Abstract
Titanium dioxide nanoparticles (TiO2NPs) are increasingly being used in various industrial applications including the production of paper, plastics, cosmetics and paints. With the increasing number of nano-related products, the concern of governments and the general public about the health and environmental risks, especially with regard to occupational and other environmental exposure, are gradually increasing. However, there is insufficient knowledge about the actual affects upon human health and the environment, as well as a lack of suitable biomarkers for assessing TiO2NP-induced cytotoxicity. Since the respiratory tract is likely to be the main exposure route of industrial workers to TiO2NPs, we investigated the cytotoxicity of the anatase and rutile crystalline forms of TiO2NPs in A549 cells, a human alveolar type II-like epithelial cell line. In addition, we evaluated the transcript and protein expression levels of two heat shock protein (HSP) members, Grp78 and Hsp70, to ascertain their suitability as biomarkers of TiO2NP-induced toxicity in the respiratory system. Ultrastructural observations confirmed the presence of TiO2NPs inside cells. In vitro exposure of A549 cells to the anatase or rutile forms of TiO2NPs led to cell death and induced intracellular ROS generation in a dose-dependent manner, as determined by the MTS and dichlorofluorescein (DCF) assays, respectively. In contrast, the transcript and protein expression levels of Hsp70 and Grp78 did not change within the same TiO2NPs dose range (25–500 μg/ml). Thus, whilst TiO2NPs can cause cytotoxicity in A549 cells, and thus potentially in respiratory cells, Hsp70 and Grp78 are not suitable biomarkers for evaluating the acute toxicological effects of TiO2NPs in the respiratory system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The industrial use of metallic oxide nanoparticles (NPs) in a wide variety of applications has rapidly expanded over the last decade. Because of its high stability, anticorrosiveness and photocatalytic properties, titanium dioxide nanoparticles (TiO2NPs) are among the most frequently used metallic oxide NPs in various human products, such as cosmetics, sunscreen, toothpaste and paints [1]. Moreover, TiO2NPs have been used in the manufacturing industry [2] and environmentally to decontaminate water, air and soil, including the decontamination of agrochemicals [3, 4]. With the rapid increase in the number of nano-related products comes an increasing concern of governments and the general public about their safety to humans and the environment. Indeed, the human health effects of TiO2NP exposure, as well as their impact on the environment, are not under regulatory control [5], which then serves to raise concern further.
Because of their very small size, TiO2NPs have specific physicochemical characteristics and can enter the human body through several potential routes, including inhalation, skin contact, ingestion and parenteral [6]. Among the different potential exposure routes, inhalation is likely to be the main entry route of manufactured TiO2 into the body of industrial workers. Up to date, the safety aspects of TiO2NPs in the respiratory system have been studied in various in vitro models that are derived from human bronchial epithelial cells or lung fibroblast cells, and in vivo models using rodent models. Most of the studies have focused on the cytotoxic effects, toxic mechanisms, accumulation and clearance of TiO2NPs. For instance, it has been reported that TiO2NPs are rapidly internalized in the A549 human alveolar derived cell line and become distributed in the cytoplasm and intracellular vesicles [7], whilst TiO2NPs induce oxidative stress and cause genotoxicity in human lung fibroblast cells via DNA-adduct formation [8]. Furthermore, it has been reported that TiO2NPs cause cell death via induction of mitochondrial apoptosis in the BEAS2B human bronchial epithelial cell derived cell line, and that this involves Bax, cytochrome C, p53 and Bcl-2 [9]. With respect to the particle uptake and clearance aspects of TiO2NPs, lung surface macrophages do not efficiently phagocytose these ultrafines, but take them up in a rather sporadic and unspecific pathway in the rat model [10]. However, there are far fewer studies on the cellular responses and possible biomarker(s) for assessing the toxicity of TiO2NPs in the respiratory system.
One of the key cellular responses to toxicant exposure which could potentially be used as an early marker of toxicity is the induction of heat shock protein (HSP) expression [11]. HSPs can function as molecular chaperones, facilitating protein folding, preventing protein aggregation and targeting improperly folded proteins to specific degradative pathways [12]. Among them, the 70-kDa HSP family (Hsp70) consist of at least 11 genes, including Hsp70, Hsc70, Grp75 and Grp78, the protein products of which have housekeeping functions in the cell and are built-in components of protein folding, signal transduction pathways and quality control functions, where they proof read the structure of proteins and repair misfolded conformations [13–15]. The Hsp70 protein family differs from the other classes of HSPs in being one of the most highly conserved members and the first to be induced under stress conditions [14]. Among the members of the Hsp70 family, Hsp70 itself is an important cytosolic protein in the HSPA chaperone machine and the strongest stress induced protein [16]. Their main chaperone functions are to help the folding of proteins and the refolding of denatured proteins [15]. Another member of the HSP family, Grp78 (glucose-regulated protein of 78 kDa; BiP) is a major endoplasmic reticulum (ER) chaperone protein that facilitates protein folding and assembly, protein quality control, Ca2+-binding and regulating ER stress signaling [17]. Grp78 is induced by physiological stresses that perturb the ER function and homeostasis and protects against tissue or organ damage under these pathological conditions [18]. Grp78, therefore, is widely used as a marker for ER stress to toxicants. However, the involvement of Hsp70 and Grp78 in biological responses to TiO2NPs in the respiratory system is currently unknown.
Therefore, in this study, we aimed to examine possible biomarker(s) in response to TiO2NP-induced toxicity in A549 cells in vitro (representative of the respiratory system) by focusing on the transcriptional and post-translational expression levels of Hsp70 and Grp78.
Materials and Methods
Nanoparticles
Anatase and rutile crystalline forms of TiO2NPs were purchased from Sigma-Aldrich (St. Louis, MO). The product insert information indicates particle sizes of less than 25 nm with a specific surface area of 200–220 m2/g.
Particles Characterization
The TiO2NP morphology was analyzed by transmission electron microscopy (TEM) using a JEM-2010 microscope (Jeol, Japan). A drop of the TiO2NP suspension (from a feedstock of 0.01 g TiO2NP powder diluted to 20 ml by ethanol and sonicated for 5 min) was placed on a carbon film supported by a 200-mesh copper grid and the solvent was removed by drying overnight in a desiccator. TEM images of the TiO2NPs were collected at 200 keV and 100,000 times magnification. An energy-dispersive X-ray analysis (EDX) was used to confirm the presence of titanium.
Particle size and polydispersity index (PDI) of TiO2NPs were measured by dynamic light scattering (DLS) with a Zetasizer Nanoseries S4700 (Malvern, UK). TiO2NPs were dispersed in ultrapure water or Ham’s F12K at a concentration of 1,000 μg/ml. To this end, after sonication for 5 or 60 min, the TiO2NP suspension was 10-fold diluted with distilled water or CM, and then analyzed by DLS.
Cell Culture and Preparation of Exposure Suspension
A549 cells, derived from the human alveolar type II-like epithelial cell line, were obtained from the American Type Culture Collection (CCL-185; ATCC, Manassas, VA). The cells were cultured in complete media (CM; Ham’s F12K medium (Invitrogen, Carlsbad, CA) supplemented with 10 % (v/v) fetal bovine serum) at 37 °C in humidified atmosphere with 5 % (v/v) CO2 and subcultured by trypsinization. Before the treatment, A549 cells at a passage number less than 40 were grown overnight to reach about 80 % confluence. TiO2NPs were dispersed in the media without FBS at a concentration of 1,000 μg/ml by sonication for 5 min and diluted with CM prior to addition to the A549 cells.
Cytotoxicity Assay
A549 cells (5 × 103 cells/well) were seeded into each well of a 96-well plate in 100 μl of CM and grown for 24 h before treatment. The medium was then removed and replaced with 100 μl of the TiO2NP suspension in CM at final concentrations of 5, 10, 25, 50, 100, 250 and 500 μg/ml, which is equivalent to 1.563, 3.125, 7.813, 15.625, 31.25, 78.13 and 156.3 μg/cm2, respectively, and incubated for 24 h. The cytotoxicity of the added TiO2NPs was subsequently assessed using the CellTiter 96 Aqueous One Solution Reagent (Promega, Madison, WI), as per the manufacturer’s instructions, which contains 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). The absorbance of the resulting solubilized formazan product was measured by monitoring the absorbance at 490 nm using a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA). Results are shown as the relative percentage cell viability (which assumes no differences between treatments in cell division or mitochondrial metabolism and hence is all due to the number of viable cells), calculated by comparing the observed absorbance to that of the control cells (no added TiO2NPs).
Intracellular Reactive Oxygen Species (ROS) Generation
The induction of ROS generation by TiO2NPs was measured by dichlorofluorescein (DCF) assay [20]. Briefly, A549 cells (1 × 104 cells/well) were seeded into each well of a 96-well culture plate and incubated at 37 °C for 24 h. The cells were subsequently incubated with 50 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCF-DA; Molecular Probes, Grand Island, NY) dispersed in phosphate buffered saline pH 7.4 (PBS) for 40 min in the dark. The cells were washed with PBS and then exposed to various concentrations of the anatase or rutile crystalline forms of TiO2NPs at final concentrations of 5, 10, 25, 50, 100, 250 and 500 μg/ml for 2 h. Hydrogen peroxide (5 mM) was used as a positive control in the assay. The fluorescent intensity of 2′,7′-dichlorofluorescein in the cells was measured at 485/528 nm by a microplate reader system (PowerWave XS2, BioTek, Winooski, VT).
Side Scatter Measurements
The degree of TiO2NPs uptake or adsorption on cellular membranes was determined by measuring side scatter pulse area (SSC-A) parameter of FACS analysis as described previously [19]. Briefly, A549 cells were plated into a 12-well plate at 6 × 104 cells/well and cultured for 24 h. After TiO2NP exposure, the cells were trypsinized, centrifuged and resuspended in PBS before subjected to FACS analysis using a flow cytometer (FACSAria II; Becton Dickinson, San Jose, CA). The mean of SSC-A was measured by FACSDiva software (BD) based on 10,000 events.
TEM Analysis
A549 cells (8 × 105 cells) were plated into a 10-cm Petri dish and cultured for 24 h before treatment with 100 μg/ml TiO2NPs for 24 h. The cell pellets were collected, fixed with 2.5 % glutaraldehyde and posted-fixed in 1 % osmium tetroxide. The specimens were subsequently dehydrated in a graded series of ethanol and embedded in a mixture of Spurr resin. After polymerized, ultra-thin sections were prepared with a Leica Ultracut microtome (Leica, Deerfield, IL), collected on copper grids, and contrasted with uranyl acetate and lead acetate. Samples were finally imaged using a TEM at 120 keV (FEI Tecnai T20, the Netherlands).
Quantification of the Expression Levels of Grp78 and Hsp70 mRNAs
A549 cells were seeded into each well of a 6-well culture plate at 1.5 × 105 cells/well and incubated at 37 °C for 24 h. The cells were then exposed to the anatase or rutile crystalline forms of TiO2NPs at a final concentration of 25, 50, 100, 250 or 500 μg/ml. Total RNA was isolated by using a FastPure RNA Kit (Takara, Japan) following the manufacturer’s instructions. The isolated total RNA was subsequently treated with DNase I (Takara) to remove contaminating genomic DNA. The RNA concentration was determined at 260 nm by Nanodrop 2000C (Thermo Scientific, Wilmington, NC). The cDNA was then generated with a random hexamer by using a high-capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster, CA). The expression level of Grp78 and Hsp70 mRNA transcripts in A549 cells were then measured by quantitative real-time PCR carried out on an ABI PRISM 7500 Fast (Applied Biosystems) using SYBR green (Applied Biosystems). Each PCR reaction mixture, at a final volume of 15 μl, contained of 1× Power SYBR green Mastermix, 0.25 μM of each of the forward and reverse primer (see Table 1) and 37.5 ng cDNA template. PCR reactions were performed at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C (62 °C for Hsp70) for 1 min. The expression levels of Grp78 and Hsp70 mRNAs were normalized to the expression level of GAPDH as an endogenous control gene. The primers were designed using the Oligo 4.0-s software (Molecular Biology Insights, Cascade, CO) and confirmed for likely gene specificity by Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) searching of the NCBI GenBank database.
Cell Lysate Preparation
A549 cells (8 × 105 cells) were seeded in a 10-cm Petri dish in 10 ml of CM and incubated at 37 °C for 24 h. The cells were then exposed to various doses of anatase or rutile crystalline forms of TiO2NPs at a final concentration of 25, 50, 100, 250 or 500 μg/ml for 6, 12 or 24 h. The cells were then washed and subsequently lysed in cell lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 % (v/v) Nonidet P-40, 1 mM EDTA, 100 mM NaF, 0.2 mM Na3VO4) supplemented with protease inhibitor cocktail (Sigma) for 30 min at 4 °C with gentle agitation. The cell debris was consequently removed by centrifugation at 10,000×g for 20 min at 4 °C. Cell lysates were kept at −80 °C until analysis. The protein concentration in each sample was measured by using a BCA protein assay kit (Thermo Scientific, Illinois, USA), as per the manufacturer’s instructions.
Western Blot Analysis
The cell lysates (10 μg protein/lane) were separated on reducing SDS–polyacrylamide gel electrophoresis (7.5 % (w/v) acrylamide resolving gel) and transferred to a polyvinylidine fluoride membrane (Pall Life Sciences, Ann Arbor, MI) by Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad, UK). The stress proteins, Grp78 and Hsp70, were specifically detected by incubating the membrane with anti-KDEL (SPA-827, Stressgen, San Diego, CA) and anti-HSP70 (SPA-820, Stressgen) antibodies (each diluted at 1:1,000) in PBS-S (5 % (w/v) skim milk in PBS) for 2 h at room temperature, respectively. Membranes were also incubated at this stage with anti-β-actin (sc-47778; Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:500 in PBS-S, for 2 h as a sample loading control. The membrane was subsequently washed with PBS and incubated with biotinylated-secondary antibodies (2,000 dilution) in PBS-S for 30 min. The membrane was then incubated with avidin–biotin complex (Santa Cruz Biotechnology) and finally incubated with 3,3′-diaminobenzidinetetrahydrochloride (DAB; a substrate for peroxidase). The protein bands were subsequently imaged by a gel imaging system (AlphaImager, San Jose, CA).
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. A value of p < 0.05 was considered statistically significant.
Results
TiO2NP Characterization
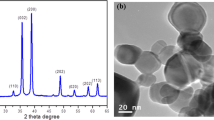
According to the supplier’s product information, the anatase and rutile TiO2NPs were each 99.9 % pure with an average particle size of less than 25 nm and a specific surface area of 200–220 m2/g. The morphology and size distribution of the anatase and rutile TiO2NPs were confirmed by dispersing TiO2NPs in an aqueous solution, and then measured in the dry and hydrated states by TEM-EDX and DLS analyses, respectively. The size of the anhydrous anatase and rutile TiO2NPs, as revealed by TEM analysis, revealed a median particle size of about 20 nm, and both forms showed a similar pattern of two peaks (high and small) of titanium in the EDX spectra (Fig. 1a and b). For the hydrated size, as evaluated by DLS, the TiO2NPs dispersed in DI water tended to form agglomerates with a net size distribution at 542 ± 8.21 and 321.53 ± 16.18 nm for the anatase and rutile forms, respectively (Table 2). Although increasing the sonication time from 5 to 60 min reduced the diameter of the anatase agglomerates ~1.1-fold, no significant change in agglomerate size was noted for the rutile NPs. Likewise, for the TiO2NP size distribution, as measured by the DLS, the PDI was not decreased by increasing the sonication time from 5 to 60 min, but rather was increased. In the experimental condition of TiO2NPs dispersed in CM, high degree of agglomeration was observed. The hydrodynamic diameter sizes of anatase TiO2NPs in CM at 5 and 60 min sonication were ~5.3- and 5-fold larger than those in DI water, respectively. The increased sonication time appeared no effect on their hydrodynamic size. In contrast, the diameter sizes of rutile TiO2NPs dispersed in CM were decreased up on increasing the sonication time from 5 to 60 min. Although diameter size of rutile TiO2NPs at 5-min sonication was about twice larger than at 60-min sonication, the long period of sonication could generate heat and induce higher degree of sedimentation. At 5-min sonication, two populations of rutile TiO2NP size distribution, in nano-sized range, were demonstrated (Suppl. 1). Therefore, a 5-min sonication of anatase and rutile TiO2NPs was used in this study.
Characterization of the a anatase and b rutile crystalline forms of TiO2NPs. The particle morphology was analyzed by TEM and imaged with a magnification of 100,000 times. The presence of titanium was confirmed by an energy-dispersive X-ray analysis (EDX). Micrographs and spectra shown are representative of those seen from five fields of view per sample
In Vitro Cytotoxicity of TiO2NPs to the A549 Cell Line
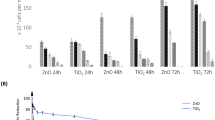
To investigate the in vitro cytotoxicity of TiO2NPs, A549 cells in tissue culture were used as an in vitro model system for the airway epithelium. The A549 cells were exposed to various concentrations of TiO2NPs for 24 h and then analyzed by the MTS assay. The anatase form of TiO2NPs caused a dose-dependent reduction in the amount of formazan produced, and thus an assumed decreased cell viability or increased cytotoxicity in A549 cells, but only the two highest concentrations tested (250 and 500 μg/ml) were statistically significant (p < 0.05) (Fig. 2a). Rutile TiO2NPs also showed a dose-dependent decrease in the cell viability, but this numerical decrease was not statistically significant at the concentrations used in this study (Fig. 2b).
In vitro cytotoxicity of the a anatase and b rutile crystalline forms of TiO2NPs against A549 cells. A549 cells were incubated with various concentrations of the anatase or rutile crystalline forms of TiO2NPs for 24 h and then the cell viability was determined by the MTS assay. Data are presented as mean ± SD of three independent experiments. *p < 0.05, using a one-way ANOVA for the comparison between untreated and TiO2NP-exposed cells
Intracellular ROS Generation
Since oxidative stress is one of the mechanisms of toxicity related to NP exposure in several cell lines, the intracellular ROS generation in A549 cells after exposure to TiO2NPs was investigated using the DCF assay. As shown in Fig. 3, both the anatase and rutile forms of TiO2NPs significantly increased the intracellular ROS levels, but this was only at the higher concentrations of 100 μg/ml numerically, and was statistically only significant at 250 and 500 μg/ml (p < 0.05). Nevertheless, consistent with the observed results for their ability to induce A549 cell cytotoxicity, the anatase and, to a lesser extent, the rutile forms of TiO2NPs tended to induced ROS generation in a concentration-dependent manner.
Intracellular ROS generation in A549 cells after treatment with the a anatase and b rutile crystalline forms of TiO2NPs. The cells were incubated with various concentrations of the anatase or rutile crystalline form of TiO2NPs for 24 h and then the intracellular ROS levels were determined by the DCF assay. H2O2 at 5 mM was used as a positive control. Data are presented as the mean ± SD of three independent experiments, and show the ROS levels relative to that of the no-TiO2NPs control. *p < 0.05, using one-way ANOVA for the comparison between untreated and TiO2NP-exposed cells
Uptake of TiO2NPs into the Cells
The uptake of TiO2NPs into A549 cells was investigated by FACS and TEM analyses. Since, increase of SSC is proportional to cell granularity or complexity, endocytosis or adsorptive nanoparticle interactions to the cells were determined by SSC measurement following TiO2NP exposure. As shown in Fig. 4, mean of SSC-A was significantly increased in cells treated with both anatase and rutile TiO2NPs at even the low concentrations (25 and 50 μg/ml) used in this study. We further confirmed the internalization of TiO2NPs to the cells by TEM analysis. Ultrastructure image demonstrated that TiO2NPs could be internalized to the cells and appeared in the cytoplasm, but not in the nucleus (Fig. 5).
TEM images of TiO2NP inside the cells. A549 cells were treated with anatase and rutile TiO2NPs at 100 μg/ml for 24 h. a Untreated control; b–d anatase TiO2NPs, where d is an increased magnification image of the circle area in b; e–g rutile TiO2NPs, where g is an increased magnification image of circle area in e and f. The arrows indicate clumps of TiO2NPs in endosomes located in the cytoplasm. The arrowheads indicate nuclear membrane. N nucleus
Effect of TiO2NPs on Grp78 and Hsp70 Transcript and Protein Expression Levels in the A549 Cell Line
To examine the alteration of stress-related gene expression after exposure to TiO2NPs, the mRNA transcript expression levels of Grp78 and Hsp70 were determined by real-time reverse transcriptase PCR. Exposure of A549 cells to TiO2NPs in the dose range of 25–500 μg/ml for 6 and 24 h did not significantly change the expression levels of either Grp78 or Hsp70 mRNA (Fig. 6). However, a slight dose-dependent numerical increase in the Hsp70 transcript levels was noted after 24 h, but not 6 h, exposure to the TiO2NPs, and especially for the anatase form, but as already noted this was not statistically significant.
Effects of the anatase and rutile crystalline forms of TiO2NPs on the Hsp70 and Grp78 transcript expression levels in A549 cells. A549 cells were treated with various concentrations of the anatase or rutile crystalline forms of TiO2NPs for 6 or 24 h and then the mRNA expression levels of Hsp70 and Grp78 were determined by real time RT-PCR. Data are shown as relative to that of the transcript housekeeping gene (GADPH), and are presented as the mean ± SD of three independent experiments
The expression level of the Grp78 and Hsp70 proteins, relative to that of the housekeeping β-actin gene, in A549 cells after TiO2NPs treatment in tissue culture were investigated by Western blot analyses. Similar to that observed for the mRNA transcript levels, both the anatase and rutile crystalline forms of TiO2NPs did not significantly change the protein expression levels of Grp78 and Hsp70 after 6, 12 and 24 h of exposure, at least in the dose range examined in this study (Fig. 7).
Effects of the anatase and rutile crystalline forms of TiO2NPs on the Hsp70 and Grp78 protein expression levels in A549 cells. A549 cells were treated with various concentrations of the anatase or rutile crystalline forms of TiO2NPs for 6, 12 or 24 h and then the expression levels of Hsp70 and Grp78 proteins were determined using Western blot analysis. Results represent blots from three independent experiments
Discussion
TiO2NPs are increasingly being used in various commercial products, but there is still an inadequate knowledge concerning their risk to human health and the environment, as well as on any suitable biomarker(s) for assessing the safety of TiO2NPs in, for example, the human respiratory system. In this study, we, therefore, investigated the in vitro cytotoxicity of both the anatase and rutile crystalline forms of TiO2NPs in A549 cells as an in vitro model of human bronchial epithelial cells and the possible biomarker(s) for evaluating the TiO2NPs-induced cytotoxicity in respiratory system by focusing on HSPs family.
In toxicological studies, the drug or toxin-sensitive genes whose expression levels correlate with, or ideally are directly responsible for, the effect of the external stimuli (such as cytotoxicity here) can be useful as biomarkers for the effect. Among the biomarkers that have been studied, the expression levels of HSPs are widely used to monitor cellular hazards, because of their sensitivity to even minor assaults and their inducible nature against a wide range of chemicals [14]. Indeed, many toxicants alter HSP gene (transcript and protein) expression levels, in addition to various other stimuli including heat, pesticides, heavy metals, solvents, industrial and municipal effluents, some drugs, and mono- and polycyclic aromatic hydrocarbons [14, 21, 22]. Among the members of the Hsp70 family, the expression of Hsp70 and Grp78 are reported to be altered in response to various metal salts (including NPs) in several organs, including the lungs, both in the in vitro and in vivo models. For instance, the induction of Hsp70 protein expression was observed following silver NP treatment in Drosophila melanogaster, an often used in vivo model [23], in cadmium chloride treatment in LLC-PK1 renal epithelial cells in vitro [24], and in copper oxide NP and nickel exposure in A549 and Calu-3 human lung epithelial A549 cells [25, 26]. Likewise, an increased expression level of the Grp78 protein has been clearly observed after cadmium chloride exposure in several cell line types, including the LLC-PK1 renal epithelial cell line and rat lung epithelial cells [24, 27].
In the present study, we further investigated the possibility to apply the transcript and protein expression levels of Hsp70 and Grp78, two members of the HSPs family, as biomarkers of TiO2NPs-induced cytotoxicity in alveolar epithelium using A549 cells as the exposure model [28]. For the TiO2NPs used in this study, TEM-EDX confirmed that the primary size of the TiO2NPs were in the “nano” range (Fig. 1). However, the TiO2NPs tended to agglomerate in the hydrated forms, as determined by DLS (Table 2). TiO2NPs dispersed in CM showed the larger agglomerated size than TiO2NPs dispersed in DI water (Table 2). This might because of the interaction between TiO2NPs and serum components such as FBS and salts in the CM resulting in changing the agglomerated state [29, 30]. Nevertheless, these agglomerates can be taken up by the cells and largely accumulated in the endosomes (Figs. 4 and 5), probably via endocytosis as reported previously shown the characteristic of lamellipodia engulfing TiO2NP aggregates [31]. Our results showed that the anatase and, to a lesser extent, the rutile crystalline forms of TiO2NPs caused cytotoxicity in a dose-dependent manner (Fig. 2), which correlates with the observed induction of intracellular ROS generation in the same cell line (Fig. 3). This is in agreement with a previous report that anatase TiO2NPs (<25 nm) can cause cytotoxicity in A549 cells via oxidative stress and that pretreatment of the cells with dimethylthiourea, an OH• scavenger, improved the cell viability [32].
Alteration of the expression levels of HSPs after exposure to toxicants associated with the induction of ROS generation has been reported previously in several studies [11, 20]. This oxidative stress is also one of the toxic insults that can disrupt normal protein folding in cells and lead to activation of the unfolded protein response [33]. In this investigation, even though we found the induction of intracellular ROS by TiO2NPs, we could not, however, observe any significant alteration in the Hsp70 and Grp78 expression levels at either the level of mRNA or protein (Figs. 6 and 7). The sensitivity in any biological response to external stimuli via these two HSPs is potentially chemical and cell type specific [24]. Heme oxygenase (Hsp32), one of the HSP family members, is very sensitive to TiO2NP exposure in the BEAS-2B human bronchial epithelial cell line, even at a dose that only causes 10 % or less cytotoxicity [34]. However, in our study, even at TiO2NP doses that caused around 40 % cytotoxicity and induced more than a 2-fold elevated ROS level (40 % of the H2O2 control level), no significant change in the Hsp70 and Grp78 mRNA or protein expression levels were observed. This is despite the fact that it has been reported that Hsp70 and Hsp72 expression levels were increased in A549 cells after treatment with other metal NPs, such as copper oxide NPs, nickel and cadmium [25, 35, 36]. Therefore, the cellular responses of Hsp70 and Grp78 to TiO2NPs in A459 cells might be metal specific, although we cannot exclude the possibility of a cell line-dependent affect, gene specific response, or due to experimental artifacts.
Up to date, the data on human exposure to TiO2NPs via inhalation are limited. Based on chronic inhalation studies in rats, NIOSH recommends airborne exposure limits of 2.4 mg/m3 for fine TiO2 and 0.3 mg/m3 for TiO2NPs for up to 10 h/day, during 40 h/week, to reduce risk of lung cancer over working lifetime [37]. It should be noted that the concentrations (up to 500 μg/ml) used in our study were considerably high, in order to determine the mechanistic toxic effects. However, since there is less correlation between toxicity of nanoparticles in vitro and in vivo [38], these biological responses might not directly reflect in vivo results.
Taken together, our results suggest that TiO2NPs can cause cytotoxicity and ROS generation, but independently of altered Hsp70 and Grp78 expression levels in A549 cells. The actual involvement of these two gene products, however, remains unknown and awaits further evaluation, such as by gene silencing. Regardless, Hsp70 and Grp78 expression levels would appear to not be useful biomarkers for the biological response to TiO2NPs in the A549 cell line (the in vitro model of this study), and so potentially not in respiratory cells, although investigation in other models is still important.
References
Kaida T, Kobayashi K, Adachi M, Suzaki F (2004) Optical characteristics of titanium oxide interference film and the film laminated with oxides and their applications for cosmetics. J Cosmet Sci 55:219–220
Allen NS, Edge M, Sandoval G, Verran J, Stratton J, Maltby J (2005) Photocatalytic coatings for environmental applications. Photochem Photobiol 81:279–290
Higarashi NM, Jardim WE (2002) Remediation of pesticide contaminated soil using TiO2 mediated by solar light. Catal Today 76:201–207
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigation-a review. Appl Catal B Environ 49:1–14
Oberdöster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle Fibre Toxicol 2:2–8
Oberdöster G, Oberdöster E, Oberdöster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Simon-Deckers A, Gouget B, Mayne-L’Hermite M, Herlin-Boime N, Reynaud C, Carrière M (2008) In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 253:137–146
Bhattacharya K, Davoren M, Boertz J, Schins RPF, Hoffmann E, Dopp E (2009) Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Particle Fibre Toxicol 6:1–11
Shi Y, Wang F, He J, Yadav S, Wang H (2010) Titanium dioxide nanoparticles cause apoptosis in BEAS-2B cells through the caspase 8/t-Bid-independent mitochondrial pathway. Toxicol Lett 196:21–27
Geiser M, Casaulta M, Kupferschmid B, Schulz H, Semmler-Behnke M, Kreyling W (2008) The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am J Respir Cell Mol Biol 28:371–376
Golli-Bennour EE, Bacha H (2011) Hsp70 expression as biomarkers of oxidative stress: mycotoxin’s exploration. Toxicology 287:1–7
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Schlesinger MJ (1990) Heat shock proteins. J Biol Chem 265:12111–12114
Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK (2010) Heat shock proteins in toxicology: how close and how far? Life Sci 86:377–384
Lanneau D, Wettstein G, Bonniaud P, Garrido C (2010) Heat shock proteins: cell protection through protein triage. Sci World J 10:1543–1552
Vos MJ, Hageman J, Carra S, Kampinga HH (2008) Structural and functional diversities between members of human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry 47:7001–7011
Ni M, Zhang Y, Lee AS (2011) Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signaling and therapeutic targeting. Biochem J 434:181–188
Lee AS (2007) GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res 67:3476–3499
Suzuki H, Toyooka T, Ibuki Y (2007) Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ Sci Technol 41:3018–3024
Yoshida Y, Shimakawa S, Itoh N, Niki E (2007) Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain. Free Radic Res 37:861–72
Maniratanachote R, Miami K, Katoh M, Nakajima M, Yokoi T (2005) Chaperone proteins involved in troglitazone-induced toxicity in human hepatoma cell lines. Toxicol Sci 83:293–302
Lu TH, Su CC, Chen YW, Yang CY, Wu CC, Hung DZ, Chen CH, Cheng PW, Liu SH, Huang CF (2011) Arsenic induces pancreatic β-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol Lett 201:15–26
Ahamed M, Posgai R, Gorey TJ, Nielsen M, Hussain SM, Rowe JJ (2010) Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol Appl Pharmacol 242:263–269
Liu F, Inageda K, Nishitai G, Matsuoka M (2006) Cadmium induces the expression of Grp78, an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial cells. Environ Health Perspect 114:859–864
Forti F, Salovaara S, Cetin Y, Bulgheroni A, Tessadri R, Jennings P, Pfaller W, Prieto P (2011) In vitro evaluation of the toxicity induced by nickel. Toxicol In Vitro 25:454–461
Ahamed M, Siddiqui MA, Akhtar MJ, Ahmad I, Pant AB (2010) Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem Biophys Res Commun 396:578–583
Timblin CR, Janssen YMW, Goldberg JL, Mossman BT (1998) Grp78, Hsp72/72 and CJUN stress protein levels in lung epithelial cells exposed to asbestos, cadmium, or H2O2. Free Radical Biol Med 24:632–642
Rothen-Rutishauser B, Blank F, Mühlfeld C, Gehr P (2008) In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Expert Opin Drug Metab Toxicol 4:1075–1089
Thio BJR, Zhou D, Keller AA (2011) Influence of natural organic matter on the aggregation and deposition of titanium dioxide nanoparticles. J Hazard Mater 189:556–63
Allouni ZE, Cimpan MR, Hol PJ, Skodvin T, Gjerdet NP (2009) Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Colloids Surf B: Biointerf 68:83–87
Singh S, Shi T, Duffin AC, Berlo DV, Hohr D, Fubini B, Martra G, Fenoglio BPJA, Schins RPF (2007) Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol Appl Pharmacol 222:141–151
Srivastava RK, Rahman Q, Kashyap MP, Lohani M, Pant AB (2011) Ameliorative effects of dimetylthiourea and N-acetylcysteine on nanoparticles induced cyto-genotoxicity in human lung cancer cells-A549. PLoS One 6:1–12
Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ (2008) Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A 105:18525–18530
Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K (2008) Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett 180:222–2229
Hfaiedh N, Allagui AS, El Feki A, Gaubin Y, Murat JC, Soleilhavoup JP, Croute F (2005) Effects of nickel poisoning on expression pattern of the 72/73 and 94 kDa stress proteins in rat organs and in the COS-7, HepG2, and A549 cell lines. J Biochem Mol Toxicol 19:12–18
Croute F, Beau B, Arrabit C, Gaubin Y, Delmas F, Murat JC, Soleilhavoup JP (2000) Environ Health Perspect 108:55–60
NIOSH. Current intelligence bulletin 63: Occupational exposure to titanium dioxide. Department of Health and Human Services. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health. DHHS (NIOSH) Publication No. 2011–160
Sayes CM, Reed KL, Warheit DB (2007) Assessing toxicity of fine and nanoparticles: Comparing in vitro measurement to in vivo pulmonary toxicity profiles. Toxicol Sci 97:163–180
Acknowledgements
This work was financial supported by the Research, Development and Engineering Fund through the National Nanotechnology, NSTDA, Thailand. We thank Dr. Robert Butcher for comments and for the English language review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 62 kb)
Rights and permissions
About this article
Cite this article
Aueviriyavit, S., Phummiratch, D., Kulthong, K. et al. Titanium Dioxide Nanoparticles-Mediated In Vitro Cytotoxicity Does Not Induce Hsp70 and Grp78 Expression in Human Bronchial Epithelial A549 Cells. Biol Trace Elem Res 149, 123–132 (2012). https://doi.org/10.1007/s12011-012-9403-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9403-z