Abstract
The aim of the study was to assess the protective effect of Picrorhiza kurroa hydroalcoholic extract (PCK), a glycoside-rich extract, against potassium dichromate (PDC)-induced liver oxidative stress in Wistar albino rats. Thirty-six male Wistar rats were divided into six groups: the control group (which received distilled water), the SIL group (which received 60 mg/kg silymarin), the PDC group (which received 30 mg/kg K2Cr2O7), and the treatment groups (which received 25, 50, 100 mg/kg PCK). Administration of PDC resulted in increased levels of liver enzymes such as alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP); up-regulated peroxidation biomarkers, i.e., thiobarbutric acid-reactive species (TBARS) and protein carbonyls in serum; and decreased activities of antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT) significantly in the liver tissue. Gene expression studies of tumor necrosis factor (TNF), mitogen-activated protein kinase (MAPK), growth arrest, and DNA damage-inducible protein (GADD45) revealed that there was a liver damage at the molecular level, and histopathological studies further confirmed the morphological changes by PDC administration. However, PCKs at 50 and 100 mg/kg promoted significant restoration of liver enzyme levels and the activities of antioxidant enzymes were kept close to the values of the control and SIL groups. Our current study confirms that the active compounds present in the PCK might have conferred a strong protection against potassium dichromate-induced oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is a naturally occurring heavy metal which exists in the environment in two valence states, i.e., trivalent Cr(III) and hexavalent Cr(VI). Chromium compounds, mostly in chromium(III) or chromium(VI) forms produced by the chemical industry, are used for chrome plating, in the manufacture of dyes and pigments, leather tanning, and wood preserving [1]. Occupational exposures often include mixed exposure to both Cr(III) and Cr(VI) [2]. Cr(VI) has been reported to cause hepatotoxicity in humans and laboratory animals primarily through an oxidative stress-mediated mechanism [3,4,5]. Among the several valence states of Cr, hexavalent chromium (Cr(VI)) is the most toxic which can readily cross cellular membranes via non-specific anion transporters and its toxicity has been associated with oxidative stress [6]. Human exposure to Cr(VI) induces several adverse health effects, such as genotoxicity, nephrotoxicity, carcinogenicity, and hepatotoxicity [7,8,9]. Oxidative stress can lead to damage of internal organs, and its major contribution is to initiate and progress liver injury [10]. Although chromium-containing compounds have gained much interest in the field of toxicology research, appropriate scientific studies are warranted to fully understand the mechanism of cytotoxicity and oxidative stress.
Picrorhiza kurroa represents one of the essential herbal drugs of an indigenous system of medicines belonging to the family Scrophulariaceae [11]. Pharmacological studies have revealed that P. kurroa has hepatoprotective, anti-inflammatory, and free-radical-scavenging-like activities [11]. Significant hepatoprotective activity of P. kurroa was reported earlier against paracetamol- and carbon tetrachloride drug-induced hepatic toxicities in albino rats [12]. Most of its pharmacological activities are attributed to novel monoterpenes-derived iridoid glycosides known as picrosides which include picroside-I, picroside-II, and other metabolites like picroside-III, apocynin, and kutkoside [13]. Silymarin (SIL) has been used worldwide for the treatment of hepatic diseases since many years. SIL maintains the hepatocyte membrane integrity and blocks the xenobiotics by forming a complex that impedes the entrance of toxins into the interior of liver cells [14]. Therefore, in this study, silymarin (SIL) has been used as the standard drug to compare the hepatoprotective activity of P. kurroa hydroalcoholic extract (PCK). Based on the above facts, the present study was focused on the protective effect of PCK against potassium dichromate (PDC)-induced liver toxicity in rats.
Materials and Methods
Chemicals
ABTS—2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid); BHT—butylated hydroxyltoluene; BSA—bovine serum albumin; DNPH—dipenylhydrazine; DPPH—2,2-diphenyl-1-picrylhydrazyl; DTNB—5,5-dithiobisnitrobenzoic acid; HCl—hydrochloric acid; EDTA—ethylenediaminetetraacetate disodium salt; FeCl3—ferric chloride; HEPES—4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid; H2O2—hydrogen peroxide; NaCl—sodium chloride; SDS—sodium dodecyl sulfate; TBA—thiobarbituric acid; and K2Cr2O7—potassium dichromate. All the chemicals used in the experiment were purchased from Sigma Aldrich, India. Picroside-I and picroside-II were purchased from Natural Remedies Pvt. Ltd., Bangalore, India.
Plant Material and Extraction
Dried rhizome powder of P. kurroa was purchased from SKP Ayurvedic Pvt. Ltd., Mysore, India, and properly authenticated by the Department of Botany, University of Mysore, Mysore, India (voucher specimen no. AND8025). The rhizome powder (100 g) was defatted with diethyl ether for 24 h, and the filtrate was suspended in 500 ml of 50% ethanol for 2 days on shaker at 520×g. This mixture was filtered using Whatman no.1 filter paper and concentrated under reduced pressure (Rotary Evaporator, Heidolph, India). The concentrated extract was lyophilized (Lyophilization System India Pvt. Ltd., India) and stored at − 20 °C for further analysis. The yield of lyophilized PCK was 5.0% (w/w) of dry rhizome.
Quantification of Picrosides by HPLC
Quantification of picrosides was performed by using high-performance liquid chromatography (JASCO, India) equipped with sample injector, column apartment, and UV detector for the visual confirmation of the presence of marker compounds in the extract. A reverse-phase column (RP-18; 3 mm × 150 mm) was purchased from Merck, India. Separation was done using methanol and water (40:60; v/v) at a flow rate of 0.7 ml/min with injection volume of 20 μl for each sample, and UV detection was set at 270 nm. Prior to use, solvents were filtered through a 0.22-mm diameter membrane filters. Satisfactory separation was obtained as shown in the chromatograms (Fig. 1). Quantification and linearity of the HPLC method were carried out for picroside content in different samples of PCK. The percentage of the picrosides was calculated by calibration curve using peak height to peak area ratio.
LC-ESI-MS/MS Analysis of PCK
The LC-ESI-MS/MS analysis of PCK was performed using 6520 accurate Q-TOF (Agilent Santa Clara, CA) mass spectrometer coupled to HPLC equipped with a UV-Vis detector. Zorbax SBC18 rapid-resolution column of 4.6 × 150 mm and 3.5-μm particle size was used under the following conditions: (A) formic acid (0.1% v/v) and 10 mM ammonium fluoride and (B) acetonitrile + 0.1% formic acid; gradient (in solvent B): (i) 30%, from 0 to 15 min; (ii) 55%, at15 min; (iii) 95%, from 25 to 45 min, and (iv) 35%, at 45–48 min; flow rate: 0.2 ml/min; injection volume was 5 μl. Both negative and positive ion modes of ESI parameters were used; mass range 100–1200 m/z, 4-kV spray voltage, gas temperature 325 °C, gas flow 10 l/min, and nebulizer 40 psi.
Determination of In Vitro Antioxidant Activities
DPPH Radical-Scavenging Activity
In vitro DPPH radical-scavenging activity of PCK was determined by the following method [15]. DPPH in methanol (0.1 mM) was added to different concentrations of PCK. The mixture was incubated at room temperature for 30 min, and the absorbance was recorded at 515 nm. Percentage inhibition was calculated as follows: DPPH scavenged (%) = [A cont − A sample]/A cont × 100, where A cont is the absorbance of the control and A sample is the absorbance of PCK. In order to calculate the IC50 value, which is the amount of sample necessary to decrease the absorbance of DPPH radical by 50%, the decolorization was plotted against the concentration of the sample extract.
ABTS Radical-Scavenging Activity
The ABTS radical-scavenging activity was performed using the method followed by Re et al. [16]. ABTS (7 mM) and potassium persulfate (2.4 mM) were used in equal quantities to prepare the working reagent. The working reagent was incubated for a period of 12 h at dark condition and was added with different concentrations of PCK. Spectrophotometric readings were recorded at 734 nm. Percentage inhibition was calculated as follows: ABTS scavenged (%) = [A cont − A sample]/A cont × 100, where A cont is the absorbance of the control and A sample is the absorbance of PCK. In order to calculate the IC50 value, which is the amount of sample necessary to decrease the absorbance of ABTS radical by 50%, the decolorization was plotted against the concentration of the sample extract.
Hydrogen peroxide (H2O2)-Scavenging Activity
Different concentrations of PCK were added to the hydrogen peroxide solution (0.6 ml, 40 mM) and incubated for a period of 10 min. The absorbance was recorded at 230 nm against a blank solution containing the phosphate buffer. The percentage inhibition was calculated as follows [17]: [A cont − A sample]/A cont × 100, where A cont is the absorbance of the control and A sample is the absorbance of PCK.
Ferric-Reducing Antioxidant Power Assay
Ferric-reducing antioxidant power (FRAP) assay was performed by the spectrophotometric method [18]. Briefly, it is a mixture of PCK, acetate buffer (300 mM; pH 3.6), TPTZ (2,4,6- tripyridyl-s-triazine; 10 mM) in HCl, and FeCl3·6H2O (20 mM). The mixture was then allowed to react with the FRAP solution for 30 min in the dark and then measured for the formation of ferrous tripyridyltriazine complex.
Experiments on Animal Models
Animals
Animal studies were conducted according to the Institute Animal Ethical Committee (IAEC) regulations approved by the Committee for the Purpose of the Control and Supervision of Experiments on Animals (CPCSEA) with the IAEC approval no. IAEC-2016/AN/12 dated 25 October 2016. Wistar albino male rats (150–200 g) were selected from the central animal facility, Defense Food Research Laboratory (DFRL), Mysore, India, and were housed in an acryl fiber cage in a temperature-controlled room (23 ± 2 °C). The animals were maintained at 12-h light/dark cycle with standard pellet diet, and water was provided ad libitum.
Experimental Design
Thirty-six Wistar albino male rats were randomly divided into six groups with six animals in each group (n = 6): control group, PDC group, SIL group, and three treatment groups (PCK). Different doses of PCK were orally administered to the treatment groups separately, such as PCK 25, 50, and 100 mg/kg p.o., respectively, for a period of 28 days. The SIL group received silymarin 60 mg/kg p.o. for a period of 28 days. All the animal groups, except control animals, received K2Cr2O7 at 30 mg/kg by oral gavage for a period of 28 days. The control group received an equal amount of distilled water during the experimental period. Dosage selection and mode of administration of both PDC and PCK were based on previous studies [19, 20]. Animals were sacrificed under mild anesthesia; the serum was separated and stored at − 20 °C for further biochemical assays. The tissue samples were excised, washed with normal saline, and stored in − 80 °C for further biochemical assays. Washed tissues were fixed immediately in 10% formalin solution for histopathology studies.
Assessment of Serum Biochemical Markers
Serum analysis of various liver marker enzymes such as alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) was estimated using commercially available kits (AGAPEE diagnostic kits, India). ALT and AST activities were measured spectrophotometrically at 340 nm, and we calculated the change in absorbance per minute (OD/min) during a 3-min period with the following formula: (U/L) = (OD/min) × 1745 (factor). ALP activity was measured by the change in absorbance per minute (OD/min) during 3 min at 405 nm and calculated using (U/L) = (OD/min) × 2750 (factor).
Estimation of TBARS
Lipid peroxidation was determined by thiobarbutric acid-reactive species (TBARS) and expressed as malondialdehyde (MDA mmol/cm/g) [21]. Liver tissues were homogenized in phosphate buffer (pH 7.0); trichloroacetic acid (TCA) (10%) and 0.5 and 2 ml of TBA mixture were added to the tissue homogenate (0.5 ml). The TBA mixture contained TBA (0.35%), SDS (0.2%), FeCl3 (0.05 mM), and BHT in the glycine-HCl buffer (100 mM, pH 3.6). The above reaction mixture was boiled at 100 °C for 30 min and then allowed to cool. The mixture was centrifuged at 8315×g for 10 min, and the absorbance was measured at 532 nm.
Protein Carbonyl Assay
Protein carbonyl content was estimated by Dalle et al. with slight modifications [22]. Homogenized liver tissue samples were diluted (1:40) with phosphate-buffered saline (PBS) containing sodium phosphate (10 mM; pH 7.4) and NaCl (0.14 M) and centrifuged twice at 25,464×g. TCA (20%; w/v) was used to precipitate the protein content. A solution of DNPH (10 mM in 2 M HCl) was added to the protein pellet to get a final protein concentration of 1–2 mg/ml. The protein pellets were washed with 20% TCA and ethanol/ethyl acetate (1:1, v/v) to remove free DNPH. Samples were then suspended in 6 M guanidine hydrochloride at 37 °C for 15 min. Carbonyl content was determined from the absorbance at 366 nm using a molar absorption coefficient of 22,000/M/cm.
Gene Expression Studies
Liver tissue samples were placed in a vial containing RNA later and stored at − 80 °C. Total RNA was extracted from ~ 100 mg of tissue by TRIzol reagent (Sigma Aldrich, India), as described the manufacturer’s protocol. RNA samples were treated with RQ1 RNase-free DNase (Applied Biosystems, India) to avoid DNA contamination. The concentration of total RNA was measured with spectrophotometer (NanoDrop-ND1000; Thermo Fischer Scientific, India), and the OD 260/OD 280 ratio was tested for the purity of RNA; further, RNA yield was confirmed by electrophoresis. The first complementary DNA (cDNA) strand was synthesized by using a high-capacity cDNA-converting kit (Applied Biosystems, India) in a one-vial procedure containing a total reaction of 20 μl having 3.2 μl of distilled H2O, 0.8 μl of dNTPs, 2 μl of the × 10 PCR buffer, 2 μl of the random primers, 1 μl of RNAse inhibitor, 1 μl of reverse transcriptase, and 10 μl of RNA sample. Further, the sample was incubated as per manufacturer’s protocol (Applied Bio systems, India).
Semi-quantitative PCR Assay
RNA was reverse-transcribed, and complementary DNAs were synthesized using specific oligo (dT) primers. Forward and reverse primers of targeted genes were as follows: glyceraldehyde 3-phosphate dehydrogenase, housekeeping gene (GAPDH: F-5′-CAACTCCCTCAAGATTGTCAGCAA-3′; R-5′-GGCATGGACTGTGGTCATGA-3′), TNF (F-5′-CTCACACTCAGATCATCTTC-3′; R-5′-GAGAACCTGGGAGTAGATAAG-3′), MAPK (F-5′-CCATTGATATTTGGTCTGTGG-3′; R-5′-ATCCAAGAATACCCAGGATG-3′), and GADD45 (F-5′-GAAGATCGAAAGGATGGAC-3′; R-5′-TGAGGGTGAAATGGATCTG-3′). PCR amplification was performed as a 20-μl reaction with 6 μmol/l of each forward and reverse primer, 2 μl of × 10 PCR buffer, 1.5 mmol/l of MgCl2, 80 μmol/l of each dNTPs and 1 unit of Taq polymerase (Sigma Aldrich, India) in a thermal cycler (Eppendorf, Germany). The PCR program consisted of initial denaturation at 94 °C for 4 min; 30 cycles of denaturation at 94 °C for 45 s; annealing at 58 °C (GAPDH), 55 °C (TNF), 60 °C (MAPK), and 59 °C (GADD45) for 30 s; and extension at 72 °C for 8 min. PCR amplicons were resolved on 1.5% agarose gel and visualized under UV transilluminator.
Western Blot Analysis
Liver tissue samples were processed for SDS-PAGE followed by Western blot for the protein expression studies of apoptosis marker GADD45 and antioxidant enzymes, viz., SOD and CAT. Tissue samples were homogenized with HEPES buffer (pH 7.4) and were centrifuged at 12,992×g for 20 min. Total protein content was estimated from the supernatant. Protein from each sample (50 μg) was separated on 12% SDS-PAGE and transferred onto a nitrocellulose membrane using an electroblotting apparatus (Bio-Rad, USA). The membrane was blocked in 5% defatted milk solution at 4 °C for 24 h, later probed by respective primary antibodies (1: 1000; Santa Cruz, India), and incubated at room temperature for 3 h. The membrane was washed four times in PBST and one time in PBS for 15 min followed by incubation for 2 h in HRP-conjugated rabbit antimouse secondary antibody (Sigma, India; 1:10,000). The membranes were washed again and developed using an enhanced chemiluminescent method by using luminal and p-coumaric acid (Sigma Aldrich, India). The band intensity was captured by exposing the membranes onto the X- ray film [23].
Histopathological Studies
Liver tissues were excised and washed with normal saline and fixed immediately in 10% formalin solution. A paraffin-embedding technique was carried out, and sections were taken at 5-mm thickness, stained with hematoxylin and eosin, and examined microscopically for histopathological changes.
Statistical Analysis
The data were expressed as mean ± SD (standard deviation). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test using GraphPad Prism version 5.03 statistics software.
Results
HPLC Analysis of PCK
Iridoid glycosides in PCK extract were quantified, and the percentages of picrosides I and II were 2.36 and 4.86%, respectively. HPLC chromatogram of PCK was calibrated from standard picroside I and II peaks (Fig. 1).
LC-ESI-MS/MS Analysis of PCK
Major bioactive compounds were identified by LC-ESI-MS/MS on their average mass, m/z, and score under mass spectrometry (Fig. 2). The major compounds were cinnamic acid, ferulic acid, kuttoside, picroside-I, picroside-II, picroside-III, scrophuloside-A, and veronicoside.
In Vitro Free Radical-Scavenging Activity of PCK
The antioxidant activity of the PCK was studied against free radicals such as DPPH·, ABTS· and H2O2 ·. The in vitro radical activity of SIL percent inhibition was increased with increasing concentration of extract. SIL and PCK were able to reduce the stable free radical DPPH to yellow-colored diphenyl-picryl-hydrazine with IC50 of 20.8 ± 0.4 and 82.6 ± 0.2 mg/ml. The results of ABTS· radical-scavenging activities of SIL and PCK were with IC50 of 9.62 ± 1.3 and 25.0 ± 3.2 mg/ml, respectively. The results of H2O2 · radical-scavenging activities of SIL and PCK were with IC50 of 14.02 ± 0.5 and 27.2 ± 0.1 mg/ml, respectively. The FRAPs of SIL and PCK were expressed as 10.38 ± 3.3 and 24.89 ± 5 units of millimoles of Fe(II) per gram.
Effects of PCK on Serum Biochemical Parameters
PDC administration had significantly increased the levels of ALP, AST, and ALT in serum when compared to the control group (p < 0.05). However, supplementation of PCK and SIL significantly decreased the serum ALP, AST, and ALT levels (Fig. 3).
Effect of PCK on serum biochemical parameters. CON, control group; SIL, silymarin (60 mg/kg); PDC, K2Cr2O7 (30 mg/kg); PCK 25, PCK 50, and PCK 100, 25, 50 and 100 mg/kg of P. kurroa extract, respectively. Units are expressed as U/L. Values represented the mean ± SD; n = 6. Significant difference was determined by one-way ANOVA followed by Tukey’s test, #p < 0.05 vs control; *p < 0.05 vs PDC group
Effect of PCK on TBARS and Protein Carbonyl
Administration of PDC significantly up-regulated the lipid peroxidation (1.23 ± 0.08 nmol MDA/mg protein) and protein carbonyl (0.86 ± 0.06 nmol/mg protein) levels when compared to the control animals (0.38 ± 0.05 and 0.4 ± 0.08, respectively; p < 0.05). However, supplementation of PCK-25, PCK-50, PCK-100 and SIL has shown significant down-regulation in the levels of TBARS and protein carbonyl content (Fig. 4).
Effect of PCK on peroxidation markers. a Lipid peroxidation (TBARS) expressed as nanomole of MDA per milligram of protein. b Protein carbonyl expressed as nanomole per milligram of protein. CON, control group; SIL, silymarin (60 mg/kg); PDC, K2Cr2O7 (30 mg/kg); PCK 25, PCK 50, and PCK 100, 25, 50 and 100 mg/kg of P. kurroa extract, respectively. Values represented the mean ± SD; n = 6. Significant difference was determined by one-way ANOVA followed by Tukey’s test, #p < 0.05 vs control; *p < 0.05; **p < 0.01 vs PDC group
Effect of PCK on Antioxidant Enzymes
Administration of PDC significantly down-regulated antioxidant enzymes like SOD and CAT when compared with control animals (p < 0.05). Further, PDC administration significantly increased the activity of GADD45 (p < 0.05). Moreover, supplementation of PCK and SIL significantly up-regulated the enzyme activities (SOD and CAT) and decreased GADD45 in a dose-dependent manner (Fig. 5).
Effect of PCK on antioxidant enzyme levels. a Western blot images. b Densitometric analysis of gel images (% expression relative to control). CON, control group; SIL, silymarin (60 mg/kg); PDC, K2Cr2O7 (30 mg/kg); PCK 25, PCK 50, and PCK 100, 25, 50 and 100 mg/kg of P. kurroa extract, respectively; SOD, superoxide dismutase; CAT, catalase; GADD 45, growth arrest and DNA damage-inducible alpha. Values represented the mean ± SD; n = 6. Significant difference was determined by one-way ANOVA followed by Tukey’s test, #p < 0.05 vs control; * p < 0.05 vs PDC group
Effect of PCK on Gene Expression
PDC stimulated the mRNA expression of genes that are involved in inflammatory and transcription pathways. Down-regulation of TNF, MAPK, and GADD45 was observed in the supplementation of PCK and SIL groups (Fig. 6). Relative expression was determined by densitometric analysis and normalized to the respective expression of the housekeeping gene GAPDH.
Effect of PCK on gene expression. a Gel images. b Densitometric analysis of gel images (% expression relative to control). CON, control group; SIL, silymarin (60 mg/kg); PDC, K2Cr2O7 (30 mg/kg); PCK 25, PCK 50, and PCK 100, 25, 50 and 100 mg/kg of P. kurroa extract, respectively; TNF, tumor necrosis factor-α; MAPK, mitogen-activated protein kinase, GADD 45, growth arrest and DNA damage-inducible alpha. Values represented the mean ± SD; n = 6. Significant difference was determined by one-way ANOVA followed by Tukey’s test, #p < 0.05 vs control; *p < 0.05 vs PDC group
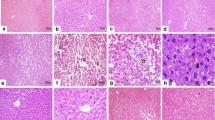
Effect of PCK on Tissue Architecture
PDC administration caused several architectural changes which include distorted lobules and dilated central veins in the liver tissue and hepatocytes with feathery degenerative morphology. Treatment of PCK-25 had no improvement in the Cr(VI)-induced liver tissue architecture. However, PCK at higher doses, i.e., 50 and 100 mg/kg, and SIL-administrated animals have shown normal morphological features (Fig.7).
Discussion
Chromium is a naturally occurring element and is released into the environment from natural and anthropogenic sources, with the largest release occurring from industrial releases [24]. Exposure to Cr(VI) is known to be toxic, mutagenic, and carcinogenic to human beings and diverse animals, which can cause a serious damage to the kidneys, liver, lungs, skin, and other vital organs [25]. In previous studies, it was observed that Cr(VI) had ill effects on the gastrointestinal system and the liver. Enlargement of cells in the liver, necrosis, and elevation of liver enzymes were reported with Cr(VI) contamination [26]. However, there is a lack of scientific data available on alternative/ prophylactic solutions in cases of Cr(VI)-associated toxicities. In such situations, possible preventive methods are to be explored. Natural and synthetic antioxidants have been reported to ameliorate or prevent K2Cr2O7-induced hepatotoxicity [27]. However, to our knowledge, the potential hepatoprotective effect of PCK on K2Cr2O7-induced hepatotoxicity has not been explored.
The present study demonstrates that oral administration of PCK could ameliorate oxidative stress and attenuate the damaging effect induced by Cr(VI) in Wistar albino rats. ALT, AST, and ALP are routine biomarkers of the liver and are located in the cytoplasm. Injuries to the liver cause the enzymes to escape into the circulatory system [27]. Therefore, elevation of these enzymes in response to PDC administration in this study could be indicative of impairment in liver function. SIL had reduced the levels of ALT, AST, and ALP when compared with the PDC-administered group. Similarly, in the present study, PCK had improved the liver function by decreasing the levels of ALT, AST, and ALP. The observed improvement in liver function may be due to the presence of bioactive compounds in the PCK extract. As per previous reports [28], picrosides are the key components in the PCK to help in liver function by altering ALT, AST, and ALP levels. HPLC and LC-ESI-MS/MS chromatograms of the present study confirmed the presence of picroside-I, picroside-II, and picroside-III. Polyphenols, flavonoids, terpenoids, etc. are the major classes among the antioxidant agents, which are also considered as major groups of medicinal plant free radical scavengers [29]. Polyphenols, such as catechins, epigallocatechin, epicatechin-3-gallate, and quercetin, were proven to capable of modulating Cr(VI)-induced oxidative stress and DNA damage [30]. Free radicals cause oxidative damage to nucleic acids, proteins, carbohydrates, and lipids. The antioxidant property of an extract neutralizes the free radicals and controls the oxidative damage of tissues. Moreover, in the present study, PCK showed excellent antioxidant activity capable of scavenging free radicals like DPPH·, ABTS· and H2O2 · and of ferrous-reducing activity. The study assumes that these scavenging activities could help in eliminating free radicals released against Cr(VI)-induced oxidative stress.
The pathogenesis of most chemical-induced liver injuries is initiated by the metabolic conversion of chemicals into reactive intermediate species, such as electrophilic compounds or free radicals, which can potentially alter the structure and function of cellular macromolecules [31]. In the present study, we observed decreased activities of the antioxidant enzymes, i.e., SOD and CAT, and also an increased expression of GADD45 by PDC administration. This might be due to increased toxic overload by the reactive oxygen species (ROS), which would have resulted in dysregulated expression of these genes. These results are in line with earlier findings, where altered gene expression was seen in energy metabolism, stress response, DNA repair, signaling pathways, apoptosis, and cell cycle regulation [32]. However, administration of PCK at 100 mg/kg had restored the antioxidant enzyme status at par with SIL.
Long-term exposure to heavy metals like cadmium (Cd), chromium (Cr), and arsenic (As) has been observed with increased oxidative stress via up-regulation of lipid peroxidation and protein oxidation, which can cause inhibition of antioxidant enzymes [33]. In the present study, Cr(VI) promoted the levels of TBARS and protein carbonyls and down-regulated antioxidant enzymes. Previous studies reported that increased TBARS and protein carbonyl levels inhibit the antioxidant system via down-regulating a group of enzymes such as SOD, catalase, glutathione peroxidase (GPx), glutathione-s-transferase (GST), and glutathione [31, 33]. Cr(VI) exposure can interfere with the antioxidant system at various levels, from the consumption of antioxidant molecules in the intracellular reduction of Cr(VI) to the disruption of the biological actions of antioxidant enzymes through interaction of these enzymes with species formed during Cr(VI) reduction [32]. As these enzymes have a protective role against oxygen free radical-induced damage, their induction can be considered as an adaptive response to oxidative stress. In the present study, antioxidant enzymes such as SOD and catalase were up-regulated by the supplementation of PCK and SIL.
When protective defenses are overwhelmed by excess toxicant insult, the effects of reactive intermediate species lead to deregulation of cell signaling pathways and dysfunction of biomolecules, leading to failure of target organelles and eventually cell death. Similarly, in the present study, we observed altered expressions of TNF, MAPK, and GADD45. TNF exerts a variety of biological effects and interacts directly or indirectly with MAPK [34]. In the present study, it was observed that PDC administration had resulted in elevated expression levels of TNF and MAPK. These elevated levels of TNF and MAPK are due to excessive oxidative stress generated by PDC, which might have further triggered the expression GADD45. These results are in line with the previous reports where p38- and JNK/stress-activated protein kinase (MAPKs) are promoted by growth-suppressive stimuli leading to cell cycle arrest, DNA repair, and/or apoptosis [35, 36]. However, administration of PCK at higher doses (50 and 100 mg/kg) showed down-regulated expressions of TNF, MAPK, and GADD45.
The liver is an organ capable of being injured by Cr(VI), and it has been demonstrated that the exposure to K2Cr2O7 induces hepatotoxicity associated to oxidative stress, lipid peroxidation, inhibition of antioxidant enzymes [37], and structural tissue injury [4]. In the present study, histopathological observations of the liver tissue showed focal necrosis, increased lymphocytic infiltration around the blood vessels, kupffer cell proliferation, appearance of numerous vacuoles and sinusoids, and distortion of hepatic cells in the PDC-treated group. Free radicals generated by PDC might have led to changes in the tissue architecture. These are in line with earlier reports where it was suggested that ROS are involved in Cr(VI)-induced cell injury [38]. On the contrary, supplementation of PCK had significantly reduced the tissue damage when compared with the PDC group. Previous studies suggested that supplementation of antioxidants could protect the liver tissues from oxidation and prevent DNA damage under various toxic conditions [4]. In our study, the down-regulated GADD45 gene expression results were in line with the previous studies. In the present study, silymarin was used as a comparative hepatoprotective drug, which has been reported to stimulate DNA biosynthesis and cell proliferation of damaged liver tissues and subsequent expressions of many cytokines and apoptotic signaling factors [39, 40]. In the present study, we observed that PCK showed a protective effect against PDC-induced liver oxidative stress proving the necessary ability compared to SIL. Results of the study confirmed that PCK has ameliorative activity from tissue damage against PDC-induced hepatotoxicity.
References
Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Heal C 34:1–32. https://doi.org/10.1080/10590501.2015.1096883
U.S. EPA. IRIS toxicological review of hexavalent chromium (2010). U.S. Environmental Protection Agency, Washington, DC, EPA/635/R-10/004A
Laborda R, Diaz-Mayans J, Nunez A (1986) Nephrotoxic and hepatotoxic effects of chromium compounds in rats. Bull Environ Contam Toxicol 36:332–336
Soudani N, Ben Amara I, Sefi M, Boudawara T, Zeghal N (2011) Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Exp Toxicol Pathol 63:541–548. https://doi.org/10.1016/j.etp.2010.04.005
Sugiyama M (1992) Role of physiological antioxidants in chromium (VI)-induced cellular injury. Free Radical Biol. Med 12:397–407. https://doi.org/10.1016/0891-5849 (92)90089-Y
Patlolla C, Barnes C, Yedjou V, Velma P (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24:66–73. https://doi.org/10.1002/tox.20395
Das AP, Mishra S (2008) Hexavalent chromium (VI): environment pollutant and health hazard. J Environ Research and Development 2:386–392
Saber TM, Farag MR, Cooper RG (2015) Ameliorative effect of extra virgin olive oil on hexavalent chromium-induced nephrotoxicity and genotoxicity in rat. Rev. Med. Vet. 166:11-19
Sahu B, Meghana K, Shriharsh R (2014) Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: involvement of oxidative stress, apoptosis and inflammation. Chem. Biol. Interact 223:69–79. https://doi.org/10.1016/j.cbi.2014.09.009
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16:26087–26124. https://doi.org/10.3390/ijms161125942
Ravishankar B, Shukla VJ (2007) Indian systems of medicine: a brief profile. Afr J Tradit Complement Altern Med 4:319–337
Jadeja R, Devkar RV, Nammi S (2014) Herbal medicines for the treatment of non-alcoholic steatohepatitis: current scenario and future prospects. Evid Based Complement Alternat Med 2014:648308. https://doi.org/10.1155/2014/648308
Stuppner H, Wagner H (1989) New cucurbitacin glycosides from Picrorhiza kurrooa. Planta Med 55:559–563. https://doi.org/10.1055/s-2006-962095
Abou Zid S (2012) Silymarin, Natural Flavonolignans from Milk Thistle. In: Venketeshwer R. Phytochemicals-A Global Perspective of Their Role in Nutrition and Health. Rijeka: Croatia. InTech 2012: 255-272
Gutteridge J, Halliwell B (2000) Free radicals and antioxidants in the year 2000: a historical look to the future. Ann N Y Acad Sci 899:136–147
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Bio Med 26:231–237. https://doi.org/10.1016/S0891-5849(98)00315-3
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10:1003–1008
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Adjroud O (2010) Protective effects of selenium against potassium dichromate-induced hematotoxicity in female and male Wistar albino rats. Ann Toxicol Anal 22:165–172. https://doi.org/10.1051/ata/2010025
Jeyakumar R, Rajesh R, Meena B, Rajaprabhu D, Ganesan B, Buddhan S, Anandan R (2008) Antihepatotoxic effect of Picrorhiza kurroa on mitochondrial defense system in antitubercular drugs (isoniazid and rifampicin)-induced hepatitis in rats. J Med Plants Res 2:017–019
Garcia N, Tapia WR, Zazueta E, Zatarain C, Barron ZL (2013) Curcumin pretreatment prevents potassium dichromate induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability transition pore opening. Evid Based Complement Alternat Med 2013:1–19. https://doi.org/10.1155/2013/424692
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38. https://doi.org/10.1016/S0009-8981(03)00003-2
Chandrasekhar Y, Ramya EM, Navya K, Kumar GP, Anilakumar KR (2017) Antidepressant like effects of hydrolysable tannins of Terminalia catappa leaf extract via modulation of hippocampal plasticity and regulation of monoamine neurotransmitters subjected to chronic mild stress (CMS). Biomed Pharmacother 86:414–425
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2:112–118
García-Niño WR, Tapia E, Zazueta C, Zatarain-Barrón ZL, Hernández-Pando R, Vega-García CC, Pedraza-Chaverrí J (2013) Curcumin pre-treatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability transition pore opening. Evid Based Complement Alternat Med 2013:424692. https://doi.org/10.1155/2013/424692
Sterekhova NP, Zeleneva NI, Solomina SN, Tiushniakova NV, Miasnikova AG (1978) Stomach pathology in the workers of chromium salts industries. Gig Trud Prof Zabol 3:19–23
Susa N, Ueno S, Furukawa Y, Ueda J, Sugiyama M (1997) Potent protective effect of melatonin on chromium(VI)-induced DNA single-strand breaks, cytotoxicity, and lipid peroxidation in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol 144:377–384. https://doi.org/10.1006/taap.1997.8151
Jayakumar T, Ramesh E, Geraldine P (2006) Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl4-induced liver injury in rats. Food Chem Toxicol 44:1989–1996. https://doi.org/10.1016/j.fct.2006.06.025
Kumar GP, Anilakumar KR, Naveen S (2015) Phytochemicals having neuroprotective properties from dietary sources and medicinal herbs. Pharmacogn J 7:1–17. https://doi.org/10.5530/pj.2015.7.1
Del Carmen G-RM, Nicolás-Méndez T, Montaño-Rodríguez AR, Altamirano-Lozano MA (2014) Antigenotoxic effects of (−)-epigallocatechin-3-gallate (EGCG), quercetin, and rutin on chromium trioxide-induced micronuclei in the polychromatic erythrocytes of mouse peripheral blood. J Tox Env Health, Part A 77(6):324–336. https://doi.org/10.1080/15287394.2013.865006
Valko MMHCM, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. https://doi.org/10.2174/0929867053764635
Abreu PL, Ferreira LMR, Alpoim MC, Urbano AM (2014) Impact of hexavalent chromium on mammalian cell bioenergetics: phenotypic changes, molecular basis and potential relevance to chromate-induced lung cancer. Biometals 27:409–443
Navya K, Phani Kumar G, Anilakumar KR (2017). Ameliorating effect of Curculigo orchoides on chromium (VI) induced oxidative stress via, modulation of cytokines, transcription factors and apoptotic genes. J Appl Biomed. doi.org/10.1016/j.jab.2017.03.003. (in press)
Tracey MD, Cerami A (1994) Tumor necrosis factor: a pleiotropic cytokine and therapuetic target. Annu Rev Med 45:491–503. https://doi.org/10.1146/annurev.med.45.1.491
Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)–from inflammation to development. Curr Opin Cell Biol 10:205–219. https://doi.org/10.1016/S0955-0674(98)80143-9
Kyriakis JM, Avruch J (1996) Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays 18:567–577. https://doi.org/10.1002/bies.950180708
Liu KJ, Shi X (2001) In vivo reduction of chromium (VI) and its related free radical generation. Mol Cell Biochem 222:41–47
Pourahmad J, Mihajlovic A, Brien PJO (2001) Hepatocyte lysis induced by environmental metal toxins may involve apoptotic death signals initiated by mitochondrial injury. Adv Exp Med Biol 500:249–252
Sonnenbichler J, Goldbero M, Hane L, Madubunyi I, Vogl S, Zetl I (1986) Stimulatory effect of silibinin on the DNA synthesis in partially hepatectomized rat livers: non-response in hepatoma and other malign cell lines. Biochem Pharmacol 35:538–541. https://doi.org/10.1016/0006-2952(86)90233-9
Tsapakos MJ, Hampton TH, Wetterhahn KE (1983) Chromium (VI)-induced DNA lesions and chromium distribution in rat kidney, liver, and lung. Cancer Res 43:5662–5667
Acknowledgements
This research was supported by the Defense Food Research Laboratory, Mysore.
Author information
Authors and Affiliations
Contributions
KN and CSY conducted the experiments and collected and analyzed the data. PKG and KRA helped in designing and discussing the study and writing the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Animal studies were conducted according to the Institute Animal Ethical Committee (IAEC) regulations approved by the Committee for the Purpose of the Control and Supervision of Experiments on Animals (CPCSEA) with the IAEC approval no. IAEC-2016/AN/12 dated 25 October 2016.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
K, N., G, P., Y, C. et al. Evaluation of Potassium Dichromate (K2Cr2O7)-Induced Liver Oxidative Stress and Ameliorative Effect of Picrorhiza kurroa Extract in Wistar Albino Rats. Biol Trace Elem Res 184, 154–164 (2018). https://doi.org/10.1007/s12011-017-1172-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1172-2