Abstract
The study was conducted to determine the effects of iron glycine chelate (Fe-Gly) on growth, tissue mineral concentrations, fecal mineral excretion, and liver antioxidant enzyme activities in broilers. A total of 360 1-day-old commercial broilers (Ross × Ross) were randomly allotted to six dietary treatments with six replications of ten chicks per replicate. Broilers were fed a control diet with no Fe supplementation, while five other treatments consisted of 40, 80, 120, and 160 mg Fe/kg diets from Fe-Gly, and 160 mg Fe/kg from ferrous sulfate, respectively. After a 42-day feeding trial, the results showed that 120 and 160 mg Fe/kg as Fe-Gly improved the average daily gain (P < 0.05) and average daily feed intake (P < 0.05) of broilers (4–6 weeks). Addition with 120 and 160 mg Fe/kg from Fe-Gly and 160 mg Fe/kg from FeSO4 increased Fe concentration in serum (P < 0.05), liver (P < 0.05), breast muscle (P < 0.05), tibia (P < 0.05), and feces (P < 0.01) at 21 and 42 days. There were linear responses to the addition of Fe-Gly from 0 to 160 mg/kg Fe on Fe concentration in serum (21 days, P = 0.005; 42 days, P = 0.001), liver (P = 0.001), breast muscle (P = 0.001), tibia (P = 0.001), and feces (21 days, P = 0.011; 42 days, P = 0.032). Liver Cu/Zn superoxide dismutase activities of chicks were increased by the addition of 80, 120, and 160 mg Fe/kg as Fe-Gly to diets at 42 days. There were no differences in liver catalase activities of chicks among the treatments (P > 0.05). This study indicates that addition with Fe-Gly could improve growth performance and iron tissue storage and improves the antioxidant status of broiler chickens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) is an essential trace element for broiler growth, which functions in numerous energy metabolism, neurotransmitter synthesis, and phagocyte antimicrobial activity, as well as the synthesis of DNA, collagen, and bile acids [1]. A level of 80 mg Fe/kg dry matter has been recommended in the diet for broiler chickens [2]. Traditionally, Fe is supplemented in the form of inorganic salts, such as sulfates, oxides, and carbonates, to allow the bird to reach its genetic growth potential [3].
In recent decades, studies have shown that chelated or proteinated sources of Fe have higher relative availability compared with inorganic Fe [4–8]. The body weights of newborn and weanling piglets increased significantly when the sows were maintained on feed supplemented with iron proteinate [9, 10]. Yu et al. [11] reported that Fe from amino acid complex increased plasma Fe and total Fe binding capacity in the blood, as well as hemosiderin and ferritin Fe in the liver and spleen of weanling pigs. Feng et al. [7] found that 90 mg iron glycine chelate (Fe-Gly) per kilogram has beneficial effects on growth and hematological and immunological functions of weanling pigs compared with those of ferrous sulfate (FeSO4).
It was demonstrated in studies with rats and humans that iron chelated with glycine was well absorbed and utilized [12–15]. Accordingly, the Fe-Gly is now used as an iron fortificant in human nutrition and especially in infant food [16]. Our previous study revealed that Fe-Gly at an appropriate dosage improves growth performance, hematological and immunological characteristics, iron tissue storage, and antioxidant enzyme activities in weanling pigs [7, 8]. But few studies have been conducted on Fe-Gly in broilers. The main objective of the present research was to investigate the effects of iron glycine chelate on growth performance, tissue mineral concentrations, fecal mineral excretion, and liver antioxidant enzyme activities in broilers.
Materials and Methods
Animals and Experimental Design
Three hundred and sixty 1-day-old commercial broiler chicks (Ross × Ross) were randomly allotted to six dietary treatments, each of which was replicated six times with ten birds per replicate. Dietary treatments were as follows: (1) control (no Fe supplementation), (2) control + 40 mg/kg of Fe as Fe-Gly, (3) control + 80 mg/kg of Fe as Fe-Gly, (4) control + 120 mg/kg of Fe as Fe-Gly, (5) control + 160 mg/kg of Fe as Fe-Gly, and (6) control + 160 mg/kg of Fe from FeSO4 (positive control).
Chicks were reared in an environmentally controlled room and fed a corn–soybean meal-based diet formulated to meet National Research Council [2] nutrient requirement estimates (Table 1) for 42 days. The initial room temperature for chicks was set at approximately 32°C and reduced by 2 to 3°C weekly until reaching 22°C at the 4th week, which was maintained for the remaining 2 weeks. In the starter (0 to 21 days) and grower (21 to 42 days) phases, each pen was offered the respective diet, and all chicks were given ad libitum access to feed and water.
Sample Collection and Analytical Methods
On days 21 and 42 of the feeding trial, all chicks were held without feed for 12 h, and the weight and feed consumption were measured to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed/gain ratio (F/G). Meanwhile, 72 chicks (two chicks per pen) were humanely killed by cervical dislocation at days 21 and 42. Plasma samples were isolated from blood by centrifuging at 3,000×g for 10 min and stored at −70°C until analysis. Liver and left breast muscle samples were removed from the carcasses and immediately stored at −70°C until analysis for mineral concentrations or antioxidant enzyme activities. Fat was removed from the left tibia by a 36-h Soxhlet extraction in ethyl alcohol followed by a 36-h extraction with diethyl ether and then stored at −70°C until analysis. Fecal samples were freeze-dried and frozen at −20°C until mineral analysis. The experiment was conducted in accordance with the Chinese guidelines for animal welfare and approved by the animal welfare committee of Animal Science College, Zhejiang University.
Determination of Mineral Concentrations in Tissues and Feces
The mineral levels in samples (liver, tibia, breast muscle, and feces) were conducted using a method described by Shelton and Southern [17]. Samples were dried at 100°C for 24 h and ashed for 10 h (liver, breast muscle, and feces) or 36 h (tibia) at 550°C. The ashed samples were dissolved in nitric acid–perchloric acid mixture (1:1) and diluted with deionized water for analysis of minerals [18]. Contents of Fe, Cu, Zn, and Mn were measured with flame atomic absorption spectrophotometry (AA-6300, Shimadzu Corp., Tokyo, Japan).
Measurement of Superoxide Dismutase and Catalase Activities
Liver samples were prepared for analysis according to the method of Feng et al. [8]. Liver Cu/Zn superoxide dismutase (SOD) activities were determined by the methods of Shaw et al. [19]. An assay for catalase (CAT) activity was performed by following the reduction in H2O2 absorbance at 240 nm as reported by Venturino et al. [20]. Protein was measured by the method of Lowry et al. [21]. Units of SOD and CAT activities were expressed as the activity of an enzyme per milligram of protein.
Statistical Analysis
Data were analyzed by ANOVA as a randomized complete block design using the GLM procedures of SAS [22]. Individual chicks were the experimental unit for all indices. The planned single degree of freedom tests included the linear and quadratic effects of Fe-Gly, the control versus FeSO4 (160 mg/kg Fe), and FeSO4 versus Fe-Gly (160 mg/kg Fe) treatments. Differences of treatment means were compared using Student’s t test. An alpha level of 0.05 was used for determination of differences among treatments.
Results
Growth Performance
The effects on growth performance are shown in Table 2. From the observed weights at day 21, it may be concluded that the level of Fe in the diet did not significantly influence broiler growth performance. High Fe supplementation (120 and 160 mg Fe/kg as Fe-Gly) improved ADG (P < 0.05) and ADFI (P < 0.05) of broilers over the birds fed basal diet at day 42. However, no effect on F/G was observed among all treatments (P > 0.05) throughout the experimental period.
Tissue Mineral Concentrations
The concentration of serum Fe was increased (P < 0.05) in chicks fed diets supplemented with 120 and 160 mg/kg Fe as Fe-Gly or 160 mg/kg Fe as FeSO4 at day 21 (Table 3). Similar effects in the liver of chicks were also observed (P < 0.05). Moreover, the Fe concentrations in serum (21 days, P = 0.005; 42 days, P = 0.001) and liver (21 and 42 days, P = 0.001) were elevated with the increasing dietary Fe-Gly levels. No significant differences on the contents of Cu, Zn, and Mn in serum and liver could be observed between the treatments.
Table 4 shows the effects of Fe-Gly on mineral contents of breast muscle and tibia in broilers. Compared with the control group, the addition of 120 and 160 mg/kg Fe as Fe-Gly or 160 mg/kg Fe as FeSO4 enhanced the Fe concentration of the breast muscle (P < 0.05). Tibia Fe concentration was improved (P < 0.05) in chicks fed 80, 120, and 160 mg/kg Fe as Fe-Gly or 160 mg/kg Fe as FeSO4. Moreover, there were linear responses to the addition of Fe-Gly from 0 to 160 mg/kg Fe on Fe concentrations in breast muscle (P = 0.001) and tibia (P = 0.001). However, there were no differences in breast muscle and tibia Cu, Zn, and Mn contents when broilers were offered different levels of iron as Fe-Gly and FeSO4 compared with the control (P > 0.05).
Fecal Mineral Excretion
Analyzed values of Fe, Cu, Zn, and Mn for fecal samples are presented in Table 5. Fecal Fe concentration elevated linearly with the increasing dietary Fe-Gly levels (21 days, P = 0.011; 42 days, P = 0.021). Moreover, supplementation of 160 mg/kg Fe-Gly or 160 mg/kg Fe as FeSO4 enhanced (P < 0.05) Fe concentration in feces compared with the control. Mineral contents of Cu, Zn, and Mn in feces of chicks did not differ among all the treatments (P > 0.05).
Liver Antioxidant Enzyme Activities
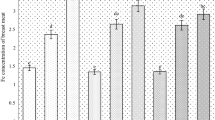
As shown in Fig. 1, SOD activity did not differ among all treatments at 21 days (P > 0.05). However, it was enhanced (P < 0.05) when the diet was supplemented with 80, 120, or 160 mg/kg Fe as Fe-Gly at day 42. In addition, CAT activity at 21 days was slightly higher for chicks fed the basal diet supplemented with iron than in the chicks fed the basal diet, but no great changes were found among all the treatments (P > 0.05).
Effects of iron glycine on SOD and CAT activities. Control (no Fe supplemental), 40, 80, 120, and 160 (Fe-Gly) group supplements with 40, 80, 120, and 160 mg Fe/kg diet from Fe-Gly, 160 p group (positive control) supplements 160 mg Fe/kg diet from ferrous sulfate. *significant difference compared with the control
Discussion
Fe deficiency is one of the most common potential mineral deficiencies in animals, which produces microcytic hypochromic anemia in chickens. For this reason, supplementation of Fe with a large safety margin to meet the dietary needs of broilers is used. It has been reported that iron from ferrous glycinate was better absorbed than that from FeSO4 [12, 23]. And, ferrous glycinate has better effect on precaution and treatment of iron deficiency anemia in humans, especially in infants or young children [13, 15]. Langini et al. [23] reported that the absorption of Fe was 30.9 % in weanling rats given infant formula labeled with [59Fe]glycine compared with 15.8 % with [59Fe]sulfate. Layrisse et al. [14] showed that the absorption of Fe in Fe-Gly is twice that of FeSO4 in a breakfast meal based on maize flour. Glycine has the lowest molecular weight of all the amino acids, which favors the stability of the chelate compounds, preventing the ferrous ion from undesirable chemical reactions in the stomach and intestines that limit the absorption of Fe [24]. Additionally, Fe from Fe-Gly has lower pro-oxidant properties since diets supplemented with additional Fe had positive effects on feed conversion ratio (FCR) in broilers [3]. Therefore, higher absorption and lower pro-oxidant properties may be both attributed to the positive effect of Fe-Gly on performance in the current study. However, our result is in conflict with Kulkarni et al. [24], who reported that significantly improved BWG and FCR were observed in boilers fed 120 mg/kg FeSO4 compared to those fed ferrous aminoate [25], which needs further investigation.
Tissue mineral concentrations are commonly used to evaluate the mineral status of animals and humans. The present study showed that the Fe concentrations in serum (21 days, P = 0.005; 42 days, P = 0.001), liver (P = 0.001), breast muscle (P = 0.001), and tibia (P = 0.001) increase with the increasing levels of Fe as Fe-Gly in chickens. The results are similar to those reported by Cao et al. [26], who also observed that the tissue iron concentrations increase with increasing levels of dietary Fe. Yu et al. [11] reported that total Fe in the liver, spleen, and muscle significantly increased as the supplement level of Fe amino acid chelate was increased in pigs. Rincker et al. [27, 28] also found pigs maintained on diets supplemented with 0, 25, 50, 100, and 150 mg/kg Fe as FeSO4 in the feed resulted in a linear increase in whole body iron stores (P = 0.001). Consistent with our results, the Fe-Gly was also better absorbed and more bioavailable in rats or humans [5, 14] Olivares et al. [29] demonstrated that Fe from glycinate was 2 to 2.5 times more bioavailable than that from FeSO4 when added to the milk. Furthermore, Bovell-Benjamin et al. [5] also found that the Fe was absorbed from Fe-Gly (6.8 %) to a greater extent than from FeSO4 (1 %). Those results may explain the increased tissue Fe concentrations and suggest that a good absorption of Fe-Gly could improve the bioavailability of Fe from Fe-Gly.
Dietary Fe (160 mg/kg Fe as Fe-Gly or FeSO4) enhanced Fe concentration in feces compared with the control. We also found linear responses to concentrations of fecal Fe with the increasing levels of Fe-Gly. This is in accordance with the results of Creech et al. [30], who observed a decreased level of fecal Fe in piglets fed with reduced levels of Fe in the diet, whereas increased dietary Fe level (0 to 150 mg/kg) as FeSO4 resulted in a linear increase of fecal Fe excretion.
The continuous presence of an excessive intake of Fe could lead to free radical formation [31, 32]. SOD and CAT are antioxidant enzymes considered as the indicators for oxidative stress [33]. SOD functions to convert the active oxygen groups into H2O2, and CAT is responsible for the destruction of excess H2O2 [34]. In the present study, the Fe-Gly diet did dramatically stress the antioxidant system of chicks, as shown by a major increase in SOD at 42 days. This agrees with other studies performed in rats. Davis and Feng [35] found that dietary Fe (140 mg Fe/kg diet) caused a significantly increased SOD activity in rat liver. However, other researchers reported that a moderate dietary Fe excess (≤400 mg Fe/kg diet) did not affect the SOD activity in rat liver [36, 37], except for rats fed Fe-deficient diets [33]. Brandsch et al. [38] also suggested that the increase of CAT activity in rat liver may be attributed to the increased Fe concentrations in the liver itself rather than the induction of oxidative stress. Therefore, in the present experiment, the Fe concentration of the basal diet (21 days, 191.60 mg/kg; 42 days, 192.57 mg/kg) probably caused the activity of SOD to be at an abnormally low level, which was restored to normal by the supplementation of Fe. This may indicate that the moderately high Fe intake does not pose a major risk for oxidative stress in chicks, although the point warrants further research.
Conclusion
In conclusion, the results of this study indicate that additions of Fe-Gly improve the growth performance, iron tissue storage, and the antioxidant status of broiler chickens. Additionally, Fe-Gly reduces the fecal Fe concentrations compared to FeSO4.
References
Brock JH (1994) Iron in infection, immunity, inflammation and neoplasia. In: Brock JH, Halliday JW, Pippard MJ, Powell LW (eds) Iron metabolism in health and disease. Saunders, London, pp 354–389
NRC (1994) Nutrient requirements of poultry, 9th Revised Edition. National Academy Press, Washington
Bao YM, Choct M, Iji PA, Brucrton K (2007) Effect of organically complexed copper, iron, manganese and zinc on broiler performance, mineral excretion and accumulation in tissues. J Appl Poult Res 16:448–455
Henry PR, Miller ER (1995) Iron availability. In: Ammerman CB, Baker DH, Lewis AS (eds) Bioavailability of nutrients for animals. Academic, San Diego, pp 169–199
Bovell-Benjamin AC, Viteri FE, Allen LH (2000) Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. Am J Clin Nutr 71:1563–1569
Kegley EB, Spears JW, Flowers WL, Schoenherr WD (2002) Iron methionine as a source of iron for the neonatal pig. Nutr Res 22:1209–1217
Feng J, Ma WQ, Xu ZR, Wang YZ, Liu JX (2007) Effects of iron glycine chelate on growth, haematological and immunological characteristics in weaning pigs. Anim Feed Sci Technol 134:261–272
Feng J, Ma WQ, Xu ZR, He JX, Wang YZ, Liu JX (2009) The effect of iron glycine chelate on tissue mineral levels, fecal mineral concentration, and liver antioxidant enzyme activity in weanling pigs. Anim Feed Sci Technol 150:106–113
Close WH (1998) The role of trace mineral proteinates in pig nutrition. In: Lyons TP, Jacques KA (eds) Biotechnology in the feed industry. Nottingham University Press, Nottingham, pp 469–483
Close WH (1999) Organic minerals for pigs: an update. In: Lyons TP, Jacques KA (eds) Biotechnology in the feed industry. Nottingham University Press, Nottingham, pp 51–60
Yu B, Huang WJ, Chiou PW (2000) Bioavailability of iron from amino acid complex in weaning pigs. Anim Feed Sci Technol 86:39–52
Allen LH, Bovell-Benjamin AC, Viteri F (1998) Ferrous bis- and ferric tris-glycinates as iron fortificants for whole maize: bioavailability and regulation by iron status. FASEB J 12:A821
Iost C, Name JJ, Jeppsen RB, Ashmead HD (1998) Repleating hemoglobin in iron deficiency anemia in young children through liquid milk fortification with bioavailable iron amino acid chelate. J Am Coll Nutr 17:187–194
Layrisse M, García-Casal MN, Solano L, Baron MA, Arguello F, Llovera D, Ramirez J, Leets I, Tropper E (2000) Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J Nutr 130:2195–2199
Pineda O, Ashmead HD (2001) Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate. Nutrition 17:381–384
Fox TE, Eagles J, Fairweather-Tait SJ (1998) Bioavailability of iron glycine as a fortificant in infant foods. Am J Clin Nutr 67:664–668
Shelton JL, Southern LL (2006) Effects of phytase addition with or without a trace mineral premix on growth performance, bone response variables, and tissue mineral concentrations in commercial broilers. J Appl Poult Res 15:94–102
Hill GM, Miller ER, Whetter PA, Ullrey DE (1983) Concentration of minerals in tissues of pigs from dams fed different levels of dietary zinc. J Anim Sci 57:130–138
Shaw DT, Rozeboom DW, Hill GM, Booren AM, Link JE (2002) Impact of vitamin and mineral supplement withdrawal and wheat middling inclusion on finishing pig growth performance, fecal mineral concentration, carcass characteristics, and the nutrient content and oxidative stability of pork. J Anim Sci 80:2920–2930
Venturino A, Anguianl OL, Gauna L, Cocca C, Bergoc RM, Pevhen AM (2001) Thiols and polyamines in the potentiation of malathion toxicity in larval stages of toad Bufo arenarum. Comp Biochem Physiol C 130:191–198
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–270
SAS Institute (1997) SAS/STAT user’s guide. SAS Institute Inc., Cary
Langini S, Carbone N, Gald IM, Barrio RME, Portela ML, Caro R, Valencia ME (1988) Ferric glycinate iron bioavailability for rats, as determined by extrinsic radioisotopic labeling of infant formulas. Nutr Rep Int 38:729–735
Kulkarni RC, Shrivastava HP, Mandal AB, Deo C, Deshpande KY, Singh R, Bhanja SK (2011) Assessment of growth performance, immune response and mineral retention in colour broilers as influenced by dietary iron. Anim Feed Sci Technol 11:81–90
Ashmead SD (2001) The chemistry of ferrous bis-glycinate chelate. Arch Latinoam Nutr 51:7–12
Cao J, Luo XG, Henry PR, Ammerman CB, Littell RC, Miles RD (1996) Effect of dietary iron concentration, age, and length of iron feeding on feed intake and tissue iron concentration of broiler chicks for use as a bioassay of supplemental iron sources. Poult Sci 75:495–504
Rincker MJ, Hill GM, Link JE, Rowntree JE (2004) Effects of dietary iron supplementation on growth performance, hematological status, and whole-body mineral concentrations of nursery pigs. J Anim Sci 82:3189–3197
Rincker MJ, Hill GM, Link JE, Meyer AM, Rowntree JE (2005) Effects of dietary zinc and iron supplementation on mineral excretion, body composition, and mineral status of nursery pigs. J Anim Sci 83:2762–2774
Olivares M, Pizarro F, Pineda O, Name J, Hertrampf E, Walter T (1997) Milk inhibits and ascorbic acid favors ferrous bis-glycine chelate bioavailability in humans. J Nutr 127:1407–1411
Creech BL, Spears JW, Flowers WL, Hill GM, Lloyd KE, Armstrong TA, Engle TE (2004) Effect of dietary trace mineral concentration and source (inorganic vs. chelated) on performance, mineral status, and fecal mineral excretion in pigs from weaning through finishing. J Anim Sci 82:2140–2147
Nicholls DG, Budd SL (2000) Mitochondria and neuronal survival. Physiol Rev 80:315–360
Zodl B, Zeiner M, Marktl W, Steffan I, Ekmekcioglu C (2003) Pharmacological levels of copper exert toxic effects in Caco-2 cells. Biol Trace Elem Res 96:143–152
Rao J, Jagadeesan V (1996) Lipid peroxidation and activities of antioxidant enzymes in iron deficiency and effect of carcinogen feeding. Free Radic Biol Med 21:103–108
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650
Davis CD, Feng Y (1999) Dietary copper, manganese and iron affect the formation of aberrant crypts in colon of rats administered 3, 2′-dimethyl-4-aminobiphenyl. J Nutr 129:1060–1067
Bristow-Craig HE, Strain JJ, Welch RW (1994) Iron status, blood lipids and endogenous antioxidants in response to dietary iron levels in male and female rats. Int J Vitam Nutr Res 64:324–329
Ibrahim W, Lee US, Yeh CC, Szabo J, Bruckner G, Chow CK (1997) Oxidative stress and antioxidant status in mouse liver: effects of dietary lipid, vitamin E and iron. J Nutr 127:1401–1406
Brandsch C, Ringseis R, Eder K (2002) High dietary iron concentrations enhance the formation of cholesterol oxidation products in the liver of adult rats fed salmon oil with minimal effects on antioxidant status. J Nutr 132:2263–2269
Acknowledgments
This work was supported by the project supported by the New-Century Training Program Foundation for Talents from the Ministry of Education of China (grant no. NCET-10-0727) and the Natural Science Foundation for Distinguished Young Scholars of Zhejiang province, China (grant no. R3110085).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, W.Q., Sun, H., Zhou, Y. et al. Effects of Iron Glycine Chelate on Growth, Tissue Mineral Concentrations, Fecal Mineral Excretion, and Liver Antioxidant Enzyme Activities in Broilers. Biol Trace Elem Res 149, 204–211 (2012). https://doi.org/10.1007/s12011-012-9418-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9418-5