenic. These findings further validate our hypothesis and are in agreement with previous reports suggesting an inverse relationship between Se, hemolysis, and oxidation of Hb to MetHb [33]. Although, we saw some improvement with Se supplementation, the differences were relatively milder indicating the involvement of other associated factors contributing to these protective effects of Se. One such mechanism may be indicating the execution of compensatory stress erythropoiesis [16] in these groups.
The changes in the levels of Hb and MetHb prompted us to investigate the possibility that Se deficiency and PHZ-mediated oxidative stress may denature the globin subunits. These oxidized and denatured globins as seen in Se-deficient and PHZ groups have been proposed as possible mechanisms of variety of hematological disorders [39]. In fact, monomeric α-Hb is known to generate ROS that damages cellular biomolecules that further aids in the propagation of free radical production [40]. This explains increased susceptibility of the Se-deficient (group I) and Se-deficient + PHZ (group IV) mice to destructive changes, which can further lead to the formation of a reactive oxygen species (ROS) [41] causing severe damage to cell membranes making them osmotically fragile and prone to hemolysis. These observations are inconsistent with the increased number of echinocytes present in Se-deficient and Se-deficient + PHZ groups suggesting potential fragmentation and spherocytosis, signifying enhanced susceptibility to lysis during circulation.
Further, to establish the fact that the protective effects of Se observed so far were mediated through the modulation of ROS-mediated oxidative stress, total reactive oxygen species (ROS), levels of protein carbonyls, lipid peroxidation, and activity and expression of enzymatic antioxidants were measured. Increased levels of free radicals in the blood of Se-deficient and PHZ-treated groups compared to PHZ-treated Se-adequate and Se-supplemented mice can be explained on the basis of decreased levels of antioxidant selenoproteins, such as GPx-1 and TR-1 in Se-deficient conditions. These studies indicate increased production of peroxides and oxidative stress in the Se-deficient conditions as reported earlier [36]. Since, superoxide radical is the main free radical produced in physiological conditions in the cell, SOD is considered to be the main element of the first level of antioxidant defense in the cell [33]. Similarly, in the context of anemia, GR also plays a very important role in protecting biological cell membranes, hemoglobin, and red cell enzymes against oxidative damage by increasing the level of reduced glutathione during the process of aerobic glycolysis. Studies suggest that GR deficiency may result in mild to moderately severe hemolytic anemia upon exposure to certain drugs or chemicals [37]. Thus, the changes in the activities of these antioxidant enzymes also support the redox modulatory effects of Se [42,43,44].
In conclusion, it is evident that Se protects against PHZ-induced hemolytic anemia via redox modulation. PHZ when administered under Se-deficient conditions exacerbates the ROS-mediated oxidative insult culminating in oxidation of membrane lipids and proteins such as Hb culminating in increased hemolysis. On the contrary, Se supplementation by increasing the pool of selenoproteins like GPx mitigates the ROS levels and, thus, protects against the oxidative damage posed by PHZ. Current study points to the direction that protective antioxidant effects of Se may be mediated through the Se-dependent selenoproteins, which may regulate the key molecular pathways responsible for maintaining homeostasis in blood cells.
References
Paqmantidis V, Bermano G, Villette S, Broom I, Arthur J, Hesketh J (2005) Effect of selenium depletion on glutathione peroxidase and selenoprotein W gene expression in the colon. FEBS Lett 579:792–796
Navarro-Alarconm M, Cabrera-Vique C (2008) Selenium in food and the human body. A review. Sci Total Environ 400:115–141
Hatfield DL, Berry MJ, Gladyshev VN (2001) Selenium: Its molecular biology and role in human health, pp 524–525
Rayman (2000) The importance of selenium to human health. Lancet 15:233–241
Zhao J, Xing H, Liu C, Zhang Z, XU S (2016) Effect of selenium deficiency on nitric oxide and heat shock proteins in chicken erythrocytes. Biol Trace Elem Res 171:208–213
Conrad M, Schweizer Y (2010) Unveiling the molecular mechanism behind selenium-related disease through knockout mouse studies. Antioxid Redox Signal 12:851–865
Semba RD, Ricks MO, Ferrucci L, Xue QL, Guralnik JM, Fried LP (2009) Low serum selenium is associated with anemia among older adults in the United States. Eur J Clin Nutr 63:93–99
Kupka R, Msamanga GI, Spiegelman D, Morris S, Mugusi F, Hunter DJ, Fawzi WW (2004) Selenium status is associated with accelerated HIV disease progression among HIV-1–infected pregnant women in Tanzania. J Nutr 134:2556–2560
Ghaffari S (2008) Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 10(11):1923–1940
Iuchi Y, Okada F, Onuma K, Onoda T, Asao H, Kobayashi M, Fujii J (2007) Elevated oxidative stress in erythrocytes due to an SOD1 deficiency causes anemia and triggers autoantibody production. Biochem J 402:219–227
Fibach E, Rachmilewitz E (2008) The role of oxidative stress in hemolytic anemia. Curr Mol Med 8:609–619
Fujii J, Kurahashi T, Konno T, Homma T, Luchi Y (2015) Oxidative stress as a potential causal factor for autoimmune hemolytic anemia and systemic lupus erythematosus. World J Nephrol 4:213–222
Johnson RM, GJr G, Ravindranath Y, Ho YS (2005) Hemoglobin autoxidation and regulation of endogenous H2O2 levels in erythrocytes. Free Radic Biol Med 39:1407–1417
Scott MD, Lubin BH, Zuo L, Kuypers FA (1991) Erythrocyte defense against hydrogen peroxide: preeminent importance of catalase. J Lab Clin Med 118:7–16
Klein EA (2004) Selenium: epidemiology and basic science. J Urol 171:S50–SS3
Kaushal N, Hegde S, Lumadue J, Paulson RF, Prabhu KS (2011) The regulation of erythropoiesis by selenium in mice. Antioxid Redox Signal 14:1403–1412
Timothy M, Sheehan T, Gao M (1990) Simplified fluorometric assay of total selenium in plasma and urine. Clin Chem 36(/12):2124–2126
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–168
Holmgren A, Björnstedt M (1995) Thioredoxin and thioredoxin reductase. Methods Enzymol 252:199–208
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Yeshoda KM (1942) Phenylhydrazine anaemia in rats. Curr Sci 11:360–363
Balasubramaniam P, Malathi A (1992) Comparative study of hemoglobin estimated by Drabkin’s and Sahli’s methods. J Postgrad Med 38:8–9
Sakata M, Yoshida A, Haga M (1982) Methemoglobin in blood as determined by double-wavelength spectrophotometry. Clin Chem 28:508–511
Sorensen S, Rubin E, Polster H, Mohandas N, Schrier S (1990) The role of membrane skeletal-associated alpha-globin in the pathophysiology of beta-thalassemia. Blood 75:1333–1336.1990
Best TM, Fiebig R, Corr DT, Brickson S, Brickson Ji L (1999) Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol 87:74–82
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99(667–676):1966
Luck H (1971) Methods of enzymatic analysis. HU Bergmeyer Academic Press, New York, p 885
Kono Y (1978) Generation of superoxide radical during auto oxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Massey V, Williams CH Jr (1965) On the reaction mechanism of yeast glutathione reductase. J Biol Chem 240:4470–4480
Luangaram S, Kukoongviriyapan U, Pakdeechote P, Kukoongviriyapan V, Pannanqpetch P (2007) Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem Toxicol 45:448–455
Scott MD, Van R, Den Berg JJ, Repka T, Rouyer-Fessard P, Hebbel RP, Beuzard Y, Lubin BH (1993) Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J Clin Investig 91:1706–1712
Rotruck JT, Pope AL, Ganther HE, Hoekstra WG (1972) Prevention of oxidative damage to rat erythrocytes by dietary selenium. J Nutr 102:689–696
Mcphail DB, Sibbald AM (1992) The role of free radicals in brassica-induced anaemia of sheep: an ESR spin trapping study. Free Radic Res Commun 16:277–284
Sachdev SW, Sunde RA (2001) Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and -4 in rat liver. J Biochem 357:851–858
Rao L, Puschner B, Prolla TA (2001) Gene expression profiling of low selenium status in the mouse intestine: transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress. J Nutr 131:3175–3181
Harley JD, Mauer AM (1960) Studies on the formation of Heinz bodies. I. Methemoglobin Production and Oxyhemoglobin Destruction. Blood 16:1722–1735
Jacob HS (1970) Mechanism of Heinz body formation and attachment to red cell membrane. Semin Hematol 7:341–354
Advani R, Sorenson S, Shinar E, Lande W, Rachmilewitz E, Schrier SL (1992) Characterization and comparison of the red blood cell membrane damage in severe human alpha-and beta thalassemia. Blood 79:1058–1063
Scott MD, Eaton JW (1995) Thalassaemic erythrocytes: cellular suicide arising from iron and glutathione dependent oxidation reactions. Br J Haematol 91:811–819
Shinar E, Rachmilewitz EA (1990) Oxidative denaturation of red blood cells in thalassemia. Semin Hematol 27:70–82
Urbankova L, Horky P, Skladanka J, Pribilova M, Smolikova V, Nevrkla P, Cernei N, Lackova Z, Hedbavny J, Ridoskova A, Adam V, Kopel P (2018) Antioxidant status of rats blood and liver affected by sodium selenite and selenium nanoparticles. PeerJ 6:e4862. https://doi.org/10.7717/peerj.4862
Hamdy MM, Mosallam DS, Jamal AM, Rabie WA (2015) Selenium and vitamin E as antioxidants in chronic hemolytic anemia: are they deficient? A case-control study in a group of Egyptian children. J Adv Res 6:1071–1077
Ines D, Sonia B, Riadh BM, Amel EG (2006) A comparative study of oxidant-antioxidant status in stable and active vitiligo patients. Arch Dermatol Res 298:147–152
Funding
Research reported in this publication was supported by the UGC-SAP (F.4-1/2015/DSA-1 (Sap-II)) and DST-FIST (SR/FST/LS1-645) programs sanctioned to the Department of Biophysics, Panjab University, Chandigarh (160014), India, by the University Grants Commission (UGC), Govt of India and the Department of Science and Technology (DST), Govt of India, respectively. The financial assistance to Panjab University by DST through DST-PURSE program is also duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experiments were performed in accordance with the guidelines of institutional ethical committee of Panjab University, Chandigarh.
Conflict Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(PNG 1092 kb)
Rights and permissions
About this article
Cite this article
Kaur, R., Ghanghas, P., Rastogi, P. et al. Protective Role of Selenium Against Hemolytic Anemia Is Mediated Through Redox Modulation. Biol Trace Elem Res 189, 490–500 (2019). https://doi.org/10.1007/s12011-018-1483-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1483-y