Abstract
Great changes have taken place in the incidence of thyroid diseases since the implementation of universal salt iodization (USI). However, the high incidence of thyroid diseases caused by the high iodine intake has been contentious. The aim of this study was to investigate the relationship between iodine intake and thyroid diseases through the comparison of urine iodine concentration (UIC) between patients with thyroid diseases and healthy volunteers and to assess the status of iodine intake among the residents. From November 2013 to May 2014, 905 patients who underwent thyroid surgeries and 272 subjects of healthy controls were enrolled and were divided into two groups: the case group and the control group, respectively. Levels of thyroid hormones and thyroid autoantibodies in serum from blood were analyzed among all the patients. UIC and thyroid B ultrasounds were performed on each participant. The median urinary iodine (MUI) concentration was 184.5 and 169.6 μg/L for case group and control group, respectively (P = 0.003). Significant differences of the MUI were found between healthy controls and patients with Hashimoto’s thyroiditis (MUI = 221.3 μg/L), nodular goiter (MUI = 193.5 μg/L), multiple nodules (MUI = 185.9 μg/L), nodule diameter ≥1 cm (MUI = 194.4 μg/L), hyperthyroidism (MUI = 258.7 μg/L), thyroid peroxidase antibody (TPOAb) (+), and thyroglobulin antibody (TGAb) (+) (MUI = 196.4 μg/L), and P values were 0.003, 0.000, 0.002, 0.000, 0.000, and 0.001, respectively. The susceptibility of the thyroid diseases among normal people was significantly associated with female sex (odds ratio (OR) = 3.3), older age (OR = 2.1), and high iodine intake (OR = 1.3). In conclusion, high iodine intake was likely to lead to the occurrence of thyroid diseases, such as Hashimoto thyroiditis, nodular goiter, and hyperthyroidism, through a long-term mechanism. USI should continue to be carried out and individual UIC detection was recommended for the disequilibrium of the iodine nutritional status among normal people.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary iodine, a main indicator of iodine nutritional status of organism, refers to the content of iodine in the urine. Iodine excretion is mainly through the kidneys and the digestive tract, the former accounts for about 90 % of the total discharge of iodine. The excretion and intake of iodine are roughly equal on the condition that the metabolism of human body is stable. As a result, the urinary iodine excretion is an ideal marker of recent iodine intake. Urine iodine concentration (UIC) in casual urine specimens is recommended for monitoring individual’s iodine status [1]. The median urinary iodine (MUI) is usually used to evaluate the iodine nutritional status of a population [2]. After the implementation of universal salt iodization (USI) in China in 1995, the MUI among children aged 8–10 increased from 164.8 μg/L in 1995 to 238.6 μg/L in 2011, which was beyond the recommended level (100.0 to 199.9 μg/L), and was more than adequate (200.0 to 299.9 μg/L) according to the World Health Organization (WHO) [1]. Meanwhile, the spectrum of thyroid diseases has undergone a significant change ranging from simple goiter toward toxic nodular goiter, Hashimoto’s thyroiditis, and thyroid cancer accompanied with the increase of iodine intake [3], especially for thyroid cancer with an annual increase of 14.51 % in China [4]. However, the high incidence of thyroid diseases caused by high iodine intake has been controversial [5]. In addition, UIC varied considerably depending on different regions, studies, and research methods. Therefore, the relationship between iodine intake and thyroid diseases deserves further exploration.
Material and Methods
Subjects
In this single-center, cross-sectional study, a total of 905 patients (209 males and 696 females) with thyroid diseases who had undergone surgical operations were recruited from the Department of Breast and Thyroid Surgery from November 1, 2013 to May 31, 2014; meanwhile, 272 healthy controls (127 males and 145 females) from the Physical Examination Center were recruited, all participates were from the Union Hospital, which were divided into two groups: the case group and the control group, respectively. The mean age were 47.5 years old (18 to 80) and 42.0 years old (17 to 81) for the case group and control group, respectively.
We excluded subjects with a previously known medical history of thyroid surgery or radioactive iodine treatment, the use of thyromimetic and/or thyrostatic drugs, and the use of medication interfering with thyroid function or iodine status such as amiodarone, kelp, oral corticosteroids, dopamine agonists, and lithium. Besides, the inclusion criterion of the control group was that the thyroids of these healthy subjects were normal in morphology. In other words, participants of the control group were excluded for the abnormal morphology of the thyroid (uneven texture, nodules, cyst, calcification, abnormal blood flow signal, and goiter) detected by professional ultrasound doctors.
Urine and Blood Collection
All the participants were asked to collect morning urine and forbidden to drink water after 10 pm for thyroid surgery or physical examination. No less than 5 mL of fasting morning urine samples was collected in iodine-free polyethylene containers from all the participants, which were tightly sealed with screw tops, stored in a refrigerator to avoid unpleasant odor, and analyzed within 24 h. For the determination of thyroid function in the case group, 5 mL of venous blood samples was collected into heparinized tubes.
Analysis of Urinary Iodine Concentration and Thyroid Hormones and Antibodies
UIC was determined in all subjects by the use of quantitative detection kit of AR type (Conson Biochemicals Co Ltd, Wuhan, China), which is based on iodine’s role as a catalyst in the reduction of ceric ammonium sulfate in the presence of arsenious acid [6]. The completion of the reaction can be monitored by using an indicator. UIC was then calculated according to the power function of quantitative relationship between the urine iodine content and the reaction time. Two standard curves were constructed with five known iodine reagents (50.0, 100.0, 200.0, 300.0, 400.0 μg/L). The absolute value of correlation coefficient of the curve regression equation should reach 0.995 or above. Moreover, two standard urine samples (145.0 and 245.0 μg/L) were then measured to ensure the reliability of the curve and one optimal curve was chosen. The urine samples collected in batches were then detected one by one. The process of the measurement was completed through a specialized computer instrument after manually adding reagents.
Serum levels of free thyroxine (FT4), free triiodothyronine (FT3), thyrotropin (TSH), thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TGAb) were measured among all the patients with a chemiluminescent immunoassay by using diagnostic kits supplied by Roche Diagnostics GmbH (Mannheim, Germany). The functional sensitivity of the TSH assay was 0.0001 mU/L.
Diagnostic Criterion
The epidemiologic criteria for assessing iodine nutritional status based on the MUI of school-aged children and adults are lower than 100 μg/L for iodine deficiency, 100 to 199 μg/L for adequate iodine nutrition, 200 to 299 μg/L for more than adequate iodine nutrition, and higher than 300 μg/L for excessive iodine nutrition according to the WHO, the United Nations Children’s Fund, and the International Council for Control of Iodine Deficiency Disorders [1].
The reference range for FT4 is 9.00 to 19.10 pmol/L, for FT3 is 2.63 to 5.70 pmol/L, for TSH is 0.35 to 4.94 mIU/L, for TPOAb is 0 to 5.10 IU/mL, and for TGAb is 0 to 4.11 IU/mL. Hyperthyroidism includes clinical hyperthyroidism (TSH < 0.35 mIU/L; FT4 > 19.10 pmol/L, FT3 > 5.70 pmol/L, or both) and subclinical hyperthyroidism (TSH < 0.35 mIU/L, FT3 and FT4 within the normal ranges). Hypothyroidism includes overt hypothyroidism (TSH > 4.94 mIU/L, FT4 < 9.00 pmol/L) and subclinical hypothyroidism (TSH > 4.94 mIU/L, FT4 within the normal range).
Statistical Analysis
SPSS 17.0 statistical software was used for all the statistical analysis. Non-normally distributed continuous variables were expressed as median and quartiles and compared with the Wilcoxon rank-sum test. Normality of data was determined using the one-sample Kolmogorov-Smirnov test and verified using histogram. Logistic regression analysis was used to identify correlates and significant interactions. A two-sided P value <0.05 was considered statistically significant.
Informed Consent
All patients provided written informed consent, and the study was approved by the Ethics Committee of the Union Hospital.
Result
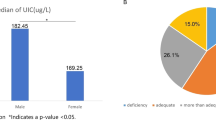
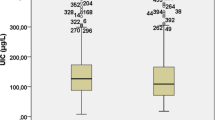
Table 1 shows the demographic characteristics of all the subjects; meanwhile, the results of the statistical analyses are also shown in the same table. The MUI was 169.6 μg/L for the control group and 184.5 μg/L for the case group (P = 0.003). The MUI of females in the control group was lowest with 144.6 ug/L, which was significantly different compared with that of males in the same group (P = 0.000) and that of females in the case group (P = 0.000). It seemed that males had a significantly higher MUI than females in both groups. However, no significant difference of the MUI was found among the males between the two groups. The MUI differed significantly both in the youth group (P = 0.003) and in the middle-aged group (P = 0.037) between the case group and control group, and a higher MUI appeared in the former. Age cohorts were classified as follows: the youth group with age ≤44 years old, middle-aged group with age 45 to 59 years old, and the elderly group with age ≥60 years old. The MUI differed significantly for all the comparisons (among the three groups, P = 0.021; the elderly group vs middle-aged group, P = 0.009; the elderly group vs youth group, P = 0.007; middle-aged group vs youth group, P = 0.744) in the control group.
Significant difference of the MUI was found among the patients with different pathological types in the case group (P = 0.029). However, the MUI of the patients with Hashimoto’s thyroiditis and patients with nodular goiter was higher than that of the control group, and P values were 0.007 and 0.001, respectively (Table 2).
Table 3 shows the comparison of the clinical characteristics of patients with thyroid diseases and healthy controls. The MUI was significantly higher in patients with multiple nodules than that of patients with solitary nodule and the healthy volunteers (P = 0.024 and P = 0.002, respectively). Meanwhile, patients with nodules in the thyroid had a significant higher MUI than the controls. What is more, patients with nodule diameter ≥1 cm had a higher MUI compared with those with nodule diameter <1 cm and healthy controls; statistical significances were found between them (both P values were 0.000). Moreover, the MUI of the patients with multiple nodules and the diameter of these nodules (at least one nodule) ≥1 cm was significantly higher than that of the healthy controls (P = 0.000).
There was a higher MUI in the patients with abnormal thyroid function than that of the healthy controls and P value was 0.000. The same significant results also appeared in patients with normal function and patients with hyperthyroidism when compared with the control group, and P values were 0.026 and 0.000, respectively. Among all the patients, the differences of the MUI for normal function, hyperthyroidism, and hypothyroidism were statistically significant (P = 0.000), and the MUI was significantly higher in patients with hyperthyroidism (MUI = 258.7 μg/L) than that of patients with normal thyroid function through further analysis. Patients who were positive for TGAb and TPOAb had a higher MUI than the healthy controls (P = 0.001).
Table 4 shows the iodine nutritional status of the two groups. The distribution of the iodine nutritional status differed a lot. In the control group, the percentage of iodine deficiency and iodine excess accounted for 23.2 and 12.9 %, respectively.
From the results of the social demographic characteristics of all the patients with thyroid diseases (Table 5), it can be observed that the highest incidence of thyroid disease (33.4 %) appeared within the age range of 41–50 years followed by the age range of 51–60 years (26.3 %), and the least incidence of thyroid abnormality (9.6 %) was observed within the age range of 18–30 years. What is more, the change of the pathological types from thyroid cancer and nodular goiter to nodular goiter indicated that the possibility of malignancy of thyroid diseases decreased with the increasing age.
Table 6 shows the comparisons of thyroid function in different iodine nutritional status. It can be found that excess iodine intake can give rise to the production of TPOAb and TGAb, especially for the latter (P = 0.039). However, no other significant differences were obtained from the analysis of thyroid function.
The bivariate logistic regression analysis showed that female sex (odds ratio, 3.3; 95 % confidence interval, 2.5 to 4.5), advanced age (odds ratio, 2.1; 95 % confidence interval, 1.7 to 2.7), and excessive iodine intake (odds ratio, 1.3; 95 % confidence interval, 1.1 to 1.6) were the risk factors for thyroid diseases in normal subjects (Table 7).
Discussion
To the best of our knowledge, the present study represented the first cross-sectional study of assuring the pathological types of all the cases with thyroid diseases through the observation of postoperative specimens. Moreover, UIC was determined by the use of quantitative detection kit of AR type and the process of measurement was performed through a specialized instrument equipped with a colorimeter, which was a substitute for artificial determination. Therefore, the UIC was more reliable than before. Enormous changes of the iodine nutritional status have taken place in China since the intervention of USI in 1995. However, certain adverse effects have been recognized [7, 8]. Moreover, the present studies on high iodine intake-induced thyroid diseases from domestic researchers were not consistent with each other.
Our study suggests that a correlation exists between iodine intake and thyroid diseases. The MUI of the patients with Hashimoto’s thyroiditis was more than adequate, statistically higher than that of the healthy people. Moreover, a significant difference of the MUI was found between the patients positive for TPOAb and TGAb and the healthy controls, and TGAb can be induced by excess iodine intake (Table 6). This means that excess iodine intake may give rise to Hashimoto’s thyroiditis or the production of thyroid autoantibodies. Similar results were reported in domestic and abroad [8–10]. Hashimoto’s thyroiditis, also known as chronic lymphocytic or autoimmune thyroiditis, autoantibodies to two primary antigens specific to thyroid thyroglobulin (Tg) and thyroperoxidase (TPO) also develop. Iodine-induced thyroid autoimmunity is associated with TGAb and the unmasking of a cryptic epitope of thyroglobulin in humans [11]. Studies on animals showed that excess dietary iodine intake can exacerbate thyroiditis in genetically susceptible hosts (the NOD.H2h4 mouse). Three possible mechanisms are as follows: First, excess iodine intake leads to an increased immunogenicity of the thyroglobulin molecule. Second, reactive oxygen species (ROS) induced by excessive iodine intake can enhance the expression of ICAM-1 which plays a vital role at the early stage in the inflammatory response in thyroidal follicle cells. Last but not least, high level of H2O2 caused by excessive iodine would damage the thyrocyte [12]. These studies suggest that chronic high iodine intake furthers thyroid autoimmunity.
Another significant difference of the MUI was found between patients with nodular goiter and the healthy controls. However, no significant differences were found for the other pathological types of thyroid diseases and the control group. Some studies reported that the dietary iodine intake and UIC of patients with thyroid cancer were significantly higher than that of other patients with thyroid diseases and normal subjects [13]. Our present study found no statistical differences of the MUI between patients with thyroid cancer and normal subjects, which were in agreement with other studies aimed to explore the correlation of the thyroid cancer and iodine intake [14–17]. In the last decade, the incidence of thyroid cancer has soared recently, and its incidence among women and men increased by 7.3 and 6.2 % every year, respectively [18]. Many experts believed that the increased detection rate of microcarcinoma of the thyroid was one of the reasons of the increased incidence of thyroid cancer [19]. Similarly, there was an ongoing epidemic of thyroid cancer in the USA. The epidemiology of the increased incidence, however, suggested that it was not an epidemic of disease but rather an epidemic of diagnosis [14, 20]. In a word, further research should be carried out to explore the relationship between the iodine intake and thyroid cancer in consideration of the high incidence of thyroid cancer and the obscure effect of iodine on thyroid cancer [21].
Thyroid nodules are commonly detected by ultrasonography, 3 to 7 % of the nodules can be found by palpation among population. When examined by ultrasonography, the detection rate is up to 20 to 76 % [22], of which about 5–15 % were malignant nodules, i.e., thyroid cancers [23]. Our present study showed that the MUI was significantly higher in patients with multiple nodules than that of the patients with solitary nodule and the healthy volunteers, what is more, patients with nodule diameter ≥1 cm had a significantly higher MUI compared with nodule diameter <1 cm and healthy controls. However, no significant differences were found between healthy controls and patients with solitary nodule and patients with nodule diameter <1 cm. There appears to be a dose-response pattern, i.e., the higher the UIC, the bigger the size and the more the numbers of thyroid nodules. Even minor differences in iodine intake among populations were associated with remarkable differences in the occurrence of thyroid disorders [24]. It seemed to be that the abnormal form (coarse texture and nodules) of the thyroid can be induced by high iodine intake through a long-term and obscure mechanism. As is known to all, the relationship between the iodine intake of a population and the occurrence of thyroid disorders is U-shaped with an increase in risk from both low and high iodine intakes. Iodine deficiency tigers endemic goiter, cretinism, and hyperthyroidism, while iodine excess can result in high iodine goiter, chronic lymphocytic thyroiditis, iodine-induced hyperthyroidism, and hypothyroidism. Both iodine deficiency and iodine excess can injure the thyroid gland. Here, we found that patients with nodular goiter had a higher iodine intake than the controls after the USI, which was in accordance with the U-shaped curve. The classic example of a high prevalence of disease caused by a high iodine intake is the endemic coast goiter in Hokkaido, Japan [25].
We found statistical difference of the MUI between the patients with normal thyroid function and healthy subjects. This implied that excess iodine intake might affect the morphology of the thyroid (thyroid goiter or thyroid nodules). Besides, the MUI of the patients with abnormal thyroid function was significantly higher than that of the healthy controls, which indicated that more than adequate iodine intake was associated with the malfunction of the thyroid, especially for hyperthyroidism [26]. However, no statistical difference of the MUI was found between patients with hypothyroidism and healthy controls. This may because of the differences of the study regions, phases of diseases, sample sizes, etc. In short, further analysis should be done for the deficient data of thyroid function in the control group.
The MUI of the females in the control group was significantly lower compared with that of the females in the case group. However, no significant difference was found among males between the two groups. This indicated a close relationship between iodine intake of females and thyroid disorders. The MUI and the distribution of iodine nutritional status among normal subjects were in agreement with other reports [27, 28]. The demand of iodine would be increased for special physiological status (such as pregnancy and lactation) of females [29]. Nevertheless, about 35.3 % of all the females in the control group were in iodine deficiency level. What is more, water iodine concentration was less than 10.0 ug/L through continuous detection of the drinking water in the studied region, which indicates an iodine deficiency area according to the Determination and classification of the areas of elevated water iodine and the endemic areas of iodine excess goiter (GB/T 19380–2003) revised by the standard committee in China. Water iodine levels in different locations have a great impact on UIC and iodine nutritional status [30]. Moreover, patients with thyroid diseases, who ate non-iodized salt blindly, were not uncommon, and this could affect the consumption of iodized salt of other family members. All in all, to prevent iodine deficiency disorders, consolidating the hard-won achievement of prevention, the USI policy should continue to be carried out. Besides, the supervision of iodized salt market should be strengthened [31]. Individual UIC should be detected when necessary and appropriate suggestions should be provided to the patients with thyroid diseases. Only in this way the iodine intake of populations can be kept within a relatively narrow interval where iodine-induced thyroid disorders were prevented, but not higher.
This study had some limitations. A selection bias was possible as patients were selected mainly for incidental thyroid nodule or mass and sought for surgery therapy. Besides, the data about the actual use of iodized salt by the subjects being studied was not collected, neither were the factors associated with thyroid disorders such as selenium and copper status in the studied region. What is more, the inclusion criteria of the control group were mainly based on B-model ultrasonography and lacked thyroid function and autoantibody detection. Last but not least, the only urine sample collected from the participants was insufficient to reflect the iodine nutritional status and the number of the studied population was limited.
In conclusion, in the present study, a correlation exists between iodine intake and thyroid diseases; high iodine intake can lead to the occurrence of thyroid diseases such as Hashimoto’s thyroiditis, nodular goiter, and hyperthyroidism through a long-term mechanism. Females with high iodine intake had a closer relationship with thyroid diseases than males. Iodine nutritional status is diverse among populations. USI should continue to be implemented, and iodine supplementation should be individualized based on UIC in the crowds. Last but not least, further exploration was recommended for the limitation of the determination of UIC from the only urine sample and the small sample size.
References
WHO/UNICEF/ICCIDD (2001) Assessment of the iodine deficiency disorders and monitoring their elimination. WHO, Geneva
Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB (2011) Ten repeat collections for urinary iodine from spot samples or 24-h samples are needed to reliably estimate individual iodine status in women. J Nutr 141:2049–2054
Zhang JY, Li SM, Leng JL, Chen YJ, Pu J, Li JM, Pang FX, Huang YH, Nong J, Cen YZ, He H, Li R, Wei LN, He HY (2013) Changes of the spectrum on thyroid disease after the ten-year implementation of universal salt iodization in Guangxi Zhuang Autonomous Region. Zhonghua Liu Xing Bing Xue Za Zhi 34:970–974
Liu YQ, Zhang SQ, Chen WQ, Chen LL, Zhang SW, Zhang XD, Zheng RS (2012) Trend of incidence and mortality on thyroid cancer in China during 2003–2007. Zhonghua Liu Xing Bing Xue Za Zhi 33:1044–1048
Camargo R, Knobel M, Medeiros-Neto G (2007) Iodine nutrition: more is better? Arq Bras Endocrinol Metabol 51:639–640
Pino S, Fang SL, Braverman LE (1998) Ammonium persulfate: a new and safe method for measuring urinary iodine by ammonium persulfate oxidation. Exp Clin Endocrinol Diabetes 106(Suppl 3):S22–27
Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C (2006) Effect of iodine intake on thyroid diseases in China. N Engl J Med 354:2783–2793
Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, Bai X, Li Y, Li N, Li Z, Wang S, Xing Q, Xue H, Zhu L, Hou X, Fan C, Teng W (2011) More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 164:943–950
Hashemipour M, Amini M, Aminorroaya A, Dastjerdi MI, Rezvanian H, Kachoei A, Moaddab MH, Mohammadi M, Kelishadi R, Amini Z, Haghighi S, Shojaee-Moradie F (2007) High prevalence of goiter in an iodine replete area: do thyroid auto-antibodies play a role? Asia Pac J Clin Nutr 16:403–410
Dogan M, Acikgoz E, Acikgoz M, Cesur Y, Ariyuca S, Bektas MS (2011) The frequency of Hashimoto thyroiditis in children and the relationship between urinary iodine level and Hashimoto thyroiditis. J Pediatr Endocrinol Metab 24:75–80
Latrofa F, Fiore E, Rago T, Antonangeli L, Montanelli L, Ricci D, Provenzale MA, Scutari M, Frigeri M, Tonacchera M, Vitti P (2013) Iodine contributes to thyroid autoimmunity in humans by unmasking a cryptic epitope on thyroglobulin. J Clin Endocrinol Metab 98:E1768–1774
Burek CL, Rose NR (2008) Autoimmune thyroiditis and ROS. Autoimmun Rev 7:530–537
Kim JY, Kim KR (2000) Dietary iodine intake and urinary iodine excretion in patients with thyroid diseases. Yonsei Med J 41:22–28
Poljak NK, Kontic M, Colovic Z, Jeroncic I, Luksic B, Mulic R (2011) Iodine intake and epidemiological characteristics of thyroid cancer: comparison between inland and littoral Croatia. Acta Clin Croat 50:329–339
Clero E, Doyon F, Chungue V, Rachedi F, Boissin JL, Sebbag J, Shan L, Bost-Bezeaud F, Petitdidier P, Dewailly E, Rubino C, de Vathaire F (2012) Dietary iodine and thyroid cancer risk in French Polynesia: a case-control study. Thyroid 22:422–429
Sehestedt T, Knudsen N, Perrild H, Johansen C (2006) Iodine intake and incidence of thyroid cancer in Denmark. Clin Endocrinol (Oxf) 65:229–233
Zhu W, Liu X, Hu X, Zhou S, Wang Y, Zhang Y (2012) Investigation on the iodine nutritional status and the prevalence of thyroid carcinoma in Zhoushan Archipelago residents. Wei Sheng Yan Jiu 41:79–82
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013:965212
Davies L, Welch HG (2014) Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322
Knobel M, Medeiros-Neto G (2007) Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol 51:701–712
Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, Vitti P, Nodules AAETFoT (2010) American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract 16(Suppl 1):1–43
American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, DS C, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carle A (2010) Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab 24:13–27
Suzuki H, Higuchi T, Sawa K, Ohtaki S, Horiuchi Y (1965) “Endemic coast goiter” in Hokkaido, Japan. Acta Endocrinol (Copenh) 50:161–176
Thomopoulos P (2002) Hyperthyroidism due to excess iodine. Presse Med 31:1664–1669
Mo Z, Lou XM, Zhu WM, Wang XF, Mao GM, Zhou JS, Ding GQ (2013) A cross-sectional study on iodine nutrition in general population from Zhejiang province, China. Zhonghua Liu Xing Bing Xue Za Zhi 34:464–470
Soriguer F, Garcia-Fuentes E, Gutierrez-Repiso C, Rojo-Martinez G, Velasco I, Goday A, Bosch-Comas A, Bordiu E, Calle A, Carmena R, Casamitjana R, Castano L, Castell C, Catala M, Delgado E, Franch J, Gaztambide S, Girbes J, Gomis R, Gutierrez G, Lopez-Alba A, Martinez-Larrad MT, Menendez E, Mora-Peces I, Ortega E, Pascual-Manich G, Serrano-Rios M, Valdes S, Vazquez JA, Vendrell J (2012) Iodine intake in the adult population. Di@bet.es study. Clin Nutr 31:882–888
Amouzegar A, Khazan M, Hedayati M, Azizi F (2014) An assessment of the iodine status and the correlation between iodine nutrition and thyroid function during pregnancy in an iodine sufficient area. Eur J Clin Nutr 68:397–400
Liu P, Liu L, Shen H, Jia Q, Wang J, Zheng H, Ma J, Zhou D, Liu S, Su X (2014) The standard, intervention measures and health risk for high water iodine areas. PLoS One 9:e89608
Xu J, Dong HJ, Lu BL, Li SM, Zheng QS, Zhuang GX (2008) Surveillance on iodized salt in China, in 2006. Zhonghua Liu Xing Bing Xue Za Zhi 29:253–257
Acknowledgments
The authors thank all of the participants in the study and wish to convey our appreciation to all staff involved.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Tian, Y., Liu, Z. et al. Correlation Between Iodine Intake and Thyroid Disorders: A Cross-Sectional Study from the South of China. Biol Trace Elem Res 162, 87–94 (2014). https://doi.org/10.1007/s12011-014-0102-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0102-9