Abstract
Iodine and iron are essential elements for healthy thyroid function. However, little is known about the association of iron and iodine with thyroid function in the general US population. We investigated iron and iodine status in relation to concentrations of thyroid hormones. We included 7672 participants aged 20 and older from three surveys (2007–2008, 2009–2010, and 2011–2012) of the National Health and Nutrition Examination Survey. Serum thyroid measures (including free and total T3 and T4, and TSH), serum iron concentration, and urinary iodine concentrations were measured. Multivariate linear regression models were conducted with serum thyroid measures as dependent variables and combinations of serum iron concentration and urinary iodine concentration as predictors with covariate adjustment. Logistic regression models were performed with TSH levels (low, normal, and high) and combinations of serum iron concentration and urinary iodine concentration. Overall, 10.9% of the study population had low iron; 32.2 and 18.8% had low or high iodine levels, respectively. Compared with normal levels of iron and iodine, normal iron and high iodine were associated with reduced free T3 and increased risk of abnormal high TSH. Combined low iron and low iodine was associated with reduced free T3 and increased TSH. In addition, high iodine was associated with increased risk of abnormal high TSH in females but not in males. Thyroid function may be disrupted by low levels of iron or abnormal iodine, and relationships are complex and sex-specific. Large prospective studies are needed to understand the mechanisms by which iron interacts with iodine on thyroid function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The incidence of thyroid cancer has been rising steadily over the past few decades in most countries [1], particularly among women. This increase may be explained partly by improved detection of papillary tumors and modification in diagnostic criteria [2]. Changes in environmental risk factors also likely play important roles in development of thyroid cancer [3]. Previous history of benign thyroid disease (e.g., nodule/adenoma, goiter) is one of the few well-established risk factors for thyroid cancer [4]. To shed light on the possible causes for rising thyroid cancer rates, we need to study and understand factors influencing thyroid function.
The thyroid gland plays important roles in regulating metabolism and promoting normal development of cardiovascular, reproductive, and nervous systems. Normal thyroid function depends on the presence of many trace elements for both synthesis and metabolism of thyroid hormones. For example, iodine is a critical element for healthy thyroid hormone production [5]. Iodine deficiency is a risk factor for goiter, development of thyroid nodules and follicular thyroid cancer, whereas papillary thyroid cancer (the most common type of thyroid cancer) seems to be more common in areas with high iodine intake (using WHO criteria for median urinary iodine concentration ≥300 μg/L) [6]. These findings indicate that the relationship between iodine and thyroid function may be non-linear and complicated [7].
Apart from iodine, other trace elements, such as iron, selenium, copper, and calcium, are also involved in regulation of this hormone network [8–10]. Iron is essential for efficient iodine utilization and thyroid hormone synthesis. The interaction between iron and iodine on thyroid function has been reported although the exact pathways are still unclear [9, 11–13]. Iron deficiency is the most common nutritional disorder affecting about 20–25% of the world’s population, predominantly children, and women [14]. Iron-deficient women have lower levels of TSH, free T4, and free T3 than the levels of controls [15, 16], as well as higher risk for isolated hypothyroxinemia [17]. Iron deficiency will impair thyroid hormone synthesis, storage, and secretion even by reducing activity of heme-dependent thyroid peroxidase if iodine intake is adequate [9, 13].
The evidence of the iron and iodine interaction has led to several randomized clinical trials in populations with elevated prevalence of goiter and iron deficiency anemia [18–23]. All studies showed greater improvement in thyroid function indices and/or thyroid volume in groups with iron and iodine treatment compared to groups with iodine treatment alone. However, all of these trials were conducted in children or in areas with elevated prevalence of goiter.
Little information is available about the possible combined effects of iron and iodine on thyroid function in the general population. We hypothesized that healthy thyroid function will occur in the presence of adequate iron intake coupled with normal range of iodine intake. People with abnormal levels of either of these elements may experience disrupted thyroid function. The present analysis investigated the combined association of serum iron concentration and urinary iodine concentration with serum thyroid hormone measures using a nationally representative sample, the National Health and Nutrition Examination Survey (NHANES).
Methods
Database
The present study analyzed measurements from three surveys (2007–2008, 2009–2010, and 2011–2012) of the National Health and Nutrition Examination Survey (NHANES) data. NHANES is an ongoing cross-sectional survey that assesses the health and diet of nationally representative samples of the US civilian non-institutionalized population [24]. It uses a complex, stratified, multistage sample design. In NHANES, participants undergo a detailed home interview, followed by a physical examination and laboratory evaluation at a local mobile examination center. Methods for survey data collection are described in detail elsewhere [24].
Study Population
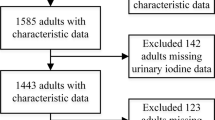
The analytic sample was restricted to survey participants aged 20 and older who had information on thyroid, iron and iodine measures. There were 8436 participants who met these inclusion criteria. We then excluded 84 women who were pregnant, 597 participants with a reported history of thyroid disease, and 83 with data missing for other covariates (age, sex, race/ethnicity, body mass index (BMI)). This resulted in 7672 total subjects available for analysis.
Serum Thyroid Measures
Serum thyroid measures in the NHANES include free and total T3 and T4, and TSH. Measures were based on immunoenzymatic assays as described in the NHANES website [24]. The distribution of TSH was right skewed and was log transformed for analysis. Since TSH analysis is the most common for diagnosing both hyperthyroidism and hypothyroidism, we also categorized TSH as below normal level (<0.45 μIU/mL), normal level (0.45–3 μIU/mL), or above normal (>3 μIU/mL) [25].
Measurement of Iron and Iodine
Serum iron concentration was abstracted from standard biochemistry profile data in the NHANES. The iron concentration was measured by the DcX800 method. The DcX800 method is a timed-endpoint method [24]. Urinary iodine concentrations in the NHANES were determined by ICP-DRC-MS (inductively coupled plasma dynamic reaction cell mass spectroscopy) [24]. There were no changes for both measurements among the three time periods in equipment, lab methods, or lab site.
Serum iron concentration was categorized based on sex-specific reference levels (55–160 μg/dL in men and 40–155 μg/dL in women) [26]. Only 3% of the study population had increased iron levels; therefore, we categorized serum iron concentration into only two categories: low (male < 55 μg/dL and female <40 μg/dL) and normal. Urinary iodine concentration was categorized as low, normal, and high based on reference ranges suggested by the WHO (low (<100 μg/L), normal (100–299), and high (≥300)) [27].
Covariates
We considered age in years, sex (male, female), race/ethnicity (Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black, others), and BMI (kg/m2) as potential confounding variables.
Statistical Analysis
Descriptive analyses examined baseline characteristics by serum iron and urinary iodine concentrations. Differences were tested using chi-squared statistics for categorical variables and ANOVA for continuous variables.
We included stratum and cluster weights to account for the complex and multistage study design of NHANES data. We also used 2-year examination sample weights for individual probabilities drawn for laboratory examination.
Multivariate linear regression models were conducted with serum thyroid measures (TT3, FT3, TT4, FT4, and TSH) as dependent variables and combinations of serum iron concentrations and urinary iodine concentrations as predictors after adjusting for age, sex, race/ethnicity, and BMI categories. Further, logistic regression models were performed with TSH levels (low, normal, and high) and combinations of serum iron concentrations and urinary iodine concentrations after adjusting for potential confounders. Models were run overall and by sex. Data analysis was performed using SAS software version 9.4.
Results
In the study population, 48.8% were females with mean age of 46.5 ranging from 20 to 80 years and mean BMI of 28.4 (SD = 7.2). Among males, the mean age was 46.0 ranging from 20 to 80 years and mean BMI was 28.6 (SD = 5.9).
Overall, 10.9% of the study population had low serum iron concentrations (10.9% in men and 10.8% in women); 32.2, 49.0, and 18.8% of the study population had low, normal and high urinary iodine concentrations, respectively. The distributions of low, normal and high iodine concentrations were 28.4, 50.2, and 21.4% in men, and 36.3, 47.8, and 16.0%, in women, respectively.
Compared to people with a normal concentrations of serum iron, people with a low level of iron were more likely to be younger (46.5 vs. 43.3), female (48.5 vs. 51.0%), non-Hispanic black (10.3 vs. 19.4%), or Mexican American (8.1 vs. 10.5%), and have high BMI (28.3 vs. 29.8). In addition, compared with people with high urinary iodine, people with low iodine were more likely to be younger, female, non-Hispanic black, and have lower BMI regardless of iron status (Table 1).
Controlling for covariates, and compared with normal levels of iron and iodine, when serum iron was normal, elevated urinary iodine concentration was associated with reduced level of free T3; when serum iron concentration was low, both low and normal levels of urinary iodine concentrations were associated with reduced level of free T3, and when serum iron concentration was low, low urinary iodine concentration was associated with increased TSH level after adjusting for age, sex, BMI and race/ethnicity (Table 2).
When TSH was analyzed as a categorical variable, we found that high urinary iodine concentration was associated with increased risk of having abnormal high TSH when serum iron was normal overall. When performing analysis stratified by sex, we observed that high iodine was associated with increasing risk of having abnormal high TSH for females regardless of iron level, but no significant association was observed for males (Table 3).
Discussion
In the present study, we found that when serum iron concentrations were normal, a high urinary iodine concentration was associated with reduced free T3, and increased risk of having abnormal high TSH. When serum iron levels were low, a low level of urinary iodine concentrations was associated with reduced free T3 level and increased TSH level. In this study, serum iron concentration modified the association between urinary iodine concentration and thyroid function. In addition, we also observed that high urinary iodine concentration was associated with increased risk of having abnormal high TSH only in females, but not in males. These results suggest that associations between iron, iodine and thyroid function may be sex-specific.
The thyroid is an endocrine gland. Measuring the TSH level is considered by the American Thyroid Association as the best way to initially test thyroid function [28]. A high TSH level often indicates that the thyroid gland is failing and does not produce enough thyroid hormones (primary or subclinical hypothyroidism). On the other hand, a low TSH level usually indicates an overactive thyroid that is producing too much thyroid hormone (hyperthyroidism) with an exception of abnormality in the pituitary gland that does not produce enough TSH to stimulate the thyroid (secondary hypothyroidism) [28].
Our data show that both low urinary iodine concentration and excess urinary iodine concentration were associated with high TSH. The high TSH associated with low iodine is well understood, because low iodine leads to a low amount of T4 and/or T3 production, and these stimulate an increase in TSH as an adaptation. However, accounting for the high TSH associated with excess iodine is less apparent, although the acute Wolff-Chaikoff effect (a transient reduction in the synthesis of thyroid hormone in rats exposed to high amounts of iodide) was described in 1948 [29]. In most individuals, the decreased production of thyroid hormones is only transient; however, vulnerable individuals (such as those with autoimmune thyroid disease, a previous history of surgery, or other pre-existing thyroid disease) might have an increased risk of failing to adapt to the acute Wolff-Chaikoff effect [30]. Exposure to high concentrations of iodine might decrease the release of thyroid hormone, and increase the serum level of TSH to the upper limit of the normal range [5, 31]. TSH is a known thyroid growth factor. Increased serum TSH concentration is associated with increased risk of differentiated thyroid cancer and advanced tumor stage [32]. Therefore, optimization of population iodine intake is an important component of preventive health care to reduce the prevalence of thyroid disorders.
Mechanisms by which iron and iodine mutually influence thyroid function are not well understood. Iron, an essential element in the body, plays a wide variety of physiological and biochemical roles. It is essential for efficient iodine utilization and thyroid hormone synthesis [9]. Iron deficiency anemia could impair thyroid metabolism through decreased oxygen transport [9]. Experimental studies on model organisms have suggested various mechanisms in which iron impacts thyroid function and iodine utilization [9, 11, 12]. For example, iron deficiency in animals alters the central nervous system control of thyroid metabolism [15], decreases T3 affinity to receptors in hepatocytes [33], lowers oxygen transport [34], and reduces thyroid peroxidase (TPO) activity [35]. TPO is an iron-dependent enzyme bound to the apical membrane of the thyrocyte. It catalyzes two initial steps in thyroid hormone synthesis, including iodination of the thyroglobulin and coupling reaction of the iodotyrosine molecules. Given the crucial role of iron in TPO activity, iron deficiency could decrease TPO activity and thereby interfere with iodine utilization and thyroid metabolism [9].
Sex differences in thyroid disease have been reported. A 2014 review of differences in thyroid disease by sex found that the prevalence of hypothyroidism was increased (two to seven fold) in women compared to men [36]. Subclinical hypothyroidism was the most common, which occurs in up to 20% of postmenopausal women [36]. These sex differences may be partially explained because thyroid function in females may be more sensitive to abnormal concentrations of iron or iodine or both. In addition, these sex differences in thyroid disease may also be because women are more likely to have iron or iodine deficiency as shown in our data.
Strengths of this study include, first, that this is the first large-scale analysis in a nationally representative adult population of combined association of iron and iodine with thyroid function, and second, that NHANES collects a range of potential confounders that we adjusted for in the models. However, several limitations should also be noted. First of all, both urinary iodine and serum iron concentrations were based on a single sample; these measures may represent short term exposure. Second, NHANES is a cross-sectional database; both iron and iodine biomarkers and thyroid function were measured at the same time, making attribution of causal direction difficult.
In conclusion, our study suggests that thyroid function may be disrupted by low levels of iron, low or high levels of iodine, and the relationships are complex and sex-specific. Large prospective studies are needed to better understand the mechanisms by which iron interacts with iodine on thyroid function, so that light may be shed on the complex nature of the associations shown here and in prior research.
References
La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E (2015) Thyroid cancer mortality and incidence: a global overview. Int J Cancer 136:2187–2195
Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal ML (2016) Worldwide thyroid-cancer epidemic? The increasing impact of Overdiagnosis. N Engl J Med 375:614–617
Enewold L, Zhu KM, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS (2009) Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomark Prev 18:784–791
Franceschi S, Preston-Martin S, Dal Maso L, Negri E, La Vecchia C, Mack WJ, McTiernan A, Kolonel L, Mark SD, Mabuchi K, Jin F, Wingren G, Galanti R, Hallquist A, Glattre E, Lund E, Levi F, Linos D, Ron E (1999) A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control 10:583–595
Zimmermann MB, Boelaert K (2015) Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 3:286–295
Zimmermann MB, Galetti V (2015) Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 8:8
Zhao W, Han C, Shi XG, Xiong CH, Sun J, Shan ZY, Teng WP (2014) Prevalence of goiter and thyroid nodules before and after implementation of the universal salt iodization program in mainland China from 1985 to 2014: a systematic review and meta-analysis. PLoS One 9:e109549
Kohrle J (2005) Selenium and the control of thyroid hormone metabolism. Thyroid 15:841–853
Zimmermann MB (2006) The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr 26:367–389
Jain RB (2014) Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol Trace Elem Res 159:87–98
Zimmermann MB, Kohrle J (2002) The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid 12:867–878
Hess SY, Zimmermann MB (2004) The effect of micronutrient deficiencies on iodine nutrition and thyroid metabolism. Int J Vitam Nutr Res 74:103–115
Hess SY (2010) The impact of common micronutrient deficiencies on iodine and thyroid metabolism: the evidence from human studies. Best Pract Res Clin Endocrinol Metab 24:117–132
McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B (2009) Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr 12:444–454
Beard JL, Brigham DE, Kelley SK, Green MH (1998) Plasma thyroid hormone kinetics are altered in iron-deficient rats. J Nutr 128:1401–1408
Kandhro GA, Kazi TG, Afridi HI, Kazi N, Arain MB, Sarfraz RA, Sirajuddin SN, Baig JA, Shah AQ (2008) Evaluation of iron in serum and urine and their relation with thyroid function in female goitrous patients. Biol Trace Elem Res 125:203–212
Yu X, Shan Z, Li C, Mao J, Wang W, Xie X, Liu A, Teng X, Zhou W, Li C, Xu B, Bi L, Meng T, Du J, Zhang S, Gao Z, Zhang X, Yang L, Fan C, Teng W (2015) Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J Clin Endocrinol Metab 100:1594–1601
Zimmermann M, Adou P, Torresani T, Zeder C, Hurrell R (2000) Persistence of goiter despite oral iodine supplementation in goitrous children with iron deficiency anemia in cote d’Ivoire. Am J Clin Nutr 71:88–93
Hess SY, Zimmermann MB, Adou P, Torresani T, Hurrell RF (2002) Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt in cote d’Ivoire. Am J Clin Nutr 75:743–748
Zimmermann MB, Zeder C, Chaouki N, Torresani T, Saad A, Hurrell RF (2002) Addition of microencapsulated iron to iodized salt improves the efficacy of iodine in goitrous, iron-deficient children: a randomized, double-blind, controlled trial. Eur J Endocrinol 147:747–753
Zimmermann MB, Zeder C, Chaouki N, Saad A, Torresani T, Hurrell RF (2003) Dual fortification of salt with iodine and microencapsulated iron: a randomized, double-blind, controlled trial in Moroccan schoolchildren. Am J Clin Nutr 77:425–432
Zimmermann MB, Wegmueller R, Zeder C, Chaouki N, Rohner F, Saissi M, Torresani T, Hurrell RF (2004) Dual fortification of salt with iodine and micronized ferric pyrophosphate: a randomized, double-blind, controlled trial. Am J Clin Nutr 80:952–959
Eftekhari MH, Simondon KB, Jalali M, Keshavarz SA, Elguero E, Eshraghian MR, Saadat N (2006) Effects of administration of iron, iodine and simultaneous iron-plus-iodine on the thyroid hormone profile in iron-deficient adolescent Iranian girls. Eur J Clin Nutr 60:545–552
NHANES. (2016) National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/index.htm. CDC
Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL, American Association of Clinical E (2002) American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract 8:457–469
Gomella LG, Haist SA (2007) Laboratory diagnosis: chemistry, immunology, serology. McGraw-Hill, New York
WHO. (2016) Urinary Iodine concentrations for determining iodine status in populations. http://apps.who.int/iris/bitstream/10665/85972/1/WHO_NMH_NHD_EPG_13.1_eng.pdf. World Health Organization.
Association AAT. Thyroid function tests. http://www.thyroid.org/wp-content/uploads/patients/brochures/nTests_brochure.pdf. American Thyroid Association
Wolff J, Chaikoff IL (1948) Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem 174:555–564
Leung AM, Braverman LE (2014) Consequences of excess iodine. Nat Rev Endocrinol 10:136–142
Paul T, Meyers B, Witorsch RJ, Pino S, Chipkin S, Ingbar SH, Braverman LE (1988) The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metabolism 37:121–124
Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H (2008) Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocr Metab 93:809–814
Smith SM, Johnson PE, Lukaski HC (1993) In vitro hepatic thyroid hormone deiodination in iron-deficient rats: effect of dietary fat. Life Sci 53:603–609
Surks MI (1969) Effect of thyrotropin on thyroidal iodine metabolism during hypoxia. Am J Phys 216:436–439
Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF (2002) Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr 132:1951–1955
Bauer M, Glenn T, Pilhatsch M, Pfennig A, Whybrow PC (2014) Gender differences in thyroid system function: relevance to bipolar disorder and its treatment. Bipolar Disord 16:58–71
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Rights and permissions
About this article
Cite this article
Luo, J., Hendryx, M., Dinh, P. et al. Association of Iodine and Iron with Thyroid Function. Biol Trace Elem Res 179, 38–44 (2017). https://doi.org/10.1007/s12011-017-0954-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0954-x