Abstract
Research has investigated the participation of zinc transport proteins and metallothionein in the metabolism of this mineral. However, studies about the genetic expression of these proteins in obese patients are scarce. The study determined the expression of zinc transporter protein codifying genes (ZnT-1, Zip-1 and Zip-3) and of metallothionein in 55 obese women, aged between 20 and 56 years. The assessment of body composition was carried out using anthropometric measurements and bioelectrical impedance. Zinc intake was obtained by recording diet over a 3-day period, and the nutritional analysis was carried out using NutWin software version 1.5. The plasmatic and erythrocytary zinc were analyzed by atomic absorption spectrophotometry (λ = 213. 9 nm). The determination of mRNA expression of the zinc transporter proteins and metallothionein was carried out using blood, using the RT-PCR method. The mean values of body mass index were 37.9 ± 5.5 kg/m2. The average intake of zinc was 9.4 ± 2.3 mg/day. The analysis of the zinc plasma concentrations showed values of 58.4 ± 10.9 μg/dL. The mean values of zinc in the erythroytes were 38.7 ± 9.1 μg/g Hb. The metallothionein gene had a higher expression in the blood, when compared to zinc transporters ZnT-1, Zip-1, and Zip-3 (p = 0.01). The study shows that there are alterations in the biochemical parameters of zinc in obese patients assessed, as well as higher expression of the codifying gene metallothionein, when compared to the investigated zinc transporters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is an essential micronutrient that plays a basic role in physiology, cellular metabolism, and in genetic expression. This mineral participates in energy metabolism as a catalytic component of more than 300 metalloenzymes [1, 2].
Recently, research carried out with the human genome database, estimated that about 10% of human proteome consists of proteins potentially linked to zinc [3]. Various biological processes are zinc dependent, and an imbalance in the homeostasis of this mineral has complex implications for some organs, which can contribute to the onset of chronic illnesses [4].
Zinc participates in the metabolism of the hormones involved in the physiopathology of obesity, such as, insulin, and the thyroid hormones [5, 6]. Studies have demonstrated low concentrations of zinc in the plasma, erythrocyte, and serum of obese individuals [7–9], associated with alterations in the metabolism of the adipose tissue of these patients.
Recent advances in methods of assessing the molecular aspects of zinc's cellular biology, using mRNA expression of zinc transporter proteins (ZnTs and Zips) and of metallothionein, have become a new tool for the investigation of zinc-related nutritional status [10, 11].
Some investigations have already demonstrated that metallothioneins play a part in the transport, storage, and distribution of zinc. It is thought that these proteins are responsible for the capture of zinc, when its level is high, protecting cells against toxicity [12, 13].
The zinc transporter proteins are transmembranous proteins that assure the carriage of zinc ions through biological membranes. They are specialized in the capture, efflux, and compartmentalization of zinc, and help maintain the intracellular levels of this mineral and its adequate distribution in the tissues [4, 13, 14]. The proteins of the Zip family transport the extracellular zinc or zinc of the intracellular vesicles into the cytoplasm, and the ZnT family controls the opposite route of this transport [4, 15].
Bearing in mind how important obesity is as a chronic illness, the secretion of diverse adipocytokines and other proteins in the adipose tissue, the interaction of these metabolytes in the metabolism of zinc, as well as the role of the mineral as a co-factor in some important enzymatic reactions for the homeostasis of the organism, the determination of the genetic expression of proteins that participate in the metabolism of zinc in obese patients can help one understand the metabolism of this mineral in obesity.
Therefore, the aim of this study is to evaluate the expression of zinc transporter protein codifying genes and metallothionein in obese female patients.
Methods
A transectional study was carried out with 55 obese women, aged between 20 and 56 years, who randomly sought treatment at an endocrinology clinic.
Patients who turned up at the clinic were selected for the study if they met the following criteria: their body mass index (BMI) was higher than 30 kg/m2, they were not taking any vitamin–mineral supplementation and/or other medicines, and they did not have any illnesses that could interfere with zinc-related nutritional status. The research was approved by the Committee of Ethics in Research of the Federal University of Piauí, and the individuals gave written consent.
Anthropometric Parameters
Body mass index was calculated using measures of weight and height. The classification of obesity according to BMI was carried out in line with the criteria of the World Health Organization [16]: The assessment of the percentage of fat of the participants was carried out using biolectrical impedance analysis.
Evaluation of Alimentary Consumption
The zinc consumption was obtained by recording alimentation over a 3-day period, and the nutritional analysis was made using NutWin software version 1.5. [17]. The Estimated Average Requirement (EAR) reference values of zinc used were 6.8 mg/day, for females [18].
Determination of Zinc in the Plasma and the Erythrocytes
Blood samples (10 ml) were collected during the morning, from 7:30 am to 9:00 am, while the patients were fasting. The blood was placed in glass test tubes containing 30% sodium citrate as an anticoagulant (10 μg/ml of blood) for zinc analysis.
The plasma was separated from the total blood by centrifugation at 3,000×g for 15 min at 4°C. Two aliquots of each plasma sample were diluted at a ratio of 1:4 with Milli-Q® water and aspirated directly into the flame of the instrument. Tritizol® (Merck), prepared by dilution with Milli-Q® water with 3% glycerol at 0.1-, 0.2-, 0.3-, 0.5-, and 1.0-μg/ml dilutions was used as a standard.
For the separation of the erythrocyte, the erythrocyte mass obtained from the total blood was washed three times with 5 mL of 0.9% saline solution, homogenized by inversion, and centrifuged at 10,000×g for 10 min (SORVALL® RC-SB) at 4°C, and the supernatant was discarded. After the last centrifugation, the saline solution was aspirated and the erythrocyte mass extracted with a micropipette, placed in demineralized “eppendorf” tubes, and stored at −20°C, for zinc and hemoglobin analyses [19]. To express the results in terms of zinc mass/hemoglobin mass (micrograms per gram Hb), a 20-μL aliquot of lysed erythrocyte was diluted in 5 mL of Drabkin solution and measured according to the cianometahemoglobin method [20].
The plasma and the erythrocytes analysis was carried out using atomic absorption spectrophotometry [21]. Tritzol was used as a reference, prepared by dilution in Milli-Q water at concentrations of 0.1, 0.2, 0.3, 0.5, and 1.0 μgZn/ml.
RNA Purification and Quantification
Total RNA was extracted from blood samples using TRIzol Reagent (Invitrogen) according to the manufacturer's protocol. The measurement of concentration and pureness of extracted RNA was carried out using a 2-μL aliquot of each sample in a NanoDrop spectrophotometer. The reading was taken at 260- and 280-nm wavelength. The obtained ratio determined the pureness of the sample, and was considered ideal when greater or equal to 1.8.
cDNA Synthesis
The first cDNA strand was synthesized from 0.1N of total RNA using Powerscript reverse transcriptase. In brief, total RNA was mixed with oligo dT15 1/10 (7.5 ρmoles), heated at 70°C for 10 min, and then cooled immediately on ice for 2 min. The content was then mixed thoroughly with DNTPs (2.5 mM), reverse transcriptase buffer (10x), 0.1 M of dithiothreitol (DTT), Rnase inhibitor (RNasin), 1 U of reverse transcriptase and ultra pure water. The reaction mixture was incubated at 42°C for at least 90 min and subsequently inactivated by heating at 70°C for 15 min. The RT-PCR reaction was processed in a GeneAmp® PCR System 9700 ThermoCycler.
Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction
The oligonucleotide primers (Invitrogen) for each gene were synthesized in line with sequences previously published by Cousins et al. [22], as shown in Table 1. The expression of Zip-1, Zip-3, ZnT1, and MT (MT1,2) genes was determined using SYBR Green assays, using the β actin gene as a constitutive. The SYBR Green assays were set up using the SYBR green PCR master mix (Applied Biosystems) according to the manufacturer's protocol, except that a 12.5-μL reaction volume was used. The quantitative reverse-transcription polymerase chain reaction assays were performed on ABI prism 7500 real-time PCR system (Applied Biosystems) with cycling conditions as follows: 50°C for 2 min, 95°C for10 min, and then 40 cycles of 95°C for 15 s, 60°C for 1 min. The dissociation curve stage was added to the reaction. The relative copy number was deduced from the corresponding cycle threshold. To correct the input RNA concentration, the relative gene copies were further normalized to the measurement for 18 s, the transcript of which has previously been established as the least variable over a range of zinc conditions (P. A. Walker, unpublished observation). The relative quantity of copies was obtained using the formula: 2−ΔΔCT [23].
Statistical Analysis
The data were processed and analyzed using SPSS software for Windows, version 15.0. ANOVA, followed by Tukey's test was applied to compare the groups regarding the studied variables, at a significance level of p <0.05.
Results
This study was carried out with 55 obese females that were selected according to criteria previously described.
Anthropometric parameters and bioimpedance results used to measure body composition are shown in Table 2. Diet analysis and plasmatic and erythrocytary zinc concentrations are shown in Tables 3 and 4.
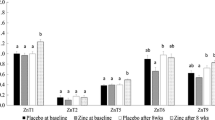
The mRNA expression of zinc transporter proteins ZnT-1, Zip-1 and Zip-3, and of metallothionein are shown in Fig. 1. It can be observed that the metallothionein gene had the highest expression in the patients that were assessed.
Discussion
This study measured the expression of zinc transporter codifying proteins and metallothionein in the blood of obese female patients. The results showed that both metallothionein and the zinc transporter proteins (Zip-1, Zip-3, and ZnT-1) were expressed in the blood of these patients.
Genetic expression was higher for metallothionein than for the analyzed codifying genes of zinc transporter proteins. According to Begin-Heick et al. [24] the visceral accumulation of adipose tissue in obese patients facilitates the secretion of cortisol and pro-inflammatory cytokines, which induces metallothionein expression.
One important factor is the body composition of the assessed patients. These women had high BMIs and waist circumference values greater than 88 cm, which could have contributed to the secretion of inductors of metallothionein expression (cortisol and pro-inflammatory cytokines). Do et al. [25] compared the genetic expression of metallothionein between obese and control patients and identified higher expression of the protein in the adipose tissue of the obese group. The high expression of metallothionein induced by the factors mentioned above can facilitate the reduction of the zinc serum concentrations and the redistribution of this mineral among different tissues, especially in the liver [26, 27].
From this perspective, the compartmentalization and the metabolism of zinc in obesity have been investigated. Begin-Heick et al. [24] verified high zinc concentrations in the adipose tissue, liver, and muscle of obese mice. According to these researchers, the concentration of hormones, such as glycocorticoids, normally high in obesity, would keep the levels of metallothionein bound to zinc high, by a “sequestration” of zinc in some specific tissues.
Another important factor is the influence of dietary zinc on the biochemical parameters of assessment of this mineral. The diets evaluated in this study had adequate concentrations of this mineral, with an EAR value of 6.8 mg/day [18], which suggests that zinc intake was not a determining factor for the reduced zinc concentrations found in plasma and erythrocytes. Considering this fact, it is possible to surmise that the elevated genetic expression of metallothionein in the obese patients was responsible for the alterations in the concentrations of the mineral in the assessed cellular compartments.
There is a scarcity of studies on the role of zinc transporter proteins in the metabolism of this mineral in obese individuals. The results of this study disclosed a high expression of the ZnT-1codifying gene, when compared to Zip-1 and Zip-3. Amongst the analyzed genes, Zip-3 had the lowest expression.
Other studies have assessed the genetic expression of these proteins in specific human blood cells. Aydemir et al. [28] determined the expression of various transporters in three subtypes of leukocytes, and verified high expression of ZnT-1, particularly in monocytes. These authors verified that, as well as ZnT-1, Zip-1, and Zip-3 had high expressions in monocytes. Another study by Overberck et al. [29], demonstrated that in the peripheral mononuclear blood cells, ZnT-1 was the transporter with highest expression, when compared to the other transporters of the ZnT group, however, these authors did not assess the genetic expression of the Zip family.
On the other hand, studies have proved that zinc intake can induce genetic ZnT-1 expression in human leucocytes [28, 29]. However, based on the results from the diet records of the sample in this study, the mean daily intake of zinc was adequate, which suggests that not diet influenced the results.
In a recent study by Smidt et al. [30] the expression of these proteins in the visceral and subcutaneous adipose tissue of obese and non-obese patients was measured. It was shown that the majority of transporters (Zips and ZnTs) had a higher expression in the subcutaneous adipose tissue of the control group. According to these authors, the differences found in the expression of the zinc transporter proteins between abdominal and subcutaneous fat of obese and non-obese individuals, can influence the zinc transport system in adipocytes. However, the possible mechanisms involved were not clarified.
Conclusions
The biochemical parameters assessed in this study indicate that the obese patients had an alteration of nutritional status in relation to zinc, with lower mean plasmatic and erythrocytary concentrations, when compared with the reference parameters. As for the determined genes, it was confirmed that the metallothionein gene had higher expression in obese patients when compared to the zinc transporters (ZnT-1, Zip-1, and Zip-3).
The results suggest that alterations in the concentrations of zinc in the cellular compartments studied can be related to the higher genetic expression of metallothionein. Further studies on the interaction between adipocytokine secretion and glycocorticoids, and the expression of metallothionein in obese patients could help clarify the metabolism and compartmentalization of zinc in obesity. Similarly, the participation of adipose tissue in the expression of zinc transporter protein codifying genes in this disease needs to be investigated further.
References
Mccall KA, Huang C, Fierke CA (2000) Function and mechanism of zinc metalloenzymes. J Nutr 130:1437S–1446S
Macdonald RS (2000) The role of zinc in growth and cell proliferation. J Nutr 130:1500S–1508S
Andreini C, Banci L, Bertini I, Rosato A (2006) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5(1):196–201
Devirgiliis C, Zalewski PD, Perozzi G, Murgia C (2007) Zinc fluxes and zinc transporter genes in chronic diseases. Mutation Res 622(1–2):84–93
Meunier N, O'connor JM, Maiani G, Cashman KD, Secker DL, Ferry M, Roussel AM, Coudray C (2005) Importance of zinc in the elderly: the zenith study. Eur J Clin Nutr 59(2):S1–S4
Gomez-Garcia A, Hernandez-Salazar E, Gonzalez-Ortiz M, Martinez-Abundis E (2006) Efecto de la administracion oral de zinc sobre sensibilidad a la insulina y niveles séricos de leptina y andrógenos em hombres con obesidad. Rev Méd Chile 134:279–284
Konukoglu D, Turhan MS, Ercan M, Serin O (2004) Relationship between plasma leptin and zinc levels and the effect of insulin and oxidative stress on leptin levels in obese diabetic patients. J Nutr Biochem 15:757–760
Marreiro DN, Fisberg M, Cozzolino SMF (2004) Zinc nutritional status and its relationships with hyperinulinemia in obese children and adolescents. Biol Trace Element Res 99:137–150
Marreiro DN, Geloneze B, Tambascia MA, Lerário AC, Halpern A, Cozzolino SMF (2006) Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol Trace Elem Res 112:109–118
Hambidge M (2000) Human zinc deficiency. J Nutr 130:1344S–1349S
Eide DJ (2006) Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta 1763:711–722
Dufner-Beatie J, Langmade SJ, Wang F, Eide D, Andrews GK (2003) Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem 278(50):50142–50150
Seve M, Chimienti F, Devergnas S, Favier A (2004) In silico identification and expression of SLC30 family genes: Na expressed sequence tag data mining strategy for the characterization of zinc transporters tissue expression. BMC Genomics 5:32
Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ (2004) Responsive transporter genes within the murine intestinal- pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci 101(40):14355–14360
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089
World Health Organization 2000 Obesity: Preventing and managing the global epidemic. Geneva. Technical report series, n.894
Anção MS, Cuppari L, Draibe AS, Sigulem D (2002) Programa de apoio à nutrição Nutwin: versão 1.5. Departamento de Informática em Saúde SPDM UNIFESP/EPM1 CD-ROM.
Institute of medicine/food and nutritional board (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, cooper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy, Washington, DC, p 650
Whitehouse RC, Prasad AS, Rabbani PI, Cossack ZT (1982) Zinc in plasma, neutrophils lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin Chem 28:475–480
Van Assendelft OW (1972) The measurement of hemoglobin. In: modern concepts in hematology. Academic, New York, pp 14–25
Rodriguez MP, Narizano A, Demczylo V, Cid A (1989) A simpler method for the determination of zinc human plasma levels by flame atomic absorption spectrophotometry. At Spectrosc 10(2):68–70
Cousins RJ, Blanchard RK, Popp MP, Liu L, Cao J, Moore JB, Green CL (2003) A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci 100(12):6952–6957
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC T method. Methods 25(4):402–408
Begin-Heick N, Dalpe-Scott M, Rowe J, Heick HMC (1985) Zinc supplementation attenuates insulin secretory activity in pancreatic islets of the ob/ob mouse. Diabetes 34:179–184
Do MS, Nam SY, Hong SE, Kim KW, Duncan JS, Beattie JH, Trayhurn P (2002) Metallothionein gene expression in human adipose tissue from lean and obese subjects. Horm Metab Res 34:348–351
Cousins RJ, Leinart A (1988) Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB 2:2884–2890
Schroeder JJ, Cousins RJ (1990) Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci 87:3137–3141
Aydemir TB, Blanchard RK, Cousins RJ (2006) Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci 103(6):1699–1704
Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L (2008) Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukocyte Biology 83(2):368–380
Smidt K, Pedersen SB, Brock B, Schmitz O, Fisher S, Bendix J, Wogensen L, Rungby J (2007) Zinc-transporters genes in human visceral and subcutaneous adipocytes: lean versus obese. Mol Cell Endocrinol 264:68–73
Gibson RS (1990) Assessment of trace element status. In: Gibson RS (ed) Principles of Nutritional Assessment. Oxford University Press, New York, pp 511–576, cap. 24
Guthrie HA, Picciano MF (1994) Micronutrient Minerals. In: Guthrie AH, Picciano MF (eds) Human nutrition. Mosby, New York, pp 351–357
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dos Santos Rocha, P.B.K., de Castro Amorim, A., de Sousa, A.F. et al. Expression of the Zinc Transporters Genes and Metallothionein in Obese Women. Biol Trace Elem Res 143, 603–611 (2011). https://doi.org/10.1007/s12011-010-8887-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8887-7