Abstract
The essential trace elements play important roles in the maintainance of the normal structure and physiology of cells. Several research groups have demonstrated that they also play important roles in states of cardiovascular diseases. Our aim is to investigate whether there is a relationship between trace elements (Zn and Cu) and the degree of atherosclerosis. The sample consisted of 67 patients with coronary artery disease and 26 clinically healthy individuals. Ninety-three subjects were separated into four groups according to their Gensini scores, the number of diseased vessels, the presence of acute coronary syndrome, and ejection fraction. Each group was divided into three subgroups, and serum zinc and copper levels were measured for each individual. The serum levels of zinc and copper were found to be significantly lower in patients with atherosclerosis than in the control group, but there were no significant differences in the serum levels of Cu and Zn between severe atherosclerosis and mild atherosclerosis. In Spearman’s rank correlation, the zinc and copper levels were correlated with the Gensini score and the number of diseased vessels. The present study revealed a relationship between the serum levels of zinc and copper and atherosclerosis, but not between these levels and the severity of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trace element status and cardiovascular disease may be associated directly, through a direct effect on the vascular system, or indirectly through lipoprotein metabolism. Investigators suggest that a decreased serum zinc (Zn) and copper (Cu) concentration may increase the risk of cardiovascular disease [1, 2].
The essential elements Zn and Cu act as microsources in biochemical reactions. Zn is involved in many biological processes, including enzyme action, regulation of gene expression, and cell signaling [3]. Zn deficiency is associated with susceptibility to oxidative stress, endothelial cell apoptosis, and atherogenesis [2]. Atherosclerosis is associated with lipid and protein peroxidation, caused by free oxygen radical generation, resulting in mechanical and bioenergetic incompatibilities [4–8]. Antioxidants have therefore been implicated as playing a preventive role in the atherosclerotic process.
Many studies are reported in the literature on the relationship between Zn and Cu levels and coronary atherosclerosis itself, but none measured any relationship with its severity. In this study, we aimed to investigate whether a relationship exists between serum levels of Zn and Cu and the severity of atherosclerosis, measured by using Gensini scores.
Materials and Methods
Patients and Controls
The study was conducted in the Department of Clinical Cardiology, University of Dicle. It was approved by the Ethical Committee on Human Research, and all participants gave an informed consent. There were 67 individuals of the patient group, with both sexes represented (49 males, 24 females). Each suffered from coronary artery disease (CAD) and had been admitted for diagnostic coronary angiography or angioplasty for typical indications, including evaluation of stable exertional angina or unstable angina pectoris.
Exclusion criteria were clinically significant valvular heart disease and significant left ventricular systolic dysfunction. None of the participants suffered from Zn and Cu deficiency caused by malabsorption (due to, for example, Crohn’s disease, persistent diarrhea, short bowel syndrome, celiac sprue, or intestinal bypass surgery), increased loss (due to, for example, alcoholism), or increased needs (due to, for example, pregnancy) [3, 9].
The control group consisted of 26 clinically healthy (group 1: control group according to Gensini scoring, group A: control group according to the number of diseased vessels, group I: control group according to the presence of acute coronary syndrome, group α: control group according to ejection fraction), nonvegetarian individuals. None had plaque in the carotid artery as assessed by ultrasound, and none reported a history of cardiac therapy or diseases of the liver, kidney, or thyroid disease.
Coronary Angiography and Severity of Coronary Atherosclerosis
Coronary angiography was performed by the Judkins technique through femoral artery access and the angiograms evaluated by two interventional cardiologists who were blinded to the study plan and to each other. A thorough review of each index coronary angiogram established lesion location and percentage of stenosis among all the coronary lesions. CAD was defined as >50% luminal diameter stenosis of at least one major epicardial coronary artery and categorized to one vessel (group B), two or more vessel disease (group C) [10].
The Gensini scoring system was used to determine the severity of coronary artery disease [11]. This method defines narrowing of the lumen of the coronary arteries as 1 for 1–25% stenosis, 2 for 26–50%, 4 for 51–75%, 8 for 76–90%, 16 for 91–99%, and 32 for total occlusion. The score is then multiplied by a factor representing the importance of the lesion’s location in the coronary artery system. For the location scores, 5 points were given for left main lesion; 2.5 for proximal left anterior descending (LAD) or left circumflex (LCX) artery; 1.5 for the mid-segment LAD and LCX; 1 for the distal segment of LAD and LCX, first diagonal branch, first obtuse marginal branch, right coronary artery, posterior descending artery, and intermediate artery; and 0.5 for the second diagonal and second obtuse marginal branches. The patients were divided into two groups according to Gensini scoring: those with mild (n = 24; Gensini score, 1–20; mild atherosclerosis, group 2) and severe atherosclerosis (n, 49; Gensini score, >20; severe atherosclerosis, group 3) [12].
Additionally, the patients were separated into two groups according to the presence of acute coronary syndrome: a stable angina group (n = 34; group II) and an acute coronary syndrome group (n = 33; group III).
Echocardiography
A GE Vivid S5 unit with a 3.5-MHz probe was used to perform the two-dimensional echocardiography. Global left ventricular (LV) systolic function was assessed by calculating the ejection fraction (EF), measured by determining LV volumes from apical four- and two-chamber views, using a modified Simpson’s method. The patients were divided into two groups according to ejection fraction: those with low (n = 24; EF < 45%; group β) and high (n = 43; EF ≥ 45%; group γ) ejection fraction.
Biochemical Evaluation
Assay kits (Boehringer Mannheim, Germany) were used to make direct measurements of total cholesterol (TC) and total triglycerides (TG). High-density lipoprotein cholesterol (HDL) was determined after precipitation of very low-density lipoprotein and low-density lipoprotein cholesterol (LDL) using sodium phosphotungstate-Mg. LDL concentrations were calculated by the Friedewald [13] formula.
Zn and Cu levels were measured by Shimadzu 6401S atomic absorption/emission spectrometer. The acetylene flow rate and the burner height were adjusted to obtain the maximum absorbance signal with a slit of 0.5 nm, at wavelengths of 213.9 nm for Zn and 324.8 nm for Cu. The radiation sources were hollow cathode lamps (Shimadzu, Japan). The operating conditions were those recommended by the manufacturer (Operation Manual, Atomic Absorption Spectrometer AA-6800, Shimadzu, 2000). The blood samples were kept at −80°C until analysis.
Statistical Analyses
Data were analyzed using the SPSS-15 software. Baseline demographic and laboratory data are presented for continuous variables as mean ± SD and for discrete variables as frequencies. The mean difference between groups and Cu, Zn levels was analyzed using a one-way ANOVA. Relationships between levels of Zn–Cu and clinical, biochemical, and echocardiographic parameters were evaluated by Spearman’s rank correlation coefficients. P < 0.05 was considered statistically significant.
Results
Baseline clinical characteristics and lipid parameters of the patients are described in Table 1.
Grouping According to Gensini Scoring: Patients’ Characteristics
We found no significant differences in gender, age, smoking habits, occurrence of diabetes mellitus (DM) and hypertension (HT), serum TC, LDL, HDL, and TG or blood glucose levels between the three groups. The patients in group 2 and 3 had suffered more significantly from previous myocardial infarction than group 1. Similarly, there was a higher incidence of coronary artery disease in group 3 than group 1 (Table 1).
Grouping According to the Number of Diseased Vessels: Patients’ Characteristics
Among the groups, there were no significant differences in gender, smoking habits, occurrence of DM and HT, serum level of TC, LDL, HDL, and TG or blood glucose levels. Significantly, more individuals from groups B and C had suffered previous myocardial infarction than individuals of group A, and individuals of Group C reported significantly higher incidence of coronary disease in the family than those of group A, who were also significantly younger (Table 1).
Grouping According to the Presence of Acute Coronary Syndrome: Characteristics of Patients
As shown in Table 1, there were no significant differences in gender, smoking habits, family history, occurrence of DM and HT, serum level of TC, LDL, and TG or blood glucose between the groups. However, significantly more individuals of groups II and III had suffered previous myocardial infarction than individuals of group I, and age and HDL in individuals of group III were significantly higher than in individuals of groups I and II (Table 1).
Grouping According to Ejection Fraction: Characteristics of Patients
Similar to other results, we found no significant differences in gender, age, smoking habits, occurrence of DM and HT, serum level of TC, LDL, and TG or blood glucose among all groups. Significantly, more individuals of groups β and γ had suffered previous myocardial infarction than individuals of group α, and individuals of group β reported significantly higher incidence of coronary disease in the family than those of group α (Table 1).
Blood Zn and Cu Levels in All Groups
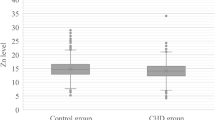
The main results are summarized in Tables 2 and 3. The serum levels of Cu and Zn in group 2, B, II, and β were significantly lower than the control groups (p < 0.01; p < 0.01; p < 0.01; p < 0.01, respectively). The serum levels of Cu and Zn Group 3, C, III, and γ were significantly lower than the control groups (p < 0.01; p < 0.01; p < 0.01; p < 0.01, respectively). There were no significant differences in serum levels of Cu and Zn between groups 2 and 3, B and C, and II and III. For members of groups β and group γ (Table 2), Cu and Zn values were correlated with Gensini scoring and the number of diseased vessels (Table 3), but not with EF or the presence of acute coronary syndrome.
Discussion
Oxidation is an important pathway in the pathogenesis of CAD through oxidation of LDL and free radical formation [14]. Oxidized LDL facilitates the evolution of early arterial wall lesions into atherosclerotic plaques by promoting the formation of foam cells from macrophages as well as the recruitment and retention of monocytes in the arterial wall [15]. Zn and Cu are essential micronutrients for enzymes that catalyze oxidation–reduction reactions [16].
We aimed to determine the relationship between serum levels of Zn and Cu and severity of the coronary atherosclerosis. In our study, we have detected lower serum levels of zinc and copper approximately 25% in patients with atherosclerosis than the control group. These findings suggest that low levels of this elements may play a role in atherosclerotic process, but it may also a result of atherosclerosis, but this association could not be correlated with the severity of the atherosclerosis, the presence of acute coronary syndrome, or EF values.
Many studies have found that serum Zn levels are significantly lower in patients with coronary disease than in healthy subjects [17, 18]. The literature is, however, controversial about the relationship between serum Cu levels and CAD [18–20]. One study has been found that diabetic patients with serum Zn concentration ≤14.1 μmol/l at baseline had a higher risk of death from CAD than >14.1 μmol/l (20.8% and 12.8%, respectively; p = 0.001) [21]. Oster et al. [22] in a small study found that serum Zn and Cu levels were significantly lower in patients with multiple CAD than in healthy subjects. Klevay [19] had proposed that Cu deficiency, rather than excess, is a risk factor for CAD and summarized its effects on various risk factors, including cholesterol level, blood pressure, glucose tolerance, and electrocardiographic abnormalities. Patients were not grouped according to the severity of atherosclerosis in any study abovementioned. Aortic occlusive disease and abdominal aortic aneurysm were compared only in one study but the control group was not. Patients with aortic occlusive disease had significantly lower serum levels of Cu than those with abdominal aortic aneurysm [23].
Ford [18] investigated mortality rates in patients with CAD and serum Cu levels. Any correlation between Cu levels and cardiovascular and total mortality were not found. Comparison of Cu levels and patients’ characteristics showed positive correlations between Cu levels and cholesterol, blood pressure, age, and BMI. Serum Cu levels were heightened in the presence of DM and in smokers. The surviving individuals were not separated into groups according to the presence or absence of CAD.
Elevated Cu concentrations may be related to CAD in at least two ways. Copper oxidizes LDL which increases its atherogenicity [24], and it may be a risk marker for inflammation, rather than a direct risk factor for CAD and may actually be involved in the pathogenesis of atherosclerosis [25].
In a study performed in patients with acute coronary syndrome, by Altekin et al. [26], a relationship between cardiac markers and trace elements was found. A positive correlation between serum Cu levels with elevated troponin T, troponin I, and CK-MB values (≥0.9, ≥1.0, and ≥30 ng/ml, respectively) and a negative correlation between troponin T and Zn were found. The same results were declared by Cikim et al. [27] in patients with acute coronary syndrome. Neither of these studies included stable coronary artery disease. In our study, serum Zn and Cu levels in patients with acute coronary syndrome were lower than in the control group. However, serum Zn and Cu levels in patients with stable coronary artery disease and acute coronary syndrome were similar.
Shokrzadeh et al. [28] determined the levels of Zn and Cu in 30 patients with ischemic cardiomyopathy diagnosed with coronary angiography. They found higher serum Cu levels in the patient groups than in the healthy subjects, but Zn levels were not. Higher serum Cu levels and lower serum Zn levels were detected in class III New York Heart Association (NYHA) patients than in class II. In another study, serum Cu, Zn, and Fe levels were determined in patients with dilated cardiomyopathy [29]. While Cu and Zn levels were consistent with the NYHA classification, there was a strong correlation between increased serum Cu levels and EF and the cardiac index. Oster et al. [22], however, found the same negative correlation between EF and serum Cu levels but not serum Zn levels. In our study, serum Zn and Cu levels were lower in ischemic patients with EF under 45% than control group, although no correlation was found between EF values and serum Zn and Cu levels. A comparison of Zn vs. Cu levels between patients with EF < 45% and those with EF > 45% showed no significant difference.
The serum Cu concentration may not reflect actual Cu status accurately [30]. It can be determined in several ways: serum or plasma Cu, ceruloplasmin, and tissue or organ Cu [31]. However, Oster et al. [22] found no association between concentrations of Zn and Cu in serum and the corresponding concentrations in heart tissue. Because of the ease of procedure, we measured concentrations of Zn and Cu in serum.
Enrolling the small number of patients to the study may be our study’s limitation, but in fact the studies performed about this subject in the literature were smaller than our study until now.
Conclusion
Our study confirmed the basic relationship between serum Zn and Cu levels and atherosclerosis, but did not reveal an association between Zn and Cu levels and severity of atherosclerosis. Hence, further and more comprehensive studies are needed to prove this relationship between these elements levels and the severity of atherosclerosis.
References
Klevay L (1975) Coronary heart disease: the zinc/copper hypothesis. Am J Clin Nutr 28:764–774
Perry DK, Smyth MJ, Stennicke HR et al (1997) Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem 272:18530–18533
King JC, Cousins RJ (2006) Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ (eds) Modern nutrition in health and disease. Lippincott Williams and Wilkins, Philadelphia, pp 271–285
Braunwald E, Kloner R (1985) Myocardial reperfusion: a double-edge sword? J Clin Invest 76:1713–1719
Goldhaber JI, Weiss J (1992) Oxygen free radicals and cardiac reperfusion abnormalities. Hypertension 20:118–127
Meerson FZ, Kagan VE, Kozlov YuP et al (1982) The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart. Basic Res Cardiol 77:465–485
Banerjee A, Grosso MA, Brown J et al (1991) Oxygen metabolite effects on creatine kinase and cardiac energetics after reperfusion. Am J Physiol 261:590–597
Neubauer S, Hamman B, Perry S et al (1988) Velocity of the creatine kinase reaction decreases in postischemic myocardium: a 31P NMR magnetization transfer study of the isolated ferret heart. Circ Res 63:1–15
Prasad AS (1991) Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr 53:403–412
Judkins MP (1967) Selective coronary arteriography. A percutaneous transfemoral technic. Radiology 89:815–824
Gensini GG (1983) A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 51:606
Oishi Y, Wakatsuki T, Nishikado A et al (2000) Circulating adhesion molecules and severity of coronary atherosclerosis. Coron Artery Dis 11:77–81
Fridewald WT, Levy RI, Frederickson DS (1972) Estimation of the low density lipoproteins separated by the three different methods. Clin Chem 1:499–502
Steinberg D, Parthasarathy S, Carew TE et al (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320:915–924
Fuster V, Badimon L, Badimon JJ et al (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 326:310–318
Linder MC, Hazegh-Azam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S–811S
Kazemi-Bajestani SM, Ghayour-Mobarhan M, Ebrahimi M et al (2007) Serum copper and zinc concentrations are lower in Iranian patients with angiographically defined coronary artery disease than in subjects with a normal angiogram. J Trace Elem Med Biol 21:22–28
Ford ES (2000) Serum copper concentration and coronary heart disease among US adults. Am J Epidemiol 151:1182–1188
Klevay LM (1989) Ischemic heart disease as copper deficiency. Adv Exp Med Biol 258:197–208
Salonen JT, Salonen R, Korpela H et al (1991) Serum copper and the risk of acute myocardial infarction: a prospective population study in men in eastern Finland. Am J Epidemiol 134:268–276
Soinio M, Marniemi J, Laakso M et al (2007) Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 30:523–528
Oster O, Dahm M, Oelert H et al (1989) Concentrations of some trace elements (Se, Zn, Cu, Fe, Mg, K) in blood and heart tissue of patients with coronary heart disease. Clin Chem 35:851–856
Koksal C, Ercan M, Bozkurt AK et al (2007) Abdominal aortic aneurysm or aortic occlusive disease: role of trace element imbalance. Angiology 58:191–195
Heinecke JW, Rosen H, Chait A (1984) Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest 74:1890–1894
Liuzzo G, Biasucci LM, Gallimore JR et al (1994) The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 331:417–424
Altekin E, Coker C, Sisman AR et al (2005) The relationship between trace elements and cardiac markers in acute coronary syndromes. J Trace Elem Med Biol 18:235–242
Cikim G, Canatan H, Gursu MF et al (2003) Levels of zinc and lipid peroxidation in acute coronary syndrome. Biol Trace Elem Res 96:61–69
Shokrzadeh M, Ghaemian A, Salehifar E et al (2009) Serum zinc and copper levels in ischemic cardiomyopathy. Biol Trace Elem Res 127:116–123
Oster O (1993) Trace element concentrations (Cu, Zn, Fe) in sera from patients with dilated cardiomyopathy. Clin Chim Acta 214:209–218
Klevay LM (1992) Re: “Serum copper and the risk of acute myocardial infarction: a prospective population study in men in eastern Finland”. Am J Epidemiol 135:832–834
Lönnerdal B (1996) Bioavailability of copper. Am J Clin Nutr 63:821S–829S
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islamoglu, Y., Evliyaoglu, O., Tekbas, E. et al. The Relationship Between Serum Levels of Zn and Cu and Severity of Coronary Atherosclerosis. Biol Trace Elem Res 144, 436–444 (2011). https://doi.org/10.1007/s12011-011-9123-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9123-9