Abstract
Most European people have selenium (Se) intake inferior to recommended values that are considered necessary to ensure the beneficial action of antioxidant selenoproteins. People could therefore tend to have recourse to Se-enriched food supplements (FS) aiming to increase their Se body level. On the Belgian market, three main types of Se-rich FS are available: Se-enriched yeast, selenate-based FS, and selenite-based FS. In the present work, in vitro tests imitating gastrointestinal digestion and intestinal absorption were used to determine the bioaccessible and bioavailable fractions of Se present in one specimen of each category of FS. The aim of the study was to verify to which extent the difference in Se speciation could influence the efficiency of FS for enhancing the human Se status. Results indicated that differences exist in both bioaccessibility and bioavailability between the three types of FS, and that these differences could be related, at least partially, to the Se species profile. Overall bioavailability of the three FS was low (maximum 14 % of the original Se content). Among the three samples, the selenate-based FS produced the highest fraction of bioavailable Se, followed by Se-yeast, and finally by the selenite-based FS for which Se was almost not available at all. These results confirm the low availability of inorganic Se but were somewhat unexpected regarding the yeast-based FS since Se-rich yeasts are usually reported to contain an important fraction of available Se.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The metalloid element selenium (Se) was discovered in 1817 and was initially considered as a toxic substance because it had been associated with poisoning of livestock and people in several Se-rich places around the world [1]. Opinions about Se radically changed 140 years later when evidence was provided that Se was actually an essential element [2] required for the synthesis of several antioxidant selenoenzymes in mammals [3]. In the USA, the recommended dietary allowance (RDA) for Se was fixed by the Institute of Medicine at 55 μg day−1 for both adult men and women [4]. This level of intake is supposed to guarantee maximal activity of glutathione peroxidases selenoenzymes [5]. In some European countries, higher intake levels are recommended: in the UK, RDA ranges between 60 and 75 μg day−1 [6] and in Belgium, recommended values are 60 μg day−1 for women and 70 μg day−1 for men [7].
Food is the most important source of human Se intake. Se content of food is determined by the element’s occurrence in local soils through the soil–plant transfer system. Thereby, Se status of a population is tightly correlated to its geographical location. Whereas Se requirements are fully met in most parts of the USA, Se intake in European countries is of higher concern as Se content of soils is considerably lower than in the USA [6]. In France, average Se intake was recently estimated at 53.7 μg day−1 [8] and in Belgium, the mean dietary intake of Se by the adult population was calculated to be 60 μg day−1 (unpublished results). A previous Belgian estimation reported a range of intake between 30 and 60 μg day−1 [7]. All these values are inferior to the RDA and many European countries are in the same situation. In Finland, a global strategy of food Se-enrichment was carried out in 1984 in order to improve Se status of inhabitants. At that moment, application of selenized fertilization to practically all Finnish crops became obligatory. This measure resulted in a significant enhancement of the Se content of food (up to a 20-fold increase in spring wheat). Consequently, the daily average Se intake by Finnish people increased from 38 μg prior crops fortification to 80 μg in 2001, with a concurrent enhancement of Se concentrations in plasma [9, 10].
The Finnish example is however an isolated case of national action against Se deficiency and, in general, people recourse more readily to Se-enriched food supplements (FS) in order to improve their Se status. During the past decade, a variety of Se-enriched FS have emerged on the European market. The large majority of them can be classified in three distinct categories: Se-enriched yeast, selenate-based FS, and selenite-based FS (personal communication from the Belgian Public Health Ministry). The last two are totally inorganic and contain either selenate (Se(VI)) or selenite (Se(IV)) usually as sodium salts surrounded by excipients. Yeast-based FS typically consist of Baker’s yeast (Saccharomyces cerevisiae) appropriately cultured in the presence of an inorganic source of Se (usually Se(IV)) that yeast can accumulate and metabolize into organic Se species [11], mostly selenomethionine (SeMet) nonspecifically incorporated into peptide chains [12, 13]. Hence, yeast represents a mean to obtain at low cost high quantities of Se under organic forms which have been reported to be less toxic than inorganic Se [14, 15]. The exact composition of yeast-based FS remains, however, uncertain because many metabolic intermediates, known or unknown, are present in variable proportions in addition to SeMet [16]. Recently, up to 49 different selenometabolites could be identified in yeast samples by advanced hyphenated techniques of 2D chromatography coupled to Se-specific detectors ICP-MS and ESI-(multi)MS [17].
The efficiency of orally ingested Se in fulfilling its essential activity of selenoenzyme production is dependent on several previous steps determining Se bioaccessibility and bioavailability, which are in turn influenced by different factors such as the kind of food matrix entrapping Se, the presence of other food components that could interact with the element and the chemical form (speciation) of Se. The bioaccessible fraction of Se can be defined as the part of ingested Se that is released from the food matrix in the lumen of the intestine, and that can thereby possibly be absorbed through the intestinal mucosa [18]. The bioavailable fraction of Se is considered to be the part that is effectively absorbed through the intestinal epithelium and that reaches the systemic bloodstream [19]. The bioavailable fraction is therefore a subfraction of the bioaccessible Se, and from this bioavailable fraction, a subsequent bioactive fraction will finally lead to selenoprotein synthesis [20].
In this study, we focussed on bioaccessibility and bioavailability of Se originating from different FS belonging to one of the three aforementioned categories (i.e., Se-enriched yeast, selenate-based FS, or selenite-based FS). We selected one FS from each category and estimated the bioaccessible and bioavailable fractions of their Se content through in vitro methods. Bioaccessibility was measured through simulated gastrointestinal digestion consisting in a first step of pepsin-mediated digestion at acidic pH, followed by a second intestinal step conducted at neutral pH with pancreatin and bile salts. Bioavailability was estimated through the use of a cellular (Caco-2) model mimicking the intestinal barrier. In this way, this study aimed to evaluate differences in Se efficiency that are suspected to exist between the three categories of most commonly taken Se-enriched FS.
Material and Methods
Samples Collection, Sample Preparation, and Experimental Approach
Food supplements were bought in a local pharmacy and an organic store. The three specimens of FS consisted in Se-enriched yeast (LepiVits, Vincennes, France), in a multimineral and multivitamin complex containing Se as sodium selenate (Bion 3, Merck, Dijon, France) and in a multimineral and multivitamin complex containing Se as sodium selenite (Azinc Optimal, Arkopharma, Wavre, Belgium). These samples are further referred to as yeast-FS, selenate-FS, and selenite-FS with reference to their type. According to the information provided by the producer, total Se concentrations of the samples were: yeast-FS, 167 μg Se g−1; selenate-FS, 30 μg Se g−1; and selenite-FS, 35 μg Se g−1. Yeast-FS and selenate-FS were formulated as pills and were crushed in powder by pillar for homogenization. Selenite-FS was a capsule-containing powder that was emptied. Homogenized samples were stored in the dark in polyethylene pots at room temperature prior to analysis. A schematic presentation of the experimental approach is presented in Fig. 1 and procedures are detailed in the following paragraphs.
Selenium Analysis
For verification of the information supplied by the producer, total Se concentration in the FS samples was measured by ICP-MS (820-MS, Varian) after acidic mineralization (50 % nitric acid (v/v)) in microwave (X-PRESS; CEM Corporation, Matthews, NC, USA). Isotopes 77 and 78 of Se were measured with use of 90 ml min−1 of H2 as reaction gas to remove interferences on these masses. Speciation analysis was realized by coupling HPLC (Pro Star, Varian, Mulgrave, Australia) to ICP-MS. Chromatographic separation of Se species was obtained with a reverse phase C8 Alltima column (250 × 4.6 mm id × 5 μm particle size; Grace Davison Discovery Science, Lokeren, Belgium) and Se was eluted with a mobile phase containing 0.2 % (v/v) heptafluorobutyric acid (HFBA; Alfa Aesar, Karlsruhe, Germany) with a gradient of 50 % (v/v) methanol (HPLC grade; VWR, Leuven, Belgium), i.e. 0–5 min 100 % HFBA, 5–10 min 70 % HFBA-30 % methanol. Total elution was obtained within 10 min at a flow rate of 1 ml min−1. All dilutions were made with milliQ water (Millipore, Overijse, Belgium). Identification of Se species was realized through matching of retention times with individual standards of corresponding Se species and quantification occurred by external calibration with the same standards. Limits of quantification values were 0.12 μg per liter of measured solution for total Se and 0.5 μg l−1 for Se species. These values were calculated respectively as six times the standard deviation of 20 Blanco’s, and as six times the baseline standard deviation.
Selenium Extraction
Proteolytic extraction of Se was achieved in order to identify Se species profile in original FS samples and in Caco-2 cells after in vitro absorption experiments. This extraction was carried out with protease (protease XIV, Sigma-Aldrich, St. Louis, MO, USA) in Tris–HCl buffer 30 mM (Sigma-Aldrich) at pH 7.5–37 °C, with application of ultrasonic probe (Vibra Cell, Bioblock Scientific, Illkirch, France). The process was repeated three times on FS samples in order to extract the maximum of Se. After that, samples were centrifuged and Se species were quantified in the liquid fraction. Details on the procedures applied to both types of samples are provided in Table 1. The integrity of Se species during the extraction process has been verified by conducting the extraction on individual Se species.
Bioaccessibility Test—In Vitro Gastric and Intestinal Digestions
Bioaccessible fraction of Se from FS was considered to be the fraction of Se that was soluble in a digestive juice during the process of an in vitro gastrointestinal digestion:
The digestion was sequenced in two successive steps, referred to as gastric and intestinal digestions (Fig. 1). The salivary digestion step was not addressed in this work owing to the fact that FS are usually directly swallowed. Quantities of sample used for digestion were dependent on the original Se content of FS and were: yeast-FS, 58 mg; selenate-FS, 320 mg; and selenite-FS, 280 mg. Such quantities were intended to permit Se concentration in final digested juices of approximately 200 ng Se ml−1. Samples were weighted in 100 ml polycarbonate Erlenmeyer flasks (Corning; Elscolab, Kruibeke, Belgium) and diluted in 40 ml of Hank’s buffered salt solution (HBSS; Lonza, Verviers, Belgium) at pH 2.0. For the gastric digestion, 2 ml of a solution of porcine pepsin from gastric mucosa (40 g l−1 in HCl 0.1M) were added to the samples. Erlenmeyer flasks were then saturated with nitrogen to create an anaerobic environment and they were placed for 2 h in an agitating (350 rpm) water bath at 37 °C. After the gastric digestion, 1 ml of the digest was taken and reserved for further Se analysis. For the intestinal digestion, pH was elevated up to 5.3 with NaHNO3 1M, and 6 ml of a solution of porcine bile extract mixture and pancreatin from porcine pancreas (both at 3.33 g l−1 in NaHCO3 0.1M; Sigma-Aldrich) were added to the samples. Pancreatin contains several enzymes including amylase, lipase, ribonuclease, trypsin, and protease. The pH was then readjusted up to pH 7.0, Erlenmeyer flasks were saturated with nitrogen, and the intestinal digestion occurred for 2 h in an agitating (350 rpm) water bath at 37 °C. At the end of the digestion, digestive enzymes were inactivated by placing Erlenmeyer flasks for 10 min at 80 °C. Finally, samples were centrifuged for 15 min at 3,000 rpm and the supernatant was kept for Se analysis and further study of bioavailability. The integrity of Se species during the in vitro digestion process has been verified by conducting the digestion on individual Se species.
Bioavailability Test—In Vitro Absorption Experiments
The bioavailable fraction of Se from FS was considered to be the sum of, firstly, the fraction of soluble Se that was transported through a monolayer of Caco-2 cells in a bicameral culture system and, secondly, the fraction that was retained in cells during the process of absorption (Fig. 1):
Caco-2 cells, originating from a human colorectal adenocarcinoma, were obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were stored in liquid nitrogen and aliquots were regularly defrosted for experiments. Caco-2 cells were cultured in Dulbecco’s modified Eagles medium (Lonza) supplemented with 10 % (v/v) heat-inactivated fetal bovine serum (FBS; HyClone; Perbio-Sciences, Erembodegem, Belgium), 1 % (v/v) non-essential amino acids (Lonza) and 1 % (v/v) l-glutamine 200 mM (Lonza). They were maintained at 37 °C in a humidified atmosphere of 5 % CO2 (v/v)–95 % air. Cells were expanded in 175-cm2 flasks and subcultured at 90 % confluence with a trypsin-EDTA solution (Lonza). Each subcultivation incremented by one the number of passage, and cells were used for experiments between passages 47 and 52. After subcultivation, cells were seeded on permeable PE Transwell® cell culture inserts (24 mm diameter, 0.4 μm pore size; Corning; Elscolab, Kruibeke, Belgium) at a density of 4 × 105 cells per insert. Inserts were placed in six-well plates (Corning; Elscolab) in which they delineated an upper (apical) and a lower (basolateral) compartments. Apical and basolateral compartments were filled respectively with 1.5 and 2.5 ml of culture medium that was changed three times a week until full differentiation of cells (21 days postseeding). Because serum contains traces of Se, last medium change before experiment was realized with 0.5 % FBS instead of 10 %. For the transport experiments, solutions of bioaccessible Se obtained from the in vitro digestion were diluted 1:1 (v/v) in HBSS (Lonza) in order to limit cytoxicity induced by bile salts, and they were added to apical compartment while HBSS containing 1 % (w/v) albumin (BSA; Sigma-Aldrich) was added to basolateral chamber. Absorption process lasted 3 h, a probable duration journey of food in the small intestine. After that, basolateral media were collected, as well as cells that were scrapped out, and both were analyzed for their Se content. Potential cytotoxicity of the substances used was estimated through importance of cell lyses, which were determined by assaying the release of intracellular enzyme lactate deshydrogenase (LDH) into the culture medium. Activity of LDH was assayed by cytotoxicity detection kit from Roche Diagnostics (Mannheim, Germany) and compared to appropriate negative (culture medium) and positive (1 % Triton, Sigma) controls. The integrity of the cell monolayer during the 3-h transport was verified by measuring the transepithelial electrical resistance (TEER) prior and after the transport experiment.
Results
Concentration of Se and its Speciation in Original FS
The acid mineralization conducted on FS permitted to recover 100 % of the Se content reported on the package, which confirmed the information provided by the producer. The triple proteolytic extraction released the totality of Se from inorganic Se-FS and 86 % of Se from yeast-FS (Table 2). Regarding yeast, the first extraction released about 90 % of extractable Se and the second extraction released the remaining 10 %. The third extraction did not release significant quantities of Se, which ensured that extraction efficiency was at its maximum.
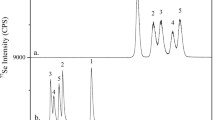
Selenate-FS and selenite-FS contained exclusively those species but Se yeast generated a variety of peaks (Fig. 2). Among them, three were identified as SeMet (the main species), Se(IV), and methylselenocysteine (MeSeCys). The other detected peaks were unknown species, probably selenopeptides that could not be quantified due to the lack of appropriate standards of calibration.
Bioaccessible and Bioavailable Fractions of Total Se from FS
A 1:1 dilution of the final FS digests in HBSS permitted to limit cytotoxicity induced by bile salts to values lower than 10 % of the positive control (LDH release test), thereby guarantying the safety for the cells (results not illustrated). TEER values prior to the start of the experiment ranged from 1,100 to 1,400 Ω cm2, which ensured the integrity of the cell monolayer. A decrease of about 10 % of initial TEER values was systematically observed at the end of the 3-h transport experiment.
Figure 3 shows the bioaccessible and bioavailable fractions of Se as percentages of the original Se content of FS. Neither the in vitro digestion nor the in vitro absorption did modify the original speciation of Se. For the three FS, the totality of Se released during the in vitro digestion was liberated during the gastric step, and no additional Se was released during the intestinal step.
Percentage of original Se from a yeast-FS, b selenate-FS, and c selenite-FS that was released in gastrointestinal juice (i.e., bioaccessible Se), and absorbed through Caco-2 monolayer or accumulated in cells during a 3-h period (i.e., bioavailable Se). Results are given as means ± standard deviations (N = 3, n = 3)
Only 57 % of Se from yeast-FS was liberated through the in vitro gastrointestinal digestion and was thus considered as bioaccessible. During the phase of absorption, Se from yeast-FS accumulated in Caco-2 cells and the bioavailable fraction of Se corresponded therefore to the sum of Se content in basolateral compartment and in cells (6 and 1 %, respectively, of original Se content). Speciation of Se in the different fractions will be addressed in the next section.
In selenate-FS, the totality of Se was bioaccessible. Fourteen percent of this quantity were absorbed through the Caco-2 monolayer and corresponded to the bioavailable fraction of Se. No Se was detected in cells upon selenate-FS presence.
As for selenite-FS, the bioaccessible fraction of Se represented 80 % of the original Se content, while its bioavailable fraction was barely of 1 %. No Se was detected in cells.
Bioaccessible and Bioavailable Fractions of Se Species from Yeast-Se
Figure 4 presents the Se species profiles in bioaccessible and bioavailable fractions of Se in yeast-FS. On the one hand, SeMet is shown to be less accessible (70 % of the original SeMet content) than Se(IV) and MeSeCys for which the totality of the original content was retrieved in the digested solution. On the other hand, SeMet was the only species detected in the basolateral compartment and in cells after in vitro absorption. The bioavailable fraction of SeMet accounted for 24 % of the original SeMet content and of about 35 % of bioaccessible SeMet.
Percentage of original Se species from yeast-FS that were released in gastrointestinal juice (i.e., bioaccessible Se), and absorbed through Caco-2 monolayer or accumulated in cells during a 3-h period (i.e., bioavailable Se). Black bar SeMet dotted bar Se(IV), and hatched bar MeSeCys. Results are given as means ± standard deviations (N = 3, n = 3)
Discussion
Because Se intake of Belgian people is lower than recommended values, and considering that the majority of local food products have low Se content [6], the use of Se-enriched FS is an evident option in order to increase Se dietary intake. In this study, the degrees of Se bioaccessibility and bioavailability from three different kinds of FS were investigated through in vitro methods that imitated the human digestive tract from the entry of the FS in the stomach, to the intestinal absorption and delivery of its Se content in the bloodstream. These in vitro approximations present the advantages to be easier, faster, cheaper and more controllable than if having monitored human subjects.
Bioaccessibility
A common observation for the three investigated FS was that the totality of the bioaccessible Se was liberated at the gastric stage of the in vitro digestion. The gastric phase consists in the first proteolytic attack of the digestion and occurs through the action of pepsin/HCl, while the further intestinal phase, mediated by bile and pancreatin (mixture of proteases, amylase and lipase), permits a second peptidic hydrolysis and the breakdown of other categories of food components [21]. The rapid release of selenite and selenate from the FS matrix was awaited considering the high degree of solubility of inorganic Se species. In yeast-FS, our model seems to indicate that the pepsin-mediated proteolysis would be sufficient to release the maximum of soluble Se present in yeast. This could illustrate the fact that SeMet, i.e., the major species present in yeast, occurs entrapped into proteins surrounded by a relatively simple matrix. In this scheme, Se compounds remaining in the matrix after the in vitro digestion should be insoluble or linked to insoluble fractions of the yeast. Significant amounts of Se have notably been detected in the cell wall constituents of Se-enriched yeasts [22].
The three FS were not equal in terms of Se bioaccessibility. The difference was especially marked between FS containing inorganic Se (selenate or selenite), and Se-rich yeast. The totality of Se contained in selenate-FS was bioaccessible and this value was 80 % in selenite-FS. In contrast, the liberation of Se from yeast-FS was only of 57 %. Different factors could explain the low bioaccessibility of Se from yeast-FS. Firstly, contrary to inorganic-based FS, yeasts constitute a matrix, though being relatively simple, from which Se must be extracted. Secondly, Se-rich yeast contains a variety of unknown species potentially nonbioaccessible (e.g., insoluble or volatile). Thirdly, the major Se species in yeast-FS is SeMet that, unlike other Se-species, is stored for a large part in the proteins of the food matrix. As a result, the majority of SeMet is more fixed into the yeast matrix than other species and is thus more difficult to extract [23]. In that sense, the importance of SeMet content in yeast may be determinant to assess its Se bioaccessibility: the more SeMet is present, the less bioaccessible the total Se content likely is.
In two previous studies where in vitro digestions were performed on yeast for 8 h, the bioaccessible fractions of Se were 89 % [13] and 80 % [12]. Based on these results, the authors concluded that Se from yeast was highly bioaccessible. However, in one of those studies, the liberation of Se was shown to be time dependent, with the release of Se being only 35 % after 2 h and 45 % after 4 h [12]. Those are more realistic durations for digestion processes and do correspond to the values found in the present study. These findings suggest that the degree of Se bioaccessibility from yeast is influenced by the rapidity of its transit in the digestive tract.
Bioavailability
The degree of Se absorption through the Caco-2 monolayer was strongly species-dependent. The bioavailability of Se(IV) was the lowest: Se(IV) present in selenite-FS was hardly absorbed through the Caco-2 monolayer (∼1 % of the original Se(IV) content; Fig. 3), and none of the Se(IV) present in yeast-FS was detected in the absorbed fraction (Fig. 4). In comparison, Se(VI) from selenate-FS was much more available (14 % of the original Se(VI); Fig. 3). Regarding yeast-FS, the presence of SeMet was crucial as it was the only species detected in the absorbed fraction of Se and in cells. The percentage of SeMet that was absorbed from the yeast digest represented 35 % of the bioaccessible SeMet and only 7 % of the total Se content of the yeast-FS. In a previous investigation conducted with the similar Caco-2 absorptive model, we had determined the degrees of bioavailability of pure individual Se species to be Se(IV) (12 ± 1 %) < Se(VI) (33 ± 2 %) < MeSeCys (46 ± 2 %) < SeMet (56 ± 4 %; unpublished results). These values are higher than those observed here for Se species present in the bioaccessible fraction of the yeast-FS. This suggests that the presence of some additional components of the intestinal bolus (digested matrix and digestive enzymes) could impede or slow down the absorption of Se species from FS. It should also be noted that the degree of bioavailability of Se from yeast-FS is likely to vary widely in function of the specimen of Se-rich yeast considered because the Se species composition—and especially the proportion of SeMet—can differ importantly from one specimen to another [11, 16, 23].
In the context of Se diet enrichment, several in vitro and in vivo studies have reported that SeMet was more efficiently absorbed and retained by the organism than other species [24–26] because of its capacity of integration to proteins [27]. Selenium can therefore be progressively released into the organism during the regular turnover of proteins and continuously meet the Se body requirements. In addition, SeMet has been showed to be less toxic than inorganic species [14, 15]. Hence, although our results did not show a very high release of bioavailable Se from the yeast-FS, this kind of dietary supplement seems to represent an interesting source of Se for long-term supplementation. Contrary to SeMet, selenate was not retained in cells and it is expected to be more readily excreted as reflected by the rapid decrease in Se plasma concentrations observed in humans at the end of period of selenate diet supplementation compared to Se-rich yeast supplementation [28]. This makes us suggest that selenate-FS, which here showed the highest bioavailable fraction of Se, would be more appropriate to respond to an acute and occasional need of Se, while Se-yeast would be convenient for a long-term supplementation.
Conclusion
Within the limits of prediction allowed by an in vitro model, our results indicate that Se from the three investigated specimens of Se-enriched FS is in general poorly available (14 % maximum of original Se content was released from the matrix and crossed the Caco-2 monolayer). The major determinant of Se bioaccessibility and bioavailability in food supplements seems to be their speciation, which sounds coherent considering their simple matrix characteristics, especially for FS based on inorganic Se. Among the FS under investigation, selenate-FS produced the highest fraction of bioavailable Se, followed by yeast-FS. Selenite-FS appeared to be of very low utility as only 1 % of its Se content was bioavailable. In yeast-FS, the fraction of SeMet may be crucial as it affects both bioaccessibility and bioavailability, but in opposite ways. Compared to selenite and methylselenocysteine, SeMet was less bioaccessible, but more bioavailable. Only Se from yeast accumulated in cells, suggesting a potential long term beneficial effect of yeast-FS compared to selenate-FS. Certainly, it became clear that different kinds of food supplements will not have the same efficiency in enhancing human Se status.
References
Navarro-Alarcón M, López-Martínez M (2000) Essentiality of selenium in the human body: relationship with different diseases. Sci Total Environ 249:347–371
Schwarz K, Foltz C (1958) Factor-3 activity of selenium compounds. J Biol Chem 233:245–251
Rotruck J, Pope A, Ganther H, Swanson A, Hafeman D, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Institute of Medicine (2000) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. A report of the Panel of Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Uses of Dietary Reference Intakes and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. National Academy Press
Monsen E (2000) Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc 100:637–640
Rayman M (2000) The importance of selenium to human health. Lancet 356:233–241
SHC (Superior Health Concil) (2009) Recommandations nutritionnelles pour la Belgique, n8309
AFSSA (2009) Avis relatif à l’évaluation des teneurs en vitamines et minéraux des denrées enrichies et des compléments alimentaires : synthèse.
Varo P, Alfthan G, Ekholm P, Aro A, Koivistoinen P (1988) Selenium intake and serum selenium in Finland: effects of soil fertilization with selenium. Am J Clin Nutr 48:324–329
Rayman MP (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100:254–268
Suhajda Á, Hegóczki J, Janzsó B, Pais I, Vereczkey G (2000) Preparation of selenium yeasts I. Preparation of selenium-enriched Saccharomyces cerevisiae. Journal of Trace Elements in Medicine and Biology 14:43–47
Yoshida M, Sugihara S, Suenaga T, Naito C, Fukunaga K, Tsuchita H (2002) Digestibility and chemical species of selenium contained in high-selenium yeast. J Nutr Sci Vitaminol 48:401–404
Reyes L, Encinar J, Marchante-Gayón J, Alonso J, Sanz-Medel A (2006) Selenium bioaccessibility assessment in selenized yeast after “in vitro” gastrointestinal digestion using two-dimensional chromatography and mass spectrometry. J Chromatography A 1110:108–116
Ammar E, Couri D (1981) Acute toxicity of sodium selenite and selenomethionine in mice after ICV or IV administration. Neurotoxicology 2:383–386
Tiwary A, Stegelmeier B, Panter K, James L, Hall J (2006) Comparative toxicosis of sodium selenite and selenomethionine in lambs. J Veterinary Diagnostic Invest 18:61–70
Rayman M (2004) The use of high selenium yeast to raise selenium status: how does it measure up? Br J Nutr 92:557–573
Preud’homme H, Far J, Gil-Casal S, Lobinski R (2012) Large-scale identification of selenium metabolites by online size-exclusion-reversed phase liquid chromatography with combined inductively coupled plasma (ICP-MS) and electrospray ionization linear trap-Orbitrap mass spectrometry (ESI-MSn). Metallomics 4:422–432
Ruby M, Schoof R, Brattin W, Post G, Harnois M, Mosby D et al (1999) Advances in evaluating the bioavailability of inorganics in soil for use in human health risk assessment. Environ Sci Technol 33:3697–3705
Schümann K, Classen H, Hages M, Prinz-Langenohl R, Pietrzik K, Biesalki H (1997) Bioavailability of oral vitamins, minerals, and trace elements in perspective. Arzneim-Forsch/Drug Res 47:369–380
Thiry C, Ruttens A, De Temmerman L, Schneider Y-J, Pussemier L (2012) Current knowledge in species-related bioavailability of selenium in food. Food Chem 130:767–784
Jackson A, McLaufhlin J (2009) Digestion and absorption. Surgery (Oxford) 27:231–236
Połatajko A, Sliwka-Kaszynka M, Dernovics M, Ruzik R, Encinar J, Szpunar J (2004) A systematic approach to selenium speciation in selenized yeast. J Anal At Spectrom 19:114–120
Schrauzer G (2006) Selenium yeast: composition, quality and safety. Pure Applied Chemistry 78:105–109
Whanger P, Butler J (1988) Effects of various dietary levels of selenium as selenite or selenomethionine on tissue selenium levels and glutathione peroxidase activity in rats. J Nutr 118:846–852
Wang Y, Han J, Li W, Xu Z (2007) Effect of different selenium source on growth performances, glutathione peroxidase activities, muscle composition and selenium concentration of allogynogenetic crucian carp (Carassius auratus gibelio). Anim Feed Sci Technol 134:243–251
Zeng H, Botnen J, Johnson L (2008) A selenium-deficient Caco-2 cell model for assessing differential incorporation of chemical or food selenium into glutathione peroxidase. Biological Trace Element Research 123:98–108
Schrauzer G (2000) Selenomethionine: a review of its biological significance, metabolism and toxicity. J Nutr 130:1653–1656
Levander O, Alfthan G, Arvilommi H, Gref C, Huttunen J, Kataja M, Koivistoinen P, Pikkarainen J (1983) Bioavailability of selenium to Finnish men as assessed by platelet glutathione peroxidase activity and other blood parameters. Am J Clin Nutr 37:887–897
Acknowledgments
This work was supported by the “Fond Special de Recherche” from the Catholic University of Louvain-la-Neuve (UCL; in vitro experiments) and by the Belgian Federal Science Policy (optimization of the Se speciation method). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiry, C., Schneider, YJ., Pussemier, L. et al. Selenium Bioaccessibility and Bioavailability in Se-Enriched Food Supplements. Biol Trace Elem Res 152, 152–160 (2013). https://doi.org/10.1007/s12011-013-9604-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9604-0