Abstract
Steatohepatitis is a common finding in chronic hepatitis C virus (HCV) infection. As in other forms of steatohepatitis, oxidative damage may play an outstanding role. However, there are conflicting results relative to the role of iron on hepatic lipogenesis. Proinflammatory cytokines up-regulate ferritin expression, probably reflecting a defensive mechanism against increased oxidative stress, capable to open haem ring and release reactive iron. On the contrary, some adipokines, such as adiponectin, are associated with low ferritin levels. The aim of this study is to analyse the relationships of the amount of liver steatosis with serum iron, transferrin and ferritin as well as with proinflammatory cytokines, such as tumour necrosis factor (TNF)-α and interleukin (IL)-6, and adiponectin levels. We included 82 HCV infected patients and assessed the amount of liver fat by histomorphometry and its relationships with serum iron, ferritin and transferrin, adiponectin and TNF-α and IL-6. Liver steatosis was observed in 67 patients out of 82; in the remaining 15 patients, no steatosis at all was found. Patients with steatosis showed significantly higher serum ferritin levels than patients without steatosis (Z = 2.14; p = 0.032). When patients were classified in quartiles according to the intensity of steatosis, we observed that both TNF-α (KW = 10.6; p = 0.014) and IL-6 (KW = 15.2; p = 0.002) were significantly different among the four groups. Patients with more intense steatosis (highest quartile) showed the highest TNF-α and IL-6 values. Patients with severe hepatitis had higher levels of serum iron than patients with mild to moderate hepatitis. Serum iron also showed a correlation with the proportion of fibrosis (ρ = 0.30; p = 0.007). Serum iron levels are related with biochemical and histological parameters derived from liver inflammation in HCV-associated liver disease. Serum ferritin is higher among those with intense steatosis and also shows a (non-significant) trend to be associated with the more severe forms of hepatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steatohepatitis is a common finding in chronic hepatitis C virus (HCV) infection, in which insulin resistance and/or deficiency leads to an increased mobilisation of fatty acids from the adipose tissue to the liver. In HCV infection, other factors may be involved. HCV genotype 3 may cause liver steatosis directly, and obesity and concomitant alcohol abuse may favour fatty acid deposition in the liver [1]. Cytokine activation and increased lipid peroxidation may trigger the progression of simple steatosis to steatohepatitis [2], although it is possible that oxidative damage may play also a role already in the initial steps of fat accumulation within the hepatocyte [3]. Iron, in its ferrous form, is able to generate free radicals, resulting in oxidative stress and lipid peroxidation, therefore theoretically favouring progression to steatohepatitis and, perhaps, also affecting initial steps of simple steatosis. However, there are conflicting results regarding the role of iron on hepatic lipogenesis [4]. In addition, it was recently shown that hepatic endothelial cells release ferritin in response to increased oxidative stress [5] as a defensive mechanism against the dangerous, highly reactive iron of the haem compound [6]. Proinflammatory cytokines up-regulate ferritin expression [7]. On the contrary, some adipokines, such as adiponectin, are associated with low ferritin levels [8].
The relation of liver steatosis with ferritin and iron levels in HCV patients as well as the potential role of several cytokines on ferritin alterations in HCV patients is incompletely known. Therefore, in this study, we analyse the relationships of the amount of liver steatosis with serum iron, transferrin and ferritin as well as with proinflammatory cytokines, such as tumour necrosis factor (TNF)-α and interleukin (IL)-6, and adiponectin levels, in treatment-naïve patients affected by HCV infection, considering also possible differences between HCV genotypes.
Methods
Patients
We included 82 patients (26 women) affected by HCV, aged 40.45 ± 9.04 years. The two diagnostic criteria for HCV infection were the following: (1) presence of anti-HCV and/or HCV RNA by reverse transcriptase polymerase chain reaction and (2) histology consistent with HCV. Most patients (62) were infected by HCV type 1 genotype; 8, by type 3 genotype; and 11, by type 4, and 1 case showed infection by several genotypes. All these patients were recruited before a treatment for virus C hepatitis was administered. Liver function was still preserved; none of them showed ascites or encephalopathy, and liver function tests were normal. Twenty-eight patients also drank alcohol, median daily amount = 200 g/day (interquartile range = 90–200 g/day) during a median time of 10 years (interquartile range = 6–20 years).
Biochemical Assessment
Blood samples were taken at 8.00 a.m. in fasting conditions and were immediately frozen at −20 °C. The following parameters were determined: TNF-α, by immunometric chemiluminescent assay (intra-assay variation coefficient = 4–6.5 %, inter-assay variation coefficient = 2.6–3.6 %, recovery = 92–112 %; Diagnostic Products Corporation (DPC), Los Angeles, CA, USA); IL-6, by chemiluminescent assay (inter-assay variation coefficient = 5.3–7.5 %, recovery = 85–104 %; DPC, Los Angeles, CA, USA); and serum adiponectin, by ELISA (sensitivity = 26 ng/ml, intra-assay variation coefficient = 3.9–5.9 %, inter-assay variation coefficient = 6.3–7 %, recovery = 92.4–102.9 %; BioVendor, Heidelberg, Germany).
Lipid Peroxidation Products
Serum malondialdehyde (MDA) levels, referred to as thiobarbituric acid-reactive substance (TBARS), were measured according to the method described by Kikugawa et al. [9]. A volume sample of 0.2 ml of plasma was added to 0.2 ml of H3PO4 (0.2 M), and the colour reaction was initiated by the addition of 25 μl of 0.11 M thiobarbituric acid solution. Samples were placed in a 90 °C heating block for 45 min. After the samples were cooled, the TBARS (pink complex colour) were extracted with 0.4 ml of n-butanol. Butanol phase was separated by centrifugation at 6,000×g for 10 min. Aliquots of the n-butanol phase were placed in a 96-well plate and read at 535 nm in a microplate spectrophotometer reader (Benchmark Plus; Bio-Rad, Hercules, CA, USA). The calibration curve was prepared with authentic MDA standards ranging from 0 to 20 μM. The intra- and inter-assay variation coefficients were 1.8 and 4.0, respectively.
In order to get a reference value, cytokines and hormones, as well as malondialdehyde, were also determined to a control group composed of 19 healthy hospital workers (seven women), aged 40.45 ± 3.57 years. As shown in Table 1, not all the variables were determined to all the patients and controls.
In addition, patients underwent a routine laboratory evaluation, including, among others, prothrombin activity, serum albumin and bilirubin, aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT). Serum ferritin (immuno-electrochemiluminiscence, normal range = 15–150 ng/ml), iron (spectrophotometry; normal range = 37–145 μg/dl) and transferrin (immunoturbidimetry; normal range = 200–360 mg/dl) were also determined (Automatic Autoanalyser; Roche, Mannheim, Germany). Reference values for ferritin in our laboratory are 15–150 ng/ml; therefore, values over 150 ng/ml are considered high values.
All these data were recorded on the day at which the patients underwent a liver biopsy before receiving an active treatment against HCV infection.
Histological Assessment
The amounts of liver steatosis and fibrosis were histomorphometrically determined, using a specific software (LEICAQWin, version 3.0; Wetzlar, Germany), at 40×, using samples stained with haematoxylin–eosin and Masson trichrome. Both parameters were measured as a proportion of fat area in relation with the total area and a proportion of fibrous tissue in relation with the total area. Knodell index [10] and Metavir score [11] were also recorded.
The study protocol was approved by the local ethical committee of our hospital and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Statistics
The Kolmogorov–Smirnov test was used to test for normal distribution. Student's t test was used to compare mean values among two different groups (i.e. men and women), and ANOVA test, to assess differences among three groups or more. Spearman's ρ and Pearson's correlation were utilised to compare quantitative variables among each other. Also, χ 2 square test was used to compare qualitative variables with each other. In those cases in which variables showed a non-parametric distribution Mann–Whitney's U test and Kruskal–Wallis test were used instead of Student's t and ANOVA tests. All these analyses were performed with the SPSS programme (Chicago, IL, USA).
Results
Serum ferritin levels were high in 21 out of 82 patients (10 out of 26 women; 11 out of 56 men, χ 2 = 2.39; not significant (NS)). No association was observed between alcoholics and non-alcoholics regarding abnormal ferritin values (χ 2 = 0), although alcoholics showed a non-significant trend to higher ferritin values (Z = 1.26; NS); similarly, no differences were observed in serum transferrin and serum iron among both groups.
Liver steatosis was observed in 67 patients out of 82; in the remaining 15 patients, no steatosis at all was found. Patients with steatosis showed significantly higher serum ferritin levels than patients without steatosis (Z = 2.14; p = 0.032; Table 2). When patients were classified in quartiles according to the intensity of steatosis, we observed that both TNF-α (KW = 10.6; p = 0.014; Fig. 1) and IL-6 (KW = 15.2; p = 0.002) were significantly different among the four groups. Patients with more intense steatosis (highest quartile) showed the highest TNF-α and IL-6 values. On the contrary, serum ferritin levels showed only a non-significant trend (KW = 6.6; p = 0.087) when compared among the four groups. Patients in the highest quartile showed significantly higher TNF-α (Z = 2.95) and IL-6 levels (Z = 3.76; p < 0.01 in both cases) when compared with patients in the lowest quartile.
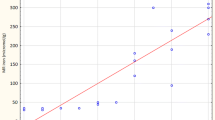
Twenty-seven patients showed features of hepatitis, according to Metavir score, which was severe in 16 of them (Knodell index > 9 and/or Metavir score > 2). The presence of hepatitis was associated to a more intense steatosis (χ 2 = 6.28; p = 0.012). Although no significant differences were observed among patients with simple steatosis or with steatohepatitis (Table 3), patients with severe hepatitis had higher levels of serum iron (133.4 ± 33.0 μg/dl) than those of patients with mild to moderate (102.3 ± 43.9 μg/dl) hepatitis (Fig. 2).
Serum iron also showed a correlation with the proportion of fibrosis (ρ = 0.30; p = 0.007); indeed, those with more intense fibrosis according to the Metavir score showed higher serum iron (128.4 ± 58.5 μg/dl) than those with less fibrosis (102.9 ± 33.9 μg/dl; t = 2.50; p = 0.015), and a similar result was observed when serum iron was compared with histomorphometrically assessed fibrosis (over the median vs below the median, Z = 2.48; p = 0.013). No differences in iron-related parameters were observed when genotype 3-infected patients were compared with those with non-genotype 3 infection, although IL-6 levels were significantly higher among the former (Z = 3.42; p = 0.001), who also showed significantly more liver fat than non-genotype 3-infected patients (Z = 2.60; p = 0.009).
We failed to find any relation among iron-related variables, cytokines and MDA, but serum iron was directly related with ASAT (ρ = 0.32; p = 0.003), ALAT (ρ = 0.40; p < 0.001) and bilirubin (ρ = 0.35; p = 0.001), and ferritin also showed a direct correlation with bilirubin (ρ = 0.32; p = 0.003), ASAT (ρ = 0.37; p < 0.001) and ALAT (ρ = 0.43; p < 0.0001).
Discussion
In this study, we have shown that liver steatosis is a frequent feature among patients with HCV (81.7 %), a figure even higher than the 50 % reported by others [12], although our series include alcoholic patients and HIV-coinfected patients (reflecting the “real world” of HCV infection in our geographical environment). We showed that liver steatosis was associated to higher serum ferritin levels. Currently, serum ferritin may be considered as a defensive resource which is capable to protect the organism from free iron, its synthesis being up-regulated by endothelial cells in response to oxidant-mediated injury [5]. Indeed, oxidative damage may be already present in early stages of liver steatosis. Although no relation was observed between MDA and liver steatosis, the patients included in this study did show higher MDA levels than the control group, suggesting that lipid peroxidation was increased; the presence of higher values also among those without hepatitis reinforces the notion that oxidative damage takes place even in early stages.
Twenty-seven out of the 82 patients also showed hepatitis. Interestingly, in these patients, serum iron was raised, especially in the more severe forms of hepatitis. It is supposed that pathogenesis of steatohepatitis requires the action of proinflammatory cytokines, shaping the so-called “two-hit” theory [13]. In this sense, although we failed to find a relation between cytokines and steatohepatitis, we did find a relation between intense steatosis and raised cytokine levels and a relation between steatohepatitis and intense steatosis. Possibly, raised cytokines associated to intense steatosis predispose to the development of steatohepatitis. There is a clear involvement of cytokine activation in the progressive accumulation of fat within liver cells. There are theoretical mechanisms which support the findings of the present work: TNF-α decreases glucose uptake by adipocytes and hepatocytes, leading to hyperglycaemia and hyperinsulinaemia. Hyperinsulinaemia decreases synthesis of apoB-100, thus preventing VLDL formation and leading to liver steatosis. Moreover, transcription of lipoprotein lipase is decreased by TNF-α, leading to hypertriglyceridaemia [14].
Ferritin is not able to separate diverse forms of non-alcoholic fatty liver disease [15], although high values have been reported in all stages of liver disease. Something similar was observed in our study; although a trend was observed for serum ferritin, only liver iron was significantly higher among those with marked fibrosis. Although, as commented, iron may exert oxidative damage, it is also involved in collagen synthesis, as a cofactor of prolyl hydroxylase [16].
Iron, in its ferrous form, is able to generate free radicals which, in turn, may stimulate stellate cells and induce its transformation into myofibroblasts, major producers of collagen [17]. Our results are therefore consistent with these statements.
In accordance with the possible effect of iron as a trigger of an inflammatory reaction, we found a direct relationship between iron parameters and aminotransferases' levels and bilirubin. Elevation of these parameters may be interpreted as indicators of hepatitis. Therefore, iron correlates not only with histological changes suggestive of hepatitis but also with biochemical ones.
In conclusion, we have shown that serum iron levels are related with biochemical and histological parameters derived from liver inflammation in HCV-associated liver disease. Serum ferritin is higher among those with intense steatosis and also shows a (non-significant) trend to be associated with the more severe forms of hepatitis, a finding which could be interpreted as reactive or defensive against the increased serum iron levels observed in these (more advanced) stages of the disease.
References
Woreta TA, Sutcliffe CG, Mehta SH, Brown TT, Higgins Y, Thomas DL, Torbenson MS, Moore RD, Sulkowski MS (2011) Incidence and risk factors for steatosis in adults coinfected with HIV and hepatitis C virus. Gastroenterol 140:809–817
James OF, Day CP (1998) Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol 29:495–501
García-Heredia A, Kensicki E, Mohney RP, Rull A, Triguero I, Marsillach J, Tormos C, Mackness B, Mackness M, Shih DM, Pedro-Botet J, Joven J, Saez G, Camps J (2013) Paraoxonase-1 deficiency is associated with severe liver steatosis in mice fed a high-fat high- cholesterol diet: a metabolomic approach. J Proteome Res (in press)
Ahmed U, Latham S, Oates PS (2012) Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J Gastroenterol 18:4651–4658
Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G (2007) Heme, heme-oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal 9:2119–2137
Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM (1992) Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem 5:18148–18153
Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, Hopkins PN, Cefalu WT, McClain DA (2012) Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest 122:3529–3540
Torti FM, Torti SV (2002) Regulation of ferritin genes and protein. Blood 99:3505–3516
Kikugawa K, Kojima T, Yamaki S, Kosugi H (1992) Interpretation of the thiobarbituric acid reactivity of rat liver and brain homogenates in the presence of ferric ion and ethylenediaminetetraacetic acid. Anal Biochem 202:249–255
Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J (1981) Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431–435
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–293
Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP (2004) Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterol 126:586–597
Basaranoglu M, Basaranoglu G, Sentürk H (2013) From fatty liver to fibrosis: a tale of “second hit”. World J Gastroenterol 19:1158–1165
Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ (2008) Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology 47:2127–2133
Chandok N, Minuk G, Wengiel M, Uhanova J (2012) Serum ferritin levels do not predict the stage of underlying non-alcoholic fatty liver disease. J Gastrointestin Liver Dis 21:53–58
Rojkind M, Dunn MA (1979) Hepatic fibrosis. Gastroenterol 76:849–863
George DK, Goldwurm SW, Mc Donald GA, CowleyLL WNI, Ward PJ, Jazwinska EC, Powell LW (1998) Increased hepatic iron concentration in non-alcoholic steatohepatitis is associated with increased fibrosis. Gastroenterol 114:311–318
Acknowledgments
Authors are indebted to the nurses and staff of the internal medicine unit and section for infectious diseases of the Hospital Universitario de Canarias.
Conflict of Interest
The authors declare that there is no conflict of interest regarding this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Prieto, J., González-Reimers, E., Alemán-Valls, M.R. et al. Iron and Proinflammatory Cytokines in Chronic Hepatitis C Virus Infection. Biol Trace Elem Res 155, 5–10 (2013). https://doi.org/10.1007/s12011-013-9760-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9760-2