Abstract
Chromium(III) picolinate, [Cr(pic)3], is a commonly used nutritional supplement in humans, which has also been approved for use in animals. Health concerns have arisen over the use of [Cr(pic)3]. At high [Cr(pic)3] doses, developmental toxicity tests in female mice have shown a higher litter incidence of split cervical arch in exposed fetuses, but this was not consistently reproducible. In the current study, male CD-1 mice were used to further assess the potential for reproductive or developmental toxicity. Four weeks prior to mating, the males were fed a diet providing 200 mg/kg/day [Cr(pic)3] for comparison with untreated controls. Females were not treated. Each male was mated with two females, which were sacrificed on gestation day 17, and their litters were examined for adverse effects. Mating and fertility indices were not significantly altered by treatment. Male exposure to [Cr(pic)3] also had no effect on prenatal mortality, fetal weight, or gross or skeletal morphology. These results suggest that paternal dietary exposure to chromium(III) picolinate has little potential for adverse reproductive effects, even at exposure levels considerably higher than expected human exposures from nutritional supplements (1 mg of Cr per day or less).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium(III) is generally thought to enhance glucose tolerance and carbohydrate and lipid metabolism in mammals when metabolism is compromised [1]. While a recent study indicates that chromium is not an essential trace element for mammals [2], it has, nevertheless, been accepted as essential for the last 30 years [3]; as a result, chromium supplements have been one of the most popular mineral supplements for the last two decades. As dietary chromium is not readily absorbed (about 0.5–2%) [4], the coupling of chromium to a suitable ligand is necessary to increase its bioavailability. Chromium picolinate [Cr(pic)3] has traditionally been one of the most popular forms of supplemental chromium. It has been added to dietary supplements, “weight loss” vitamins, and “health” shakes for years and is touted as an aid in promoting fat loss and lean muscle gain. [Cr(pic)3] has also been suggested as a treatment for metabolic disorders, such as type 2 diabetes, as some studies suggest that it is able to increase insulin sensitivity and lower serum glucose. However, the heterogeneity across these studies and their overall limitations restrict their ability to provide firm conclusions [5].
The safety of [Cr(pic)3] supplementation remains controversial. Stearns and coworkers were the first to report deleterious effects of [Cr(pic)3] when they demonstrated that it was clastogenic [6] and later that it was mutagenic in Chinese hamster ovary cells [7]. Chromium from [Cr(pic)3] accumulates in cells and has nuclear affinity, implying that supplemental levels over long periods of time might be harmful [8]. While [Cr(pic)3] was not found to be mutagenic in Salmonella typhimurium, it was found to induce mutagenic responses in the L5178Y mouse lymphoma mutation assay [9]. Chromium picolinate has also been found to be capable of cleaving DNA under physiologically relevant conditions [10]. Recent studies have failed to demonstrate that commercially prepared [Cr(pic)3] was clastogenic or mutagenic [11, 12]. However, these apparently conflicting results have recently been reconciled, as the studies in which damage was not observed used dimethyl sulfoxide, a free radical trap which could quench reactive oxygen species, as a solvent for [Cr(pic)3] [13]. Our laboratory has published findings that mated female mice exposed to high levels of chromium picolinate resulted in progeny with an increased incidence of cervical arch defects [14]. However, although a similar trend was observed in more recent studies, the differences from control values for this parameter were not statistically significant [15, 16].
Concerns about potential effects on fertility due to exposure of the male parent have also arisen. Studies by Hepburn et al. [17, 18] indicated that exposure of male and female Drosophila melanogaster to [Cr(pic)3] resulted in defects to offspring, including developmental delays, lethality during development, and significant levels of germ-line lethal and semilethal mutations. For example, placing male and female Drosophila on food containing [Cr(pic)3] at concentrations similar to human supplemented diets resulted in delays in the development of progeny, and the number of progeny successfully reaching the pupal stage was also diminished. The effect was dependent on the concentration of [Cr(pic)3] in the food. A systematic analysis of germ-line mutagenicity from exposing male Drosophila to [Cr(pic)3] via the food revealed that the compound enhanced the rate of appearance of lethal mutations and dominant female sterility [17].
No mammalian studies have ever examined the effects of male exposure to [Cr(pic)3] on these parameters despite the fact that [Cr(pic)3] is marketed as a fat burner and muscle gainer (products typically attractive to males). This raises the question of whether [Cr(pic)3] is significantly more deleterious as a developmental toxin when administered to male subjects or whether the mechanism of the deleterious activity in Drosophila is not manifested in mammals. Thus, the current study examined the effects on fertility and fecundity of premating exposure of males to Cr(pic)3.
Materials and Methods
Breeding and Exposure
Male and female CD-1 mice, obtained from Charles River Breeding Laboratories, International (Wilmington, MA) were housed in an AAALAC-approved animal facility in rooms maintained at 22 ± 2°C, with 40–60% humidity and a 12-h photoperiod. Males were randomly assigned to one of two treatment groups: (1) control, untreated rodent diet or (2) [Cr(pic)3], 200 mg/kg body mass per day (25 mg Cr per kilogram body mass per day). After a 2-week acclimation period, males were fed the appropriate test diet for 4 weeks prior to mating. Animals were mated naturally, two females with one male, for 1 week. Observation of a copulation plug in the vagina designated gestation day 0 (GD 0). Mated females were individually housed in shoe box-type cages with hardwood bedding and given Harlan Teklad rodent chow and water ad libitum.
Chromium(III) picolinate, [Cr(pic)3], was synthesized according to the method of Press et al. [19], and authenticity was established by high-resolution electron impact mass spectrometry [20]. LM-485 milled rodent diet was purchased from Harlan Teklad (Madison, WI). [Cr(pic)3] was added to milled rodent chow in sufficient quantities to achieve the appropriate concentration of the test compound. All calculations were based on data from previous studies, which indicated that CD-1 mice consume an average of 7 g diet per day. The dosage of [Cr(pic)3] was based on results from a previous study in which treatment of dams during gestation with 25 mg of Cr per kilogram per day as [Cr(pic)3] was associated with a significant increase in the incidence of cervical arch defects compared to control animals [14]. Extensive stability studies indicate that [Cr(pic)3] is extremely stable and that no degradation in the diet would be expected [20].
Because the purpose of this study was to determine the effects of pharmaceutical levels of chromium, no special measures were taken to prevent exposure of the mice to small amounts of chromium that may be introduced into the diet through methods of feed preparation or from the cage hardware. The diet purchased, Teklad LM-485 (7012), contained added chromium in the form of chromium potassium sulfate (0.48 mg/kg of diet).
Data Collection
On GD 17, mated females were euthanized by CO2 overdose; their uteri were exposed, and the number of resorptions and dead or live fetuses were recorded. Live fetuses were removed from the uterus, weighed individually, and examined for gross malformations. Maternal body weight, minus the gravid uterine weight, was then obtained. Fetuses were initially fixed in 70% ethanol and then cleared and stained by the double-staining technique described by Webb and Byrd [21]. All fetuses were subsequently examined for skeletal abnormalities.
Statistical Methods
The litter was used as the experimental unit for statistical analysis. This study was performed in multiple replicates. The data from each study replicate were calculated independently, tested for homogeneity of variance by means of the Levene statistic, using SPSS (SPSS, Inc., Chicago, IL), and then pooled and analyzed to give the results reported. All tabular data are presented as the mean ± standard error. Data were analyzed by one-way analysis of variance followed by a least significant difference post hoc test to determine specific significant differences (P ≤ 0.05).
Results
Fertility Studies
The mating indices for control and treated males were identical (100%, Table 1). The fertility indices for males treated with [Cr(pic)3] was lower compared to control males (95.7% versus 100%), but the difference was not statistically significant. Exposure of the males to [Cr(pic)3] did not affect the average number of implantations in females (Table 1).
Fetal Development
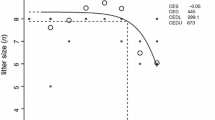
An increase in the average number of total resorbed or dead fetuses was observed in the treated group as compared to controls; however, the increase was not statistically significant (Table 2). No significant effect of exposure was seen on gross or skeletal malformations (split cervical arch) or skeletal variations (rudimentary or supernumerary ribs). Fetuses sired by exposed males weighed significantly more than fetuses of unexposed males (Table 2). However, this difference is extremely small and is probably a statistical anomaly.
Discussion
The potential toxicity of [Cr(pic)3] has recently been an area of intense debate, but a consensus is currently being realized. In in vitro studies, [Cr(pic)3] has reproducibly been shown to interact with reductants and dioxygen to generate reactive species capable of causing oxidative damage, including DNA damage [1]. The picolinate ligands alter the redox properties of the Cr center such that it is more susceptible to undergoing redox chemistry in the body than hexaaquoCr(III). In mammalian cell culture studies and studies in which the complex is given intravenously, [Cr(pic)3] is clearly toxic and mutagenic, unlike other commercial forms of Cr(III) [1] (Recently studies funded by the company selling the supplement observed no effects in cell culture studies [11, 12]; however, subsequent research has shown that DMSO (used as a solvent for the supplement and one that can serve as a radical trap) quenched the deleterious effects [13]). However, when given orally to mammals, the complex does not appear to be toxic nor appear to be a mutagen or carcinogen [1]. An NIH study of the effects of up to 5% of the diet (by mass) of rats and mice for up to 2 years found no harmful effects on female rats or mice and, at most, ambiguous data for one type of carcinogenicity in male rats (along with no changes in body mass in either sex of rats or mice) [22]. These studies can readily be reconciled [1]. Only 1% of absorbed Cr from the supplement is found in the bloodstream as [Cr(pic)3], suggesting that little of the intact molecule is absorbed [23]. When ingested, the complex probably hydrolyzes near the stomach lining, releasing the Cr, which is subsequently absorbed. The hydrolysis of the complex in the stomach is probably fortuitous, releasing the chromium before the intact complex can be absorbed to an appreciable level, in contrast to the cell studies where the very stable, neutral complex could be absorbed intact [1]. Recently, the European Food Safety Authority determined that chromium picolinate added for nutritional purposes to foodstuffs for particular nutritional purposes and to foods intended for the general population “would not be of concern provided that the amount of total chromium does not exceed 250 μg/day” [24].
In contrast, studies with D. melanogaster found that [Cr(pic)3] generated appreciable delays in the development of larvae and lower success rates in pupation and eclosion [17]. [Cr(pic)3] had detrimental effects on the longevity of male and female Drosophila at a nutritionally relevant dosage [18]. Thus, [Cr(pic)3] and picolinic acid have detrimental effects on essentially all stages of the life cycle of Drosophila. At the same dosage, [Cr(pic)3] was found to generate approaching one mutation per chromosome per individual and 12% sterility. The ability of [Cr(pic)3] to generate chromosomal aberrations in polytene chromosomes of the salivary glands of Drosophila larvae was also examined [18]. In the chromium picolinate-treated group, 53% of the identified chromosomal arms were positively identified as containing one or more aberrations while no aberrations were observed for the identified chromosomal arms of the control group. Thus, in contrast to mammals, [Cr(pic)3] is a developmental toxin and clastogen, suggesting that [Cr(pic)3] is absorbed intact by Drosophila.
However, the current work utilizing male mice, in combination with earlier studies by Bailey, et al. [14–16], indicates that in mammals, independent of whether [Cr(pic)3] is administered to breeding male or female mice, the compound is not a developmental toxin at any reasonable dose.
References
Vincent JB (2010) Chromium: celebrating 50 years as an essential element? Dalton Trans 39:3787–3794
Di Bona KR, Love S, Rhodes NR et al (2011) Chromuim is not an essential trace element for mammals: effects of a “low chromium” diet. J Biol Inorg Chem (in press)
National Research Council (1980) Recommended dietary allowances, 9th ed. Report of the Committee on Dietary Allowances, Division of Biological Sciences, Assembly of Life Science, Food and Nutrition Board, Commission on Life Science, National Research Council. National Academy Press, Washington, D.C
Anderson RA, Kozlovsky AS (1985) Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am J Clin Nutr 41:1177–1183
Balk E, Tatsioni A, Lichtenstein A et al (2007) Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diab Care 30:2154–2163
Stearns D, Wise SJ, Patierno S et al (1995) Chromium(III) picolinate produces chromosome damage in Chinese hamster ovary cells. FASEB J 9:1643–1648
Stearns D, Silveira S, Wolf K et al (2002) Chromium(III) tris(picolinate) is mutagenic at the hypoxanthine (guanine) phosphoribotransferase locus in Chinese hamster ovary cells. Mutat Res 513:135–142
Stearns D, Belbruno J, Wetterhahn K (1005) A prediction of chromium(III) accumulation in humans from chromium dietary supplements. FASEB J 9:1650–1657
Whittaker P, San R, Clarke J et al (2005) Mutagenicity of chromium picolinate and its components in Salmonella typhimurium and L5178Y mouse lymphoma cells. Food Chem Toxicol 43:1619–1625
Speetjens JK, Collins RA, Vincent JB et al (1999) The nutritional supplement chromium(III) tris(picolinate) cleaves DNA. Chem Res Toxicol 12:483–487
Gudi R, Slesinski R, Clarke J et al (2005) Chromium picolinate does not produce chromium damage in CHO cells. Mutat Res 587:140–146
Slesinski R, Clarke J, San R et al (2005) Lack of mutagenicity of chromium picolinate in the hypoxanthine phosphoribosyltransferase gene mutation assay in Chinese hamster ovary cells. Mutat Res 585:86–95
Coryell V, Stearns D (2006) Molecular analysis of hprt mutations induced by chromium picolinate in CHO AA8 cells. Mutat Res 610:114–123
Bailey MM, Boohaker JG, Sawyer RD et al (2006) Exposure of pregnant mice to chromium picolinate results in skeletal defects in their offspring. Birth Defects Res B 77:244–249
Bailey MM, Jernigan PL, Townsend MB et al (2008) Comparison of the potential for developmental toxicity of prenatal exposure to two dietary chromium supplements, chromium picolinate and [Cr3O(O2CCH2CH3)6(H2O)3]+, in mice. Birth Defects Res B 83:27–31
Bailey MM, Bohaker JG, Jernigan PL et al (2008) Effects of pre- and postnatal exposure to chromium picolinate and picolinic acid on neurological development. Biol Trace Elem Res 124:70–82
Hepburn DDD, Xiao J, Bindom S et al (2003) Nutritional supplement chromium picolinate causes sterility and lethal mutations in Drosophila melanogaster. Proc Natl Acad Sci USA 100:3766–3771
Stallings DM, Hepburn DDD, Hannah M et al (2006) Nutritional supplement chromium picolinate generates chromosomal aberrations and impedes progeny development in Drosophila melanogaster. Mutat Res 610:101–113
Press R, Gellar J, Evans G (1990) The effects of chromium picolinate on serum cholesterol and apolipoprotein fractions in human subjects. West J Med 152:41–45
Chakov NE, Collins RA, Vincent JB et al (1999) A re-investigation of the electronic spectra of chromium(III) picolinate complexes and high yield synthesis and characterization of Cr2(m-OH)2(pic) .4 5H2O (Hpic = picolinic acid). Polyhedron 18:2891–2897
Webb G, Byrd R (1994) Simultaneous differential staining of cartilage and bone without glacial acetic acid. Biotech Histochem 69:181–185
Stout MD, Nyska A, Collins BJ et al (2009) Chronic toxicity and carcinogenicity studies of chromium picolinate monohydrate administered in feed to F344/N rats and B6C3F1 mice for 2 years. Food Chem Toxicol 47:729–733
Research Triangle Institute, Project Report (2002) [14C]Chromium picolinate monohydrate: disposition and metabolism in rats and mice, submitted to National Institutes of Environmental Health Sciences
EFSA panel on Food Additives and Nutrient Sources added to Food (ANS) (2010) Scientific opinion on the safety of chromium picolinate as a source of chromium added for nutritional purposes to foodstuff for particular nutritional uses and to foods intended for the general population. EFSA J 8(12):1883
Acknowledgments
F.B. and C.G. were supported in part by NSF REU grant (CHE 0647789) to J.B.V. and Stephen A. Woski.
Conflict of Interest Statement
J.B.V. is the inventor or co-inventor of four patents on the use of Cr3, [Cr3O(O2CCH2CH3)6(H2O)3]+, as a nutritional supplement or therapeutic agent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McAdory, D., Rhodes, N.R., Briggins, F. et al. Potential of Chromium(III) Picolinate for Reproductive or Developmental Toxicity Following Exposure of Male CD-1 Mice Prior to Mating. Biol Trace Elem Res 143, 1666–1672 (2011). https://doi.org/10.1007/s12011-011-9002-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9002-4