Abstract
Prostate cancer is the most common fatal cancers in men, and exposure to toxic elements is the most important factor in the aetiology for prostate cancer. Selected elements (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) were analyzed in the blood, scalp hair and nails of prostate cancer patients and counterpart healthy donors by atomic absorption spectrometry. Average concentrations of Cd, Mn, Ni and Pb were found to be significantly higher (p < 0.05) in the blood, scalp hair and nails of the patients compared with those of the healthy subjects who exhibited significantly higher concentrations of Zn. The correlation study revealed significantly diverse relationships of the elements in the blood, scalp hair and nails of the two donor groups. Variations in the elemental concentrations were also noted for various types of prostate cancer (adenocarcinoma, squamous cell carcinoma, transitional cell carcinoma and small cell carcinoma), as well as for different stages of the cancer. Multivariate apportionment of trace elements in the blood, scalp hair and nails of the patients was also significantly different than that in the healthy donors. The study evidenced considerably divergent variations in the elemental concentrations in prostate cancer patients in comparison with healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trace elements play a vital role in various biochemical processes in the human body, and the deficiency or excess of these elements can affect health and may be involved in the development of various diseases such as cancer [1]. Prostate cancer continues to be the most frequently diagnosed neoplasm and the second leading cause of cancer-related mortality in men. Globally, prostate cancer is the sixth most common cancer and occurs when cells in the prostate gland grow and multiply uncontrollably [2]. The main histological types of prostate cancer are adenocarcinomas, squamous cell carcinoma, signet-ring carcinoma, transitional cell carcinoma, small cell carcinoma, neuroendocrine carcinoma or sarcoma [3]. Although its cause is not well understood, several risk factors including age, diet, race and genetic/family history have been identified [4]. All the factors might be tracked to the difference of chemical concentrations, especially the trace elements [5]. Even though the molecular mechanism of trace elements as the cause of cancer is not very clear, the studies on the relationship between the cancer causes and the concentrations of elements are of great importance from the perspective of cancer prevention and diagnosis [6].
Determination of elements in the various body fluids/tissues indicates susceptibility to certain diseases, support therapeutic interventions and explain disturbances associated with many pathological conditions. However, the choice of the appropriate specimen depends on several factors, such as toxico-kinetics, the convenience or invasiveness of the sample collection procedure and the potential for specimen contamination [7]. The blood, hair and nails are most commonly used materials which, in comparison to other body tissues, demonstrate many advantages [8]. Blood analysis provides reliable information about what the body has recently absorbed and the concentrations are largely independent of tissue deposition [9]. Hair and nails provide long-term information and are the materials easily accessible for noninvasive sampling. They are a stable matrix, of low cost, and collection and transportation are far simpler, painless and less hazardous to handle [10]. Additionally, they are partially independent of the influence of metabolic processes and homeostatic mechanisms [11]. Many epidemiological studies in humans have demonstrated the carcinogenic effects of trace element exposure including prostate cancer [1, 12–16]. Limited data are available in Pakistan, concerning the trace elemental concentrations in the prostate cancer patients. Thus, there is a dire need to study the interrelationship of trace elements, which could have clinical and diagnostic significance.
The present study is based on the measurement of various essential/toxic elements (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in the blood, scalp hair and nail samples of prostate cancer patients in comparison with those of healthy subjects/controls with matching age, habitat and food habits. Mutual variations among the elemental concentrations were evaluated by correlation study, whereas multivariate cluster analysis was employed for the apportionment of elementals in the blood, hair and nails of the patients and controls. Plausible differences in the elemental concentrations with respect to cancer stages and types were also assessed, thereby investigating whether these elements had any presumptive benefits in the diagnosis/prognosis of prostate cancer.
Materials and Methods
Study Population
The blood, scalp hair and nail samples were collected from the newly diagnosed prostate cancer patients admitted in Nuclear Oncology & Radiotherapy Institute (NORI), Islamabad, Pakistan. Subjects were selected on volunteer basis, and their ages ranged from 32 and 85 years. Prior to sample collection, the protocol of study was approved by the ethical review committee of the institute. The samples were collected from the patients prior to any treatment (i.e. surgery, chemotherapy or radiotherapy), and they were not taking any mineral supplement during last 3 months. Prostate cancer was confirmed by the serum prostate-specific antigen (PSA) test.
The healthy subjects/controls were also selected on volunteer basis from the same localities with matched age groups, similar socioeconomic status and food habits. The subjects were initially briefed about the purpose and objectives of the study, and then a written consent was obtained. A proforma was filled to record the information such as age, gender, habitat, ailment duration, food habits, smoking habits, type of ailment, medicine, hobbies, occupation and tumour grade etc., at the time of sample collection from the subjects. Physical examinations were performed in the hospital to measure participant’s weight, height, blood pressure and biochemical data.
Collection and Processing of the Blood Samples
The blood samples were collected from an antecubital vein by using appropriate precautions to prevent exogenous contamination. Approximately 3 mL venous blood was withdrawn and transferred to the evacuated blood collection tubes. The samples were kept in a refrigerator until further processing. For digestion, an exactly known amount of blood sample was transferred from storage tube to the digestion flask and digested with nitric acid–perchloric acid (10:1 v/v) mixture with subsequent heating to a soft boil until white dense fumes evolved. Samples were then cooled to room temperature and diluted to proper volume with doubly distilled water [6]. Blanks containing all the reagents in the same sequence (without blood sample) were also processed with each batch of the samples.
Collection and Processing of the Hair Samples
About 3 g of hair was cut from the nape of the neck close to scalp, as strands 3–5 cm long, with a pair of plastic scissors. The samples were directly stored in zip-mouthed polythene bags, duly labelled with relevant codes. Scalp hair samples were thoroughly washed to remove any exogenous impurities [11]. The samples were cut into small pieces of 2–3 cm in length and mixed with 50 mL of detergent solution in a conical flask and shaken on an autoshaker for 30 min at 320 vibrations per minute. The samples were left for 2 h undisturbed and then washed with plentiful water until all detergent was leached out. It was followed by the addition of 30 mL Triton X-100 (0.5 %, v/v) and again shaken on an autoshaker for 20 min. Now samples were washed with excess of doubly distilled water and finally dried in electric oven for overnight at 70 °C and cooled to room temperature in a desiccator containing silica gel as the desiccant [6].
For digestion, an accurately weighed portion (∼1 g) of the hair sample was treated with 10 mL of concentrated (65 %) nitric acid and heated at 80 °C for 10 min. It was cooled to room temperature, followed by addition of 5.0 mL of perchloric acid (70 %) with subsequent heating to a soft boil until white dense fumes evolved marking the completion of the digestion process. Sample was cooled to room temperature and diluted to 50 mL with distilled water [6]. The blank was also prepared the same way but without the hair sample.
Collection and Processing of the Nail Samples
For collection of nail samples, the subjects were asked to wash their hands and feet thoroughly with double-distilled water and medicated soap, followed by drying with a clean towel or tissue paper to remove external contamination, if any. All the fingernails and toenails of the subject were clipped in order to obtain the measurable quantity. Then samples were stored in sealed labelled plastic bags. For washing of the nails, the samples were scratched with a quartz knife to remove the surface contamination and were placed in labelled conical flasks and soaked in 5 % (w/v) detergent solution overnight to weaken the bound dirt and shaken on an autoshaker for 20 min at 320 vibrations per minute followed by washing with plenty of tap water. Any loosely bound impurity was removed manually at this stage. Then acetone was added in the conical flasks containing nail sample and was shaken on the autoshaker for 20 min. This step was followed by the addition of 30 mL Triton X-100 (0.5 %, v/v) solution and again shaken for 20 min. Now samples were washed with excess of doubly distilled water and finally dried in electric oven overnight at 70 °C [17].
The dried nail samples were taken in a conical flask and concentrated nitric acid (65 %) and perchloric acid (70 %) (5:1, v/v) were added and left undisturbed for 30 min at room temperature. After predigesting at room temperature, the samples were heated at 80 °C until white dense fumes evolved. At this time, it was observed that the samples were completely digested. Samples were again cooled to room temperature. The digested samples were transferred to volumetric flasks, and the final volume was adjusted with 0.1 N of HNO3 [18]. A blank was also prepared in the same way along with each batch but without the nail sample. After dilution, the samples were coded and stored in screw-tight plastic bottles for elemental analysis.
Quantification of the Elements
The quantitative measurement of selected essential/toxic elements (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in the digested samples was performed using flame atomic absorption spectrophotometer (Shimadzu AA-670, Japan), with automatic background compensation and under optimum analytical conditions as shown in Table 1. All the reagents used were of ultrahigh purity. Doubly distilled water was used throughout the study for the preparation of the samples and standards. Stock solution (1000 mg/L) of each element was used to prepare the fresh working standards just before the analysis. Three subsamples of each sample were treated and run separately onto the spectrophotometer to pool the mean concentrations. The samples were also analyzed at an independent laboratory for comparison of the results, and a maximum of 5 % difference was observed in the results of two laboratories. Parallel routine check on the accuracy of quantified results was ensured through the use of standard reference materials and interlaboratory comparison. Generally, the contribution of the blank was <5 % of the measured concentrations in the samples.
Statistical Analysis
STATISTICA software was used for statistical analysis of the elemental data [19]. The quantified results were subjected to univariate and multivariate analysis in order to classify the relationship among the measured variables. Statistical analysis of the data comprised of the basic statistical parameters, including, range, mean, standard error (SE) and skewness, while mutual variations in the elemental concentrations were computed by correlation study. Cluster analysis (CA) was used for the multivariate apportionment of the elemental data.
Results and Discussion
Characteristics of the Subjects
The demographic characteristics related to the prostate cancer patients (hereafter called ‘patients’) and matched healthy persons (hereafter called ‘controls’) are presented in Table 2. Majority of them in both groups were vegetarians. Almost half of the patients were drawn from rural areas, and most of the patients were not using tobacco on continuous basis. The relative proportion of the healthy donors was more or less the same as those of the patients (Table 2). Patients included in the present study were commonly suffering from adenocarcinoma (55–57 %), followed by squamous cell carcinoma (16–19 %), transitional cell carcinoma (14–15 %) and small cell carcinoma (10–12 %), whereas 25–32 % of the patients were diagnosed at stage I, 24–27 % at stage II, 13–16 % at stage III and 30–36 % at stage IV. The PSA levels in the patients ranged from 317 to 1640 ng/mL with the mean value of 643 ng/mL.
Distribution of the Elements in the Blood
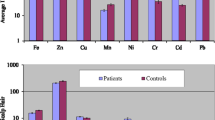
Basic statistical distribution parameters of the trace elements in blood samples of the patients and controls are depicted in Table 3. Most of the elements exhibited large spread/range in their concentrations in both groups. In blood samples of the patients, mean concentration of Fe (850.8 μg/g) was predominantly high, followed by Ni (4.335 μg/g), Zn (4.283 μg/g) and Pb (3.658 μg/g). However, relatively lower concentrations were observed for Cu (1.903 μg/g), Mn (1.524 μg/g), Cr (1.230 μg/g) and Cd (1.084 μg/g). On the average basis, elemental concentrations in the blood of patients showed the following order: Fe > Ni > Zn > Pb > Cu > Mn > Cr > Cd. Basic statistical parameters for the distribution of trace elements in the blood of controls are also shown in Table 3. Predominantly higher mean concentrations were found for Fe (464.3 μg/g), followed by relatively lower concentrations of Zn (6.571 μg/g), Ni (2.687 μg/g), Pb (2.248 μg/g) and Cu (1.951 μg/g). The lowest average concentrations were recorded for Cr (0.976 μg/g), Mn (0.895 μg/g) and Cd (0.774 μg/g). On the average basis, trace elements revealed the following order in their concentrations in the blood of controls: Fe > Zn > Ni > Pb > Cu > Cr > Mn > Cd. Relatively larger dispersion in terms of SE was shown by Fe; however, a noticeably higher asymmetry was observed for Zn and Fe in the blood of controls. Overall, most of the elements manifested random distribution in the blood of both donor groups, although comparatively higher dispersion was observed for the patients.
Two-tailed Student’s t test (p < 0.05) of the data showed that mean concentrations of Fe, Mn, Ni, Cd and Pb in the blood of the patients were significantly higher than those observed in controls; however, there were no significant differences in the concentrations of rest of the elements. Only Zn exhibited considerably higher mean concentration in the blood of controls, whereas Cu showed almost equivalent concentrations in the patients and controls.
Distribution of the Elements in the Scalp Hair
Basic statistical parameters related to the distribution of trace elements in the scalp hair of the patients and controls are shown in Table 3. Most of the elements exhibited a large spread in their concentrations. In the scalp hair of the patients, Zn revealed the highest mean concentration at 160.7 μg/g, followed by Fe (76.85 μg/g), Ni (37.21 μg/g), Pb (35.87 μg/g) and Cu (14.10 μg/g). However, relatively lower mean concentrations were observed for Mn (4.653 μg/g), Cr (4.276 μg/g) and Cd (1.629 μg/g). Overall, the elemental contents in the scalp hair of the patients revealed the following decreasing order: Zn > Fe > Ni > Pb > Cu > Mn > Cr > Cd. Among the selected elements, Zn, Fe, Ni and Pb showed relatively higher dispersion as manifested by SE values. Large skewness values for Zn and Cr indicated their asymmetric distribution in the scalp hair of patients. The distribution of trace elements in the scalp hair of the controls showed markedly higher mean concentration of Zn (582.7 μg/g), followed by Fe (38.37 μg/g), Ni (19.35 μg/g), Cu (14.37 μg/g) and Pb (13.03 μg/g). The lowest concentrations were recorded for Cr, Mn and Cd. The average concentrations of the elements in the scalp hair of controls revealed the following order: Zn > Fe > Ni > Cu > Pb > Cr > Mn > Cd. Higher SE values of Zn, Fe, Ni and Pb indicated their random distribution pattern in the scalp hair of controls.
Comparison of average concentrations of the elements by Student’s t test in the scalp hair of the patients and controls revealed that mean concentrations of Fe, Mn, Ni, Cr and Pb were significantly higher (p < 0.05) in the scalp hair of the patients; nonetheless, the average contents of Cu were more or less similar in the patients and controls. In the case of controls, average concentration of Zn was noticeably higher (p < 0.05) than that in the patients. The comparative study thus indicated an imbalance of the elements in the scalp hair of the patients compared with the controls.
Distribution of the Elements in the Nails
Average concentrations of trace elements in the nails of the patients and controls along with relevant statistical distribution parameters are presented in Table 3. Mostly the elements exhibited a large spread of concentrations in the nails of both groups. Among the elemental concentrations in the nails of patients, Fe revealed the highest average contribution at 226.2 μg/g, followed by Zn (114.2 μg/g) and Ni (101.1 μg/g). However, average concentrations of Pb (24.49 μg/g), Cr (23.88 μg/g), Cu (23.48 μg/g), Mn (11.98 μg/g) and Cd (5.655 μg/g) were relatively lower. Overall, most of the elements exhibited relatively symmetrical distribution in the nails of patients supported by low skewness values. In case of controls, the highest average concentration was noted for Fe (239.4 μg/g), followed by those of Zn (122.7 μg/g), Ni (56.65 μg/g), Cu (24.40 μg/g), Pb (23.83 μg/g), Cr (17.91 μg/g), Mn (8.629 μg/g) and Cd (3.063 μg/g). Predominantly random distribution pattern supported by the large dispersion was shown by most of the elements; however, some of the elements (Cd and Mn) exhibited relatively lower dispersion and narrow range. On the average basis, the elemental concentrations in the nails of controls revealed the following order: Fe > Zn > Ni > Cu > Pb > Cr > Mn > Cd.
Average concentrations of Mn, Ni, Cr and Cd were found to be significantly higher (p < 0.05) in the nails of patients compared with those in the controls, while mean concentrations of Zn, Fe and Cu were somewhat lower in the nails of the patients than those in the controls, but the differences were not statistically significant. The proportional variations of the elemental concentrations in the nails of the patients and controls indicated imbalances of the elements which may be linked with the onset and/or progress of the cancer.
Correlation Study
Spearman correlation coefficients between trace elements in the blood, scalp hair and nails of the patients and controls are shown in Table 4, wherein significant r values are shown in bold at p < 0.001. In case of the blood of the patients, significant positive correlations were observed between Cd-Mn (r = 0.535), Cd-Cr (r = 0.503), Pb-Cr (r = 0.421), Zn-Fe (r = 0.411) and Cr-Ni (r = 0.379). All other pairs exhibited insignificant relationships, which manifested their independent variations in the blood of patients. The counterpart data for the controls (Table 3) showed a significant relationship between Cd-Cu (r = 0.377). A few negative correlations were also observed, but they were not significant. Thus, the correlation study revealed significantly dissimilar pattern of mutual dependence of the elements in the blood of patients and controls.
In case of the scalp hair of the patients, significant positive correlations were observed between Cr-Ni (r = 0.487), Cu-Mn (r = 0.420), Cd-Cu (r = 0.403) and Cd-Pb (r = 0.400) as shown in Table 4. The correlation study revealed mutual associations among Cd, Cr, Cu and Pb manifesting their common origin in the scalp hair of patients. On contrary, no significant correlation was observed among the elements in the scalp hair of controls (Table 4).
In case of nail samples of the patients, only significant positive correlation was found between Cu-Zn (r = 0.419), while the rest of the pairs exhibited independent behaviour (Table 4). Likewise, trace elements in the nails of controls mostly revealed an independent pattern as manifested by insignificant correlations except Cd-Ni (r = 0.427). A few negative correlations were also observed, but they were not significant.
Multivariate Analysis
Another important aspect of the present study was the apportionment of trace elements using multivariate cluster analysis [6, 10, 20]. The dendrogram of the elements based on Ward’s method for blood samples of the patients and controls is shown in Fig. 1a. Four major clusters of the elements were found in the blood of the patients: the first two clusters were composed of Fe-Mn and Zn-Pb, while Ni-Cr formed the third cluster and Cu-Cd constituted the last cluster. The corresponding dendrogram of the elements in the blood of controls showed two major clusters which were considerably different than those in the previous cases: the first cluster was composed of Fe-Zn-Cu, and the second cluster consisted of Cd-Cr-Mn-Pb-Ni. The former group of the elements were mainly regulated by internal body metabolism and linked with nutritional sources, while the latter group was mostly contributed by external environmental conditions, especially the anthropogenic contributions.
The findings of CA for the scalp hair of the subjects are displayed in Fig. 1b. In case of the scalp hair of the patients, two major clusters were identified: Pb-Cd-Fe-Mn-Cu and Cr-Ni-Zn. On the contrary, the CA revealed fairly dissimilar clustering of the elements in the scalp hair of controls, which exhibited four diverse clusters: Fe-Ni, Zn-Cu, Cd-Mn and Cr-Pb. Thus, CA exhibited significantly different apportionment of trace elements in the scalp hair of the patients and controls.
The CA of the elemental data pertaining to the nails of patients and controls is shown in Fig. 1c. The elemental concentrations in the nails of the patients revealed very strong clusters of Cr-Pb-Mn, Cu-Ni-Fe and Cd-Zn. In case of the controls, two major clusters of the elements were observed: Cd-Ni-Mn-Cr-Fe and Zn-Cu-Pb. The CA thus showed significant disparities in the elemental concentrations in the nails of the patients in comparison with the controls. Multivariate cluster analysis evidenced notable disparities in elemental apportionment in the blood, scalp hair and nails of the patients and controls. In case of the patients, mostly the toxic elements shared the clusters with essential elements, thereby indicating the interferences which may result in physiological disorder. These differences may be attributed to the elemental imbalances in the patients compared with the healthy subjects. Based on these deliberations, it can be assumed that CA may be used as a diagnostic tool in clinical studies although it required further support and justification by detailed studies comprising larger population segments.
Comparison Among Various Types of Prostate Cancer
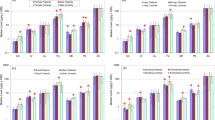
Comparative evaluation of mean elemental concentrations in the blood of various types of prostate cancer patients (i.e. adenocarcinoma, squamous cell carcinoma, transitional cell carcinoma and small cell carcinoma) is shown in Fig. 2. In case of small cell carcinoma patients, Zn, Cu, Mn and Cd exhibited comparatively higher concentrations in the blood, while mean concentrations of Fe and Ni were appreciably higher in the blood of transitional cell carcinoma patients. However, Pb and Cr showed relatively higher concentrations in the blood of squamous cell carcinoma patients. Average concentrations of Cr were not appreciably different in the blood of transitional cell carcinoma and small cell carcinoma patients.
Average concentrations of trace elements in the scalp hair of various types of the patients are shown in Fig. 2. In case of adenocarcinoma, Fe exhibited elevated concentration, while mean concentration of Mn was appreciably higher in the scalp hair of squamous cell carcinoma patients. However, Zn, Cu, Pb and Cd showed higher concentrations in the scalp hair of transitional cell carcinoma patients and Cr exhibited the highest concentration in the scalp hair of small cell carcinoma patients. Average concentrations of Mn, Cd and Pb were not appreciably different in the scalp hair of adenocarcinoma and squamous cell carcinoma patients.
Mean concentrations of trace elements in the nails of various types of the patients are also displayed in Fig. 2. The highest Mn concentration was found in the nails of adenocarcinoma patients, while mean concentrations of Fe, Zn, Cr and Ni were relatively higher in the nails of squamous cell carcinoma patients. Furthermore, Cu and Pb concentrations were markedly higher in transitional cell carcinoma patients. Conversely, some of the elements (Zn, Pb, Mn and Ni) exhibited the lowest concentrations in the nails of small cell carcinoma patients.
Comparison Based on Stages of Prostate Cancer
Mean concentrations of trace elements in the blood, scalp hair and nails of the patients at different stages of the cancer are shown in Fig. 3, for comparative evaluation. Mean blood concentrations of Pb, Cr, Mn and Cu were considerably higher at stage IV, while average concentration of Ni was relatively higher at stage III. However, mean concentration of Fe was considerably higher at stage II, and Zn was found elevated at stage I. Average concentrations of Pb, Cd and Zn followed the increasing concentration order: stage III > stage II > stage I > stage IV. Mean concentrations of Cu, Mn and Ni were comparable at stage I and stage II, whereas Fe, Zn and Cr concentrations were almost equivalent at stage I and stage IV. Some of the elements (Pb, Cd, Cu, Zn and Fe) exhibited the lowest blood concentrations at stage III.
Average concentrations of Fe, Mn, Pb and Cd in the scalp hair were relatively higher at stage IV, while mean concentrations of Ni and Cr were relatively higher at stage III and those of Zn and Cu were relatively higher at stage I. Among the elements, Cu, Mn and Ni concentrations were not significantly different at stage I and stage II. The average concentrations of Pb, Cd, Cr and Zn exhibited almost a similar trend for different stages of the cancer.
In case of nail samples, mean concentrations of Cu and Mn were comparatively higher at stage I, while elevated concentrations of Ni, Cr and Cd were observed at stage II. Likewise, elevated concentrations of Zn and Pb were found at stage IV; however, the average concentrations of Fe and Cd were more or less similar at stages I and IV. Mean concentrations of Pb in the nails were nearly comparable at stage I and stage III (Fig. 3).
Oxidative stress has been associated with prostate cancer development and progression due to an increase of reactive oxygen species (ROS). However, the precise mechanism whereby ROS participate in the cancer progression is poorly understood. ROS are derived from endogenous and exogenous sources [21]. Increased ROS generation has been associated with tissue injury or DNA damage which are general manifestations of pathological conditions associated with infection, mitochondrial DNA mutations and cellular proliferation [22]. However, the actual effects of oxidative stress may depend on the cellular genetic background, the types of ROS involved, and the extent and time of interference [23]. Various elements (such as, Cr, Ni, Pb, Cd) are reported as catalysts in the oxidative damage of biological systems, and therefore, the toxicity associated with these elements may be due to their ability to generate the free radicals [24, 25]. It is also well established that free radicals are known to react with all components of DNA, thus damaging its bases and the deoxyribose backbone causing mutations in crucial genes, which ultimately may lead to cancer [26, 27]. Exposure to high concentrations of Cd has been suggested to increase prostate cancer risk factor due to its ability to simulate the growth of human prostate epithelial cells even at low concentration and to induce their malignant transformation [28]. It may induce free radical toxicity as a result of reaction with thiols or enzymes, which normally protect against the reactive species. The results of present study were consistent with the other reports in which Cd concentrations in the blood, scalp hair and nails of prostate cancer patients were higher than those of healthy donors [14, 16, 24, 29]. West et al. [30] reported a statistically significant positive association with ingested Cd and prostate cancer risk. Armstrong and Kazantzis [31] and Platz et al. [32] analyzed data from two separate US cohort studies and reported no association between high Cd exposure and prostate cancer risk. Kazantzis et al. [33] found no association between occupational exposure to Cd and prostate cancer mortality in a British cohort. In contrast, Sorahan and Watherhouse [34] observed a statistically significant increased risk of prostate cancer mortality among Ni-Cd alloy workers in the UK. Similarly, Vinceti et al. [35] also reported an elevated Cd concentration in the nails of prostate cancer patients in comparison with the healthy subjects.
Epidemiological studies showed that exposure to Ni and its compounds produced a high risk of various types of cancer [24, 36–38]. Sorahan and Watherhouse [34] reported a statistically significant increased risk of prostate cancer mortality with relatively high occupational Ni exposure. Average concentrations of Ni and other elements (Cu and Fe) in the blood of prostate cancer patients were reported to be higher than those of controls [39]. Concentration of Cr was also reported to be significantly higher in prostate cancer patients than that in normal subjects [40]. In a previous study, the blood, hair and nail Pb concentrations were reported to be significantly higher in prostate cancer patients than those in controls [41]. Generally, Mn is not considered carcinogenic to humans [42]; however, Prasad et al. [43] reported that Mn toxicity damages the brain and may have caused prostate cancer. Kwiatek et al. [44] reported an increase of Mn, Ni and Co concentrations in prostate cancer.
Zinc plays a key role in maintaining antioxidant defences and DNA repair systems, failure to which may result in human carcinogenesis [45]. It has been known that Zn concentration in the prostate gland is much higher than that in any other organ, which suggested that Zn may play a role in prostate function and health. Zinc is well known to induce production of metallothionein, which is very rich in cysteine, and this is an excellent scavenger of hydroxyl radical. Epidemiological studies provided contradictory findings on the effectiveness of Zn in prevention against prostate cancer; there were studies that showed that Zn reduces the risk of developing prostate cancer [46], while others showed that there were neither beneficiary nor potential adverse effects of Zn on prostate cancer risk [47]. However, many studies showed that Zn deficiency was considered as possible risk factor for prostate cancer [48]. It is notable that the hair Zn concentration between patients with malignancy was also significantly lower than that in normal patients [1, 4]. In the present study, Zn concentrations in the blood, scalp hair and nails were considerably lower in prostate cancer patients than those in healthy donors. It is important to note that oxidative stress markers are prognostically important in prostate cancer, but these biomarkers are not analyzed in the present study. Although accumulation of some elements has been clearly demonstrated in the cancer patients, it is still unclear whether this is a consequence of the disease or a need for its development. It was also observed in the present study that socioeconomic factors also play a role in higher mortality rates in patients, such as poor nutrition, irregular screening, late diagnosis and unequal access to health care. The cost factor of cancer treatment is also very high. The local hygiene centre facilities are poor in Pakistan, and there are no any routine monitoring and screening carried out for those people living in small towns. Trace element analysis of the cancer patients could become a powerful diagnostic tool. Considering risk assessment of metal toxicology, future research should be focused on the relevance of respective mechanisms in experimental animals and exposed humans, especially with respect to effective metal concentrations. Further study having a large number of subjects is warranted to more clearly define the risks associated with specific exposures with different histological types of prostate cancer. A better understanding of the molecular basis for the development and progression of prostate cancer is needed. The fact that the risk factors of prostate cancer are uncertain provides additional setbacks and halts the search for preventative measures that could benefit for potential patients. Although, further studies are required in this area, these reports, nevertheless, raised concern for potential detrimental outcomes of long-term exposure of trace elements in human.
Conclusions
The concentration of trace elements in the blood, scalp hair and nails of the cancer patients in comparison with that in the controls were considerably divergent. In the blood and scalp hair of prostate cancer patients, Pb, Cd, Ni, Mn and Fe were significantly higher compared to those of the controls, but the concentration of Zn was considerably lower in the patients than that in the controls. The average concentrations of Fe, Zn and Cu were appreciably higher in the scalp hair and nails of controls; nonetheless, some of the elements (Cd, Ni and Mn) were significantly higher in the nails of patients than those of controls, thus indicating the imbalance of these elements in the patients. Likewise, the average concentration of Cr was not significantly different in the blood, scalp hair and nails of patients and controls. Trace elements also exhibited significant disparities in the blood, scalp hair and nails of the patients and controls based on abode, food habits, smoking habits, cancer types and stages. The correlation study revealed appreciably different mutual variations of the elements in the blood, scalp hair and nails of the two groups. CA revealed different apportionment mechanisms of trace elements in the blood, scalp hair and nails of the cancer patients and controls which evidenced that the carcinogenic processes were significantly affecting the trace element balance in humans.
References
Tan C, Chen H (2011) Screening of prostate cancer by analyzing trace elements in hair and chemometrics. Biol Trace Elem Res 144:97–108
Zaichick V, Nosenko S, Moskvina I (2012) The effect of age on 12 chemical element contents in the intact prostate of adult men investigated by inductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res 147:49–58
Bracarda S, de Cobelli O, Greco C, Prayer-Galetti T, Valdagni R, Gatta G, de Braud F, Bartsch G (2005) Cancer of the prostate. Crit Rev Oncol Hematol 56:379–396
Guo JK, Deng W, Zhang L, Li C, Wu P, Mao P (2007) Prediction of prostate cancer using hair trace element concentration and support vector machine method. Biol Trace Elem Res 116:257–271
Zaichick S, Zaichick V, Nosenko S, Moskvina I (2012) Mass fractions of 52 trace elements and zinc/trace element content ratios in intact human prostates investigated by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 149:171–183
Pasha Q, Malik SA, Shaheen N, Shah MH (2010) Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comparison with controls. Clin Chim Acta 411:531–539
Rodrigues JL, Batista BL, Nunes JA, Passos CJS, Barbosa F Jr (2008) Evaluation of the use of human hair for biomonitoring the deficiency of essential and exposure to toxic elements. Sci Total Environ 405:370–376
Schiffer E (2007) Biomarkers for prostate cancer. World J Urol 25:557–562
Polkowska Z, Kozlowska K, Namiesnik J, Przyjazny A (2004) Biological fluids as a source of information on the exposure of man to environmental agents. Crit Rev Anal Chem 34:105–119
Przybylowicz A, Chesy P, Herman M, Parczewski A, Walas S, Piekoszewski W (2012) Examination of distribution of trace elements in hair, fingernails and toenails as alternative biological materials. Application of chemometric methods. Cent Eur J Chem 10:1590–1599
Rodushkin I, Axelsson MD (2000) Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part II. A study of the inhabitants of northern Sweden. Sci Total Environ 262:21–36
Guzel S, Kiziler L, Aydemir B, Alici B, Ataus S, Aksu A, Durak H (2012) Association of Pb, Cd, and Se concentrations and oxidative damage-related markers in different grades of prostate carcinoma. Biol Trace Elem Res 145:23–32
Gray MA, Centeno JA, Slaney DP, Ejnik JW, Todorov T, Nacey JN (2005) Environmental exposure to trace elements and prostate cancer in three New Zealand ethnic groups. Int J Environ Res Public Health 2:374–384
Silvera SAN, Rohan TE (2007) Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control 18:7–27
Yaman M, Atici D, Bakırdere S, Akdeniz I (2005) Comparison of trace metal concentrations in malign and benign human prostate. J Med Chem 48:630–634
Wu CC, Pu YS, Wu HC, Yang CY, Chen YC (2011) Reversed association between levels of prostate specific antigen and levels of blood cadmium and urinary cadmium. Chemosphere 83:1188–1191
Were FH, Njue W, Murungi J, Wanjau R (2008) Use of human nails as bio-indicators of heavy metals environmental exposure among school age children in Kenya. Sci Total Environ 393:376–384
Moses MF, Prabakaran JJ (2011) Evaluation of occupational exposure to toxic metals using fingernails as biological indicators. Res J Environ Toxicol 5:65–70
StatSoft (1999) STATISTICA for Windows, computer program manual. StatSoft, Tulsa
Jobson JD (1991) Applied multivariate data analysis. Springer, New York
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Khandrika L, Kumar B, Koul S, Maroni P, Koul HK (2009) Role of oxidative stress in prostate cancer. Cancer Lett 282(2):125–136
Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK (2008) Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68:1777–1785
Mulware SJ (2013) Trace elements and carcinogenicity: a subject in review. 3. Biotech 3:85–96
Henkler F, Brinkmann J, Luch A (2010) The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers 2:376–396
Rahman K (2007) Studies on free radicals, antioxidants and co-factors. Clin Interv Aging 2:219–236
Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H (2002) Free-radical-induced damage to DNA: mechanisms and measurement. Free Rad Bio Med 32:1102–1115
Brys M, Nawrocka AD, Miekod E, Zydek C, Foksinski M, Barecki A, Krajewska WM (1997) Zinc and cadmium analysis in human prostate neoplasms. Biol Trace Elem Res 59:145–152
Chen YC, Pu YS, Wu HC, Wu TT, Lai MK, Yang CY, Sung FC (2009) Cadmium burden and the risk and phenotype of prostate cancer. BMC Cancer 9:429
West DW, Slattery ML, Robison LM, French TK, Mahoney AW (1991) Adult dietary intake and prostate cancer risk in Utah: a case–control study with special emphasis on aggressive tumors. Cancer Causes Control 2:85–94
Armstrong BG, Kazantzis G (1985) Prostatic cancer and chronic respiratory and renal disease in British cadmium workers: a case–control study. Br J Ind Med 42:540–545
Platz EA, Helzlsouer KJ, Hoffman SC, Morris JS, Baskett CK, Comstock GW (2002) Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. Prostate 52:288–296
Kazantzis G, Lam TH, Sullivan KR (1988) Mortality of cadmium-exposed workers. Scand J Work Environ Health 14:220–223
Sorahan T, Watherhouse JAH (1983) Mortality study of nickel–cadmium battery workers by the method of regression models in life tables. Br J Ind Med 40:293–300
Vinceti M, Venturelli M, Sighinolfi C, Trerotoli P, Bonvicini F, Ferrari A, Bianchi G, Serio G, Bergomi M, Vivoli G (2007) Case–control study of toenail cadmium and prostate cancer risk in Italy. Sci Total Environ 373:77–81
Salnikow K, Zhitkovich A (2008) Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol 21:28–44
IARC (1990) Chromium, nickel and welding. In: IARC monographs on the evaluation of carcinogenic risks to humans, nickel and nickel compounds, vol 49. World Health Organization – International Agency for Research on Cancer, Lyon
Boffetta P (1993) Carcinogenicity of trace elements with reference to evaluations made by the International Agency Research on cancer. Scand J Work Environ Health 19:67–70
Ozmen H, Erulas FA, Karatas F, Cukurovali A, Yalcin O (2006) Comparison of the concentration of trace metals (Ni, Zn, Co, Cu and Se), Fe, vitamins A, C and E, and lipid peroxidation in patients with prostate cancer. Clin Chem Lab Med 44:175–179
Guntupalli JN, Padala S, Gummuluri AV, Muktineni PK, Byreddy SR, Sreerama L, Kedarisetti PC, Angalakuduru DP, Satti BR, Venkatathri V, Pullela VB, Gavarasana S (2007) Trace elemental analysis of normal, benign hypertrophic and cancerous tissues of the prostate gland using the particle-induced X-ray emission technique. Eur J Cancer Prev 16:108–115
Siddiqui MKJ, Srivastava S, Mehrotra PK (2002) Environmental exposure to lead as a risk for prostate cancer. Biomed Environ Sci 15:298–305
Pearson GF, Greenway GM (2005) Recent developments in manganese speciation. Trends Anal Chem 24:803–809
Prasad AS, Sandstead HH, Frederickson C (2010) Vitamin and mineral use and risk of prostate cancer: misleading? Letter to the Editor. Cancer Causes Control 21:1743–1744
Kwiatek WM, Banas A, Gajda M, Galka M, Pawlicki B, Falkenberg G, Cichocki T (2005) Cancerous tissues analyzed by SRIXE. J Alloys Compd 401:173–177
Lin Y, Caffrey JL, Lin J, Bayliss D, Faramawi MF, Bateson TF, Sonawane B (2013) Increased risk of cancer mortality associated with cadmium exposures in older americans with low zinc intake. J Toxicol Environ Health Part A 76:1–15
Epstein MM, Kasperzyk JL, Andren O, Giovannucci EL, Wolk A, Hakansson N, Andersson SO, Johansson JE, Fall K, Mucci LA (2011) Dietary zinc and prostate cancer survival in a swedish cohort. Am J Clin Nutr 93:586–593
Andersson SO, Wolk A, Bergstrom R, Giovannucci E, Lindgren C, Baron J, Adami HO (1996) Energy, nutrient intake and prostate cancer risk: a population-based case–control study in Sweden. Int J Cancer 68:716–722
Zaichick V, Sviridova TV, Zaichick SV (1997) Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol 29:565–574
Acknowledgments
The funding by Higher Education Commission, Government of Pakistan, to carry out this project is thankfully acknowledged. We are also grateful to the administration of Nuclear Oncology & Radiotherapy Institute (NORI), Islamabad, Pakistan, for their invaluable help during sample collection. Technical and financial help by Quaid-i-Azam University, Islamabad, Pakistan, to execute this project is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qayyum, M.A., Shah, M.H. Comparative Study of Trace Elements in Blood, Scalp Hair and Nails of Prostate Cancer Patients in Relation to Healthy Donors. Biol Trace Elem Res 162, 46–57 (2014). https://doi.org/10.1007/s12011-014-0123-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0123-4