Abstract
The present study was to evaluate the hepatotoxicity effects in mice exposed to copper (Cu) used as dietary supplements for 95 days. Cu-treated mice showed increased body weight, and no toxic symptoms were observed at the beginning, but the tendency gradually changed with progress of experiment. In the liver, beneficial metals [Cu, iron (Fe), zinc (Zn), manganese (Mn), and molybdenum (Mo)] were analyzed by flame atomic absorption spectrometry. The content of Cu maintained at the same level during the experiments, but not resulting in the imbalance of Fe, Zn, Mn, and Mo being distributed. The activities of alkaline phosphatase (AKP) and super oxidation dismutase (SOD) showed significantly improvement during the first 30 days in Cu-supplemented group (P < 0.01) but declined rapidly from 30th to 60th days, and later, they stabilized and were not statistically significant compared with control (P > 0.05). No statistically significant correlation of ceruloplasmin (CPL) activity was appreciated during the experiment. The histopathological and ultrastructural abnormalities changes were observed in the liver of mice including vacuolar degeneration, necrosis, karyorrhexis, and endolysis. Many hepatocytes showed increased collagenic fibers, appearance of triglyceride droplets, and swollen mitochondria due to oral route of copper, which may lead to lipid peroxidation and free radicals. In conclusion, our study showed that exposure to copper influenced behavioral pattern and body weight, affected several enzymatic activities, and led to the physiological and considerable structural changes in the liver of mice. The public should pay more attention to avoid being exposed to copper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies showed that metal contamination in the environments is closely associated with accelerated economic growth in the past decades [1]. China has been able to maintain an annual GDP growth of over 8 % in the past few years [2]. The rapid growth of the economy in China has been coupled with increasing environmental pollution. The ratio of pollution haze days in central and eastern regions of China was about 10 % in 2013. Air quality in China is notoriously poor and recently has become an issue associated with increasing social unrest. Some researchers have recently reported that long-term exposure to an additional 100 μg/m3 of total suspended particulates (TSPs) is associated with a reduction in life expectancy at birth of about 3.0 years [3]. It is estimated that 350,000–500,000 people died prematurely every year because of air pollution [4, 5]. Rapid urbanization and industrialization are accompanied by a burst of production and usage of chemicals including metals. Heavy metals contaminate the environment and enter the food chain. Alarmingly, high metal concentrations are observed in the sediments, water, and organisms collected from the heavily industrialized areas and coastal and estuarine ecosystems in China, which draw worldwide attention [1, 6–8]. Elevated levels of metal contamination in China’s environment can increase the threat to human and animals’ health because of toxicity, bioaccumulation, and biomagnification in the food chain. It will significantly influence the oxidative stress biomarkers when the metal is over a critical concentration [9]. The contents of these metals in vegetables and meat foodstuffs may vary depending on the general (varieties, maturity, genetics, and age) and environmental (soils, geographical locations, season, water source, and use of fertilizers) conditions of plants and animals and on methods of handling and processing [10].
Concentration of trace elements must be maintained within narrow limits to safeguard the functional and structural integrity of tissues and animals’ growth, health, and fertility [11]. They are given special attention due to their toxic effect in the body when their concentrations exceed limits of safe exposure. As a cofactor for important enzymes, copper (Cu) is an essential element for all living organisms [12]. However, various biological functions can be impaired when Cu intakes are deficient. In contract, it can be toxic mainly because it can exist in two oxidation states and catalyze production of oxygen radicals when taken in high doses [13, 14]. Cu excess also alters gene expression, affecting the synthesis of cholesterol as well as the expression of enzymes involved in fatty acid metabolism and bile acid synthesis [15]. Potential risks associated with high chronic Cu intake from environment, foods, and water has been a concern to health researchers and regulators. However, to date, except for liver content, there are no good indicators for identifying individuals with excess liver copper.
These previous studies led us to investigate Cu-induced liver toxicity in mice, especially involving hepatic trace element levels and morphological characteristic of hepatocytes. The changes in contents of trace amounts (Cu), histopathology, and ultrastructure of the liver were observed. Evaluation also included levels of alkaline phosphatase (AKP), super oxidation dismutase (SOD), and ceruloplasmin (CPL) in plasma.
Materials and Methods
Design and Copper Dosing
All experiments were carried out humanely and with respect for alleviation of suffering following protocols approved by the Institutional Animal Care and Use Committee of Lanzhou Institute of Husbandry and Pharmaceutics Sciences of Chinese Academy of Agricultural Sciences (animal use permit: SCXK20008-0003). Mice were obtained from the Experimental Animal Center of Lanzhou University, China.
One hundred Kunming mice (20 days old) were randomly divided into two groups, 50 in each group; all of them were housed and maintained under specific pathogen-free conditions in temperature-controlled rooms (25 °C) with 24-h light cycles. Food and non-ionic water were consumed ad libitum. The experiment lasted for 95 days. During 0–30, 31–60, 61–80, and 81–95 experimental days, mice diet was supplemented with copper at 4.5 mg/kg dry matter (DM) in control and 15.93, 31.86, 63.72, and 127.44 mg/kg DM in high-copper group (H–Cu). Surviving animals were weighed regularly, and visual observations for mortality, behavioral pattern, changes in physical appearance, injury, pain, and signs of illness were conducted daily during the period.
Colorimetric Examination for AKP, SOD, and CPL in Plasma
On the 30th, 60th, 80th, and 95th day of the experiment, respectively, ten mice in each group were selected randomly. Blood samples were collected via decapitation from each group. Plasma was separated and stored at −80 °C before analysis. The colorimetric assays detected quantify AKP, SOD, and CPL with kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instruction by ultraviolet spectrophotometer (UV-2100, Shimadzu Corporation, Japan).
Cu, Zinc, Iron, Manganese, and Molybdenum Content Assay
The livers were immediately excised, blotted, and then rinsed with ice-cold 0.9 % NaCl solution. One biopsy was snap-frozen at −80 °C and analyzed for total copper. The contents of Cu in liver tissue were detected using flame atomic absorption spectrometry (FAAS) (ZEEnit 700; Analytik Jena, Germany). The samples of liver organs were cut into small pieces and dried at 110 °C for 12 h in baking oven; then, 1.0 g of samples was placed in PTFE digestion tubes, and 12 mL diacid mixture (HNO3:HCl, 3:9) was added to each digestion tubes. The optimal operating condition was developed as described in our previous publication [16].
Histopathology and Ultrastructure
The second liver specimen was fixed in 10 % buffered neutral formalin and was processed for paraffin wax sectioning. Sections were stained with hematoxylin and eosin for light microscopy [17]. For electron microscopy, liver organ specimens were fixed with 2.5 % glutaraldehyde as previously described [18, 19]. Ultrathin sections were stained with 4 % uranyl acetate and lead citrate for transmission electron microscope evaluation.
Statistical Analyses
The SPSS procedures (version 17.0; SPSS, Inc., Chicago, USA) were used for all statistical analyses of data. We performed initial descriptive statistics, including mean and standard deviation (SD). Significance of differences between two groups was evaluated using Student’s t test. The P values were two-tailed, and two significant levels were P = 0.05 and 0.01.
Results
Behavior, Symptoms, and Body Weight Statistical Analysis
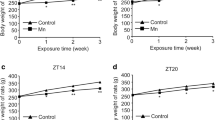
The toxic effect of Cu on the appearance and general behavioral pattern of mice was observed during the period. No toxic symptoms or mortality was observed at the beginning of the experiment (0–20 days), but the behavioral patterns displayed significant changes in behavior with progress of experiment, such as panicky frenzy, biting each other, and death in experimental group. The body weight was calculated and shown in Fig. 1. The body weight of mice between Cu-treated and control groups shown statistically significant differences (P < 0.05) during the period of 45–60 days; copper-treated mice showed increased body weight. All animals exhibited a normal increment in body weight without a drastic difference during the other experiments (P > 0.05).
Body weight of mice receiving control or supplemented high-copper diet. During the period of 45–60 days, body weight increased in the copper-treated group. During 0–30, 31–60, 61–80, and 81–95 experimental days, mice diet was supplemented with copper at 4.5 mg/kg DM in control and 15.93, 31.86, 63.72, and 127.44 mg/kg DM in high-copper group (H–Cu). Double asterisks indicate significance at 0.01 probability level. Asterisk indicates significance at 0.05 probability level
Determination of the Concentrations of Cu, Zinc, Iron, Manganese, and Molybdenum in Liver Tissue
The contents of Cu, its main antagonists [zinc (Zn), iron (Fe), and molybdenum (Mo)], and manganese (Mn) during experiment periods (n = 10) in liver of both groups were analyzed (Figs. 2 and 3). Element contents in the body of livestock and poultry mainly come from the diet and water. The change of Cu in diet had an obvious effect on the elements in the liver and blood. When the concentration of Cu in diet increased from 15.93 to 31.86 mg/kg DM during 60 days, the accumulation of Cu in the liver was in saturation compared to control (P < 0.05 and P < 0.01). The results revealed that the Cu content in the liver appeared to be in a dose- and time-dependent fashion during 60 days. While the Cu level in diet constantly rose up from 63.72 to 127.44 mg/kg DM, the level of Cu was rapidly reduced in the liver (Fig. 2).
The Cu content in the liver of mice. During 0–30, 31–60, 61–80, and 81–95 experimental days, mice diet was supplemented with copper at 4.5 mg/kg DM in control and 15.93, 31.86, 63.72, and 127.44 mg/kg DM in high-copper group (H–Cu). Double asterisks indicate significance at 0.01 probability level. Asterisk indicates significance at 0.05 probability level
The Zn, Fe, Mn, and Mo contents in the liver of mice. During 0–30, 31–60, 61–80, and 81–95 experimental days, mice diet was supplemented with copper at 4.5 mg/kg DM in control and 15.93, 31.86, 63.72, and 127.44 mg/kg DM in high-copper group (H–Cu). Double asterisks indicate significance at 0.01 probability level. Asterisk indicates significance at 0.05 probability level
There were no statistically significant differences in liver composition in any of the Zn, Mn, and Mo analyzed during experiments (P > 0.05) (Fig. 3), but the mean concentration of Fe in the liver of Cu-supplemented group increased rapidly from beginning to 30th day, and it was statistically significant compared with that of control (Fig. 3, P < 0.05); then, it declined, but this change was not statistically significant (Fig. 3, P > 0.05).
AKP, SOD, and CPL Expression
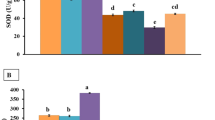
The animals from the Cu-supplemented group showed significantly improvement of the activities of AKP and SOD during the first 30 days (Fig. 4, P < 0.01), but the mean activities declined rapidly from 30th to 60th day; later, they stabilized and were not statistically significant compared with those of control (Fig. 4, P > 0.05). No statistically significant correlations of activity of CPL were appreciated between Cu- and non-supplemented groups (Fig. 4).
Histopathology and Ultrastructure
Histology of the liver in Cu-treated mice was shown in Fig. 5a–f. Histology of the liver in control mice showed intact structure with regular morphology. The gallbladder was filling, and intestinal tympanites in several mice in Cu-supplemented group (Fig. 5a) were observed. The cloudy swelling, necrosis, and cytoplasm crack of liver cells emerged obviously in Cu-supplemented group, and the form of nucleus was vague (Fig. 5b–f). The pathological changes appeared in the liver cells around portal areas, and then, the areas and degree of damages were extended to all liver lobules with improvement of copper concentration in the diet. Taken together, all these results revealed that Cu poisoning could induce liver apoptosis.
Histology (hematoxylin and eosin staining) of liver in Cu-supplemented group; b × 100, c × 200, d × 200, e × 200, f × 200 diseased liver in the Cu-supplemented group, respectively. a The gallbladder was filling, and intestinal tympanites were present. b Liver cells were in vacuolar degeneration and necrosis. c Liver cells were in extensive vacuolar degeneration. d Liver cells were in necrosis and karyorrhexis. e Liver cells were in vacuolar degeneration. f Liver cells were in areas of coagulative necrosis. CV central vein
Electron microscopy showed normal liver ultrastructure in the control group (Fig. 6a). Cells in control group distinctly exhibited normal chromatin. The plentiful organoid and ribosome were observed in smooth endoplasmic reticulum (SER). Cu-supplemented group had extensive liver tissue damage. Cells in Cu-supplemented group displayed dissolution of cytoplasm, increasing collagenic fibers, appearance of triglyceride droplets, decreasing cell organelle, and markedly swollen mitochondria with degeneration (Fig. 6b–f).
Transmission electron microscopy of Cu-supplemented group liver cells. a The structure of normal liver cell (×13,000). b Cytoplasm dissolution (×8,300). c, d The collagenic fibers were increased in liver cell (c × 6,600; d × 13,000). e The mitochondria were swelling in liver cell (×16,000). f Triglyceride droplets appeared in liver cell (×13,000). MI mitochondria, NU nucleus APO apoptosis
Discussion
Longer Cu exposure time was used in the present study, which would provide a further foundation for the environmental risk assessment of Cu chronic toxicity under natural conditions. The metal trace elements are involved in the basic biochemistry of aerobic life because these atoms participate as a part of the active center of vital proteins, hormone systems, and enzymes. Adequate intake and balance of trace elements are required for proper functioning of metabolic processes including immune response and reproduction. Cu is an essential trace element required for enzyme systems, iron metabolism, connective tissue metabolism, and mobilization, plus integrity of the central nervous and immune systems. Concerning Cu atoms, they are a half part of the binuclear center of cytochrome oxidase that accommodates and reacts with O2. Moreover, Cu redox changes is the basis of the dismutation chemistry catalyzed by Cu, Zn–superoxide dismutase in which two superoxide radicals (O2 −) yield hydrogen peroxide (H2O2), and O2. Cu levels in the liver are often used to diagnose Cu deficiencies or excesses because it is a better indicator of Cu status than blood levels, and Cu levels in the liver are also affected by the amount of Cu in the diet [20].
In this study, a model of Cu poisoning was established to study the effects of dietary Cu on liver damage in mice. Surviving animals were weighed, and visual observations for mortality, behavioral pattern, and changes in physical appearance were conducted regularly during the period. No toxic symptoms or mortality was observed at the beginning of the experiment (0–20 days), but the behavioral patterns displayed significant changes in behavior with progress of experiment, such as panicky frenzy, biting each other, and death in experimental group. Although the Cu-treated mice showed increased body weight during the period of 45–60 days, after that, the tendency decreased gradually. Longer Cu exposure time may influence the behavioral pattern, growth, and development of animals.
Our results revealed that Cu content in the liver increased gradually along with the doses of Cu-supplemented diets from 15.93 to 31.86 mg/kg DM during 60 days. The accumulation of Cu in the liver was in saturation compared to that of control (P < 0.05 and P < 0.01). The results revealed that the Cu content in the liver appeared to be in a dose- and time-dependent fashion during 60 days. While the Cu level in the diet constantly rose up from 63.72 to 127.44 mg/kg DM, the Cu level in the liver was rapidly reduced. Cu absorption is negatively affected by antagonistic elements in the diet such as Mo, Fe, and Zn and can be further exaggerated when Mo, Fe, and Zn levels are elevated. There were basically no statistically significant differences in liver composition in any of the Mo, Fe, and Zn analyzed during experiments. Mn can exert serious neurotoxic effects on both human beings and experimental animals at higher concentrations [21]. The cellular, intracellular, and molecular mechanisms underlying the Mn-induced neurotoxicity are both dose- and time-dependent [22]. Our results illustrated that a high level of Cu in diets led to accumulation in the liver of mice, but not resulting in the imbalance of trace elements.
AKP is an important enzyme that regulates a number of essential functions in all living organisms [23]. This enzyme plays an important role in phagotrophy and sterilization ability of macrophages. AKP activity can reflect the growth performance of animals, improving AKP activity that is helpful for improving average daily gain [24]. AKP activity can be used as a reliable index in the assessment of immune status [25] and also can enhance the non-specific immunity function. It is widely recognized that elevated levels of free radicals derived from oxygen (ROS) are related to the pathogenesis of various human diseases. Although copper ions activate signaling cascades of the antiapoptotic PI3K/Akt pathway that are known to protect cells against oxidative stress-induced apoptosis [26], many studies have reported that Cu overload leads to oxidative stress and subsequent oxidative damage to proteins, lipids, and nucleic acids [27, 28]. Organisms have developed antioxidant defense mechanisms to compensate for oxidative damage caused by exposure to metals. Prominent among antioxidant defense system of the organism is SOD which catalyzes the dismutation of the superoxide anion to molecular oxygen and hydrogen peroxide [22, 29] and involved in protective mechanisms in tissue injury following oxidation and phagocytosis. In the present study, the animals of the Cu-supplemented group showed significantly improvement on the activities of AKP and SOD during the first 30 days (P < 0.01), but the mean activities declined rapidly from 30th to 60th day along with increasing long-term exposure to dietary Cu and later stabilized. This study indicated that the total antioxidative capability and immune status of mice plasma in the Cu diet-fed group were gradually increased during the first 30 days. After that, the tendency was not significant, but most likely impaired. The vast majority of serum Cu is transported bound to CPL, which is synthesized by hepatocytes. CPL carries Cu from the liver to numerous tissues [30]. Cu is a cofactor of CPL which exhibits an anti-inflammatory activity and also plays a critical role in the prevention of oxidative damage resulting from infections and inflammation [31]. Alterations in CPL levels are currently regarded as one of the mechanisms underlying the development of a number of neurodegenerative disorders [32]. This study indicated that plasma CPL activity was not changed by increasing the dietary Cu levels.
In the present work, the histopathological and ultrastructural abnormality changes were observed in the liver of mice including vacuolar degeneration, necrosis, karyorrhexis, and endolysis. Many hepatocytes showed increased collagenic fibers, appearance of triglyceride droplets, and swollen mitochondria due to oral route of Cu, which may lead to lipid peroxidation and free radicals. Free radical may propagate damage in the endoplasmic reticulum and oxidation of the membrane component of the liver cells consistent with ultrastructural changes observed in the present work. Oxidation has been shown to be associated with apoptosis (programmed cell death) [33]. Cell death can result from naturally occurring apoptosis (physiological apoptosis) or from irreparable cell injury (pathological apoptosis) [34]. Apoptosis is a common feature of hepatotoxicity induced by many chemicals, or it may occur concurrently with necrosis as in hepatotoxicity [35]. Cu deposition occurs in the hepatic parenchymal cells, brain, periphery of the iris, and kidney [30]. Initially, Cu accumulates in the liver; thus, hepatic presentations are common. It is reported that a high concentration of Cu resulted in the derangement of lipid and lipoprotein metabolism [36]. Mitochondria is an important target organelle that is easily influenced by heavy metal toxicity. In this study, markedly swollen mitochondria with degenerated or missing cristae were observed in the liver cells by electron microscopy.
In conclusion, our study showed that exposure to copper influenced the behavioral pattern, reduced several enzymatic activities, and led to the physiological and considerable structural changes in the liver of mice. Air pollution may have a great harmful effect to human health. The state and the public should pay more attention to this problem, especially in China.
References
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421–422:3–16
NBSC (National Bureau of Statistics of China) (2012) China statistical yearbook 2012. China Statistic Press, Beijing
Chen Y, Ebenstein A, Greenstone M, Li H (2013) Evidence on the impact of sustained exposure to air pollution on life expectancy from China’s Huai River policy. Proc Natl Acad Sci U S A 110:12936–12941
Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ (2013) Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 381:1987–2015
Chen Z, Wang JN, Ma GX, Zhang YS (2013) China tackles the health effects of air pollution. Lancet 382:1959–1960
Yuan CG, Shi JB, He B, Liu JF, Liang LN, Jiang GB (2004) Speciation of heavy metals in marine sediments from the East China Sea by ICP–MS with sequential extraction. Environ Int 30:769–783
Wang CY, Wang XL (2007) Spatial distribution of dissolved Pb, Hg, Cd, Cu and As in the Bohai Sea. J Environ Sci (China) 19:1061–1066
Zhang L, Ye X, Feng H, Jing Y, Ouyang T, Yu X, Liang R, Gao C, Chen W (2007) Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Mar Pollut Bull 54:974–982
Pytharopoulou S, Grintzalis K, Sazakli E, Leotsinidis M, Georgiou CD, Kalpaxis DL (2011) Translational responses and oxidative stress of mussels experimentally exposed to Hg, Cu and Cd: one pattern does not fit at all. Aquat Toxicol 105:157–165
Stef DS, Gergen I (2012) Effect of mineral-enriched diet and medicinal herbs on Fe, Mn, Zn, and Cu uptake in chicken. Chem Cent J 6:19
Castillo-Durán C, Cassorla F (1999) Trace minerals in human growth and development. J Pediatr Endocrinol Metab 12:589–601
Franco R, Sánchez-Olea R, Reyes-Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: ménage à trois. Mutat Res 674:3–22
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Huster D, Lutsenko S (2007) Wilson disease: nor just a copper disorder. Analysis of a Wilson disease model demonstrates the link between copper and lipid metabolism. Mol Biosyst 3:816–824
Wang H, Liu Y, Qi Z, Wang S, Liu S, Li X, Wang H, Wang X, Xia X, Zhu X (2014) The estimation of soil trace elements distribution and soil–plant–animal continuum in relation to trace elements status of sheep in Huangcheng area of Qilian mountain grassland, China. J Integ Agri 13:140–147
Araya M, Núñez H, Pavez L, Arredondo M, Méndez M, Cisternas F, Pizarro F, Sierralta W, Uauy R, González M (2012) Administration of high doses of copper to capuchin monkeys does not cause liver damage but induces transcriptional activation of hepatic proliferative responses. J Nutr 142:233–237
Liu X, Zuo N, Guan H, Han C, Xu SW (2013) Manganese-induced effects on cerebral trace element and nitric oxide of Hyline cocks. Biol Trace Elem Res 54:202–209
Sierralta WD (2001) Immunoelectron microscopy in embryos. Methods 24:61–69
Fry RS, Spears JW, Lloyd KE, O’Nan AT, Ashwell MS (2013) Effect of dietary copper and breed on gene products involved in copper acquisition, distribution, and use in Angus and Simmental cows and fetuses. J Anim Sci 91:861–871
Eybl V, Kotyzová D (2010) Protective effect of manganese in cadmium-induced hepatic oxidative damage, changes in cadmium distribution and trace elements level in mice. Interdiscip Toxicol 3:68–72
Villalobos V, Castro F, Bonilla E, Estévez J, Dávila JO (1994) Manganese toxicity: muscarinic receptor binding in the mouse brain. J Toxicol Environ Health 42:185–191
Zhou C, Liu B, Ge X, Xie J, Xu P (2013) Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of Wuchang bream, Megalobrama amblycephala. J Appl Ichthyol 29:1348–1356
Zhou Y, Diao QY, Tu Y, Yun Q (2010) Effects of yeast β-glucan on growth performance, serum biochemical and gastrointestinal characteristics in pre-ruminant calves. Chin J Anim Sci 46:47–51
Sarlin PJ, Philip R (2011) Efficacy of marine yeasts and baker’s yeast as immunostimulants in Fenneropenaeus indicus: a comparative study. Aquaculture 321:173–178
Ostrakhovitch EA, Lordnejad MD, Schliess F, Sies H, Klotz LO (2002) Copper ions strongly activate the phosphoinositide–3–kinase/Akt pathway independent of the generation of reactive oxygen species. Arch Biochem Biophys 397:232–239
Boveris A, Musacco-Sebio R, Ferrarotti N, Saporito-Magriñá C, Torti H, Massot F, Repetto MG (2012) The acute toxicity of iron and copper: biomolecule oxidation and oxidative damage in rat liver. J Inorg Biochem 116:63–69
Arnal N, de Alaniz MJ, Marra CA (2012) Cytotoxic effects of copper overload on human-derived lung and liver cells in culture. Biochim Biophys Acta 1820:931–939
Wang H, Liu YM, Qi ZM, Wang SY, Liu SX, Li X, Wang HJ, Xia XC (2013) An overview on natural polysaccharides with antioxidant properties. Curr Med Chem 20:2899–2913
Twomey PJ, Viljoen A, House IM, Reynolds TM, Wierzbicki AS (2005) Relationship between serum copper, ceruloplasmin, and non-ceruloplasmin-bound copper in routine clinical practice. Clin Chem 51:1558–1559
Jones DG, Suttle NF (1981) Some effects of copper deficiency on leukocyte function in sheep and cattle. Res Vet Sci 31:151–156
Virit O, Selek S, Bulut M, Savas HA, Celik H, Erel O, Herken H (2008) High ceruloplasmin levels are associated with obsessive compulsive disorder: a case control study. Behav Brain Funct 4:52
Mohan M, Taneja TK, Sahdev S, Mohareer K, Begum R, Athar M, Sah NK, Hasnain SE (2003) Antioxidants prevent UV-induced apoptosis by inhibiting mitochondrial cytochrome c release and caspase activation in Spodoptera frugiperda (Sf9) cells. Cell Biol Int 27:483–490
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd RF, Gaur RL, Fernando A, Raj MH, Ouhtit A (2009) Histopathological effects of cisplatin, doxorubicin and 5-fluorouracil (5-FU) on the liver of male albino rats. Int J Biol Sci 5:466–473
Liu XJ, Luo Z, Xiong BX, Liu X, Zhao YH, Hu GF, Lv GJ (2010) Effect of waterborne copper exposure on growth, hepatic enzymatic activities and histology in Synechogobius hasta. Ecotoxicol Environ Saf 73:1286–1291
Acknowledgments
The financial supports from the Special Fund for Agro-scientific Research in the Public Interest (No. 201303040) and Central Public Interest Scientific Institution Basal Research Fund (No. 1610322013003) are greatly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xuezhi Wang and Hui Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, X., Wang, H., Li, J. et al. Evaluation of Bioaccumulation and Toxic Effects of Copper on Hepatocellular Structure in Mice. Biol Trace Elem Res 159, 312–319 (2014). https://doi.org/10.1007/s12011-014-9970-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-9970-2