Abstract
To explore the copper uptake mechanisms by the Cu-tolerant plant Commelina communis, the contents of Cu and other metals (including Fe, Zn, and Mn) in roots were detected using atomic absorption spectrometer under transporter inhibitors, partial element deficiency, or Cu excess treatments, while distribution characters of Cu and other metals in root growth zones were investigated by synchrotron radiation X-ray fluorescence spectroscopy (SRXRF). Cu uptake was inhibited by the uncoupler DNP and P-type ATPase inhibitor Na3VO4, not by the Ca2+ ion channel inhibitor LaCl3, suggesting that Cu could probably be assimilated actively by root and be related with P-type ATPase, but not through Ca2+ ion channel. Fe or Zn deficiency could enhance Cu uptake, while 100 μM Cu inhibited Fe, Zn, and Mn accumulation in roots significantly. Metal distribution under 100 μM Cu treatment was investigated by SRXRF. High level of Cu was found in the root meristem, and higher Cu concentrations were observed in the vascular cylinder than those in the endodermis, further demonstrating the initiative Cu transport in the root of C. communis. Under excess Cu stress, most Fe was located in the epidermis, and Fe concentrations in the endodermis were higher than those in the vascular cylinder, suggesting Cu and Fe competition not only in the epidermal cells but also for the intercellular and intracellular transport in roots. Zn was present in the meristem and the vascular cylinder similar to Cu. Cu and Zn showed a similar pattern. Mn behaves as Zn does, but not like Fe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper is an essential element for normal plant metabolism and has also been reported to be among the most toxic metals [1]. Accumulation of Cu in soils results from various sources including the following: the use of fertilizers, the application of pig and poultry slurries rich in Cu, fungicides, atmospheric deposition from industrial and urban activities, metaliferous mining, or metal processing, which inhibits plant growth and development [2, 3].
Plants can accumulate heavy metals in different plant tissue or cell organelles [4–6] and also can detoxify heavy metals by chelating them to organic acids or proteins [7, 8]. The roots are the sole organs penetrating the soil and thus the ones to be in direct contact with the toxic Cu. It is, therefore, expected that roots are the first to develop the responding symptoms. Studies suggested that much of the Cu accumulated in root tissue is cell wall associated [9]. The casparian strip and endodermis seems to provide selectivity against Cu, with reduced concentrations entering the stele of root [10]. Ni et al. [11] found that most Cu in protoplasts of Elsholtzia splendens was compartmented in the vacuoles. In some plants, Cu tolerance was related to metallothioneins (MTs) and phytochelatins (PCs) [8, 12, 13].
Metal interactions affecting distribution have been noted in various species of plants with additional metals having both positive and negative effects on uptake and deposition of a given metal [14]. The increasing soil Cu concentration was found in oregano to relate to increasing root bio-accumulation of Cu, Fe, and Mn and decreasing of Ca, K, and Zn, respectively [15]. Fe deficiency in the growth medium increased Cu concentration in maize [16] and pea [17]. Fe increase in the nutrient medium suppressed Cu concentration in apple plants [18]. Lin and Wu [19] found that in Lotus purshianus, elevated Cu in soil reduced Zn uptake. However, Luo and Rimmer [20] found that copper increased the uptake of Zn by barley. Bernal et al. [3] reported foliar and root Cu supply affecting differently Fe and Zn uptake in soybean plants. All these studies demonstrated that cation homeostasis in the root will be controlled by metal interactions.
Commelina communis is a Cu-tolerant plant that grows in copper mine areas along the middle and lower streams of the Yangtze River [21, 22]. However, its mechanisms of Cu uptake and tolerance need further elucidation. Many techniques have been studied for the element localization and heavy metal tolerance mechanisms in plants such as electron dispersive X-ray spectroscopy, electron energy loss spectroscopy, and proton-induced X-ray analysis. Compared with these techniques, synchrotron radiation X-ray fluorescence spectroscopy (SRXRF) microprobe is more sensitive and less injurious to cells and also has excellent characteristics such as very high brightness, collimation, polarization, and lower bremsstrahlung, which is the electromagnetic radiation produced by an accelerated electrically charged subatomic particle. Up to now, SRXRF has been extensively applied in many fields such as materials science, life science, medical science, and environmental science [23, 24]. In this study, we investigated the copper uptake mechanisms in root of C. communis under transporter inhibitors and partial Fe or Zn deficiency treatments and revealed Cu and other metal distribution characters in root growth zones by SRXRF.
Materials and Methods
Plant Material and Culture
The seeds of C. communis were collected from the Huajiachi campus of Zhejiang University, China. They were soaked in distilled water overnight and then germinated on moistened cellulose tissue in a porcelain dish at 25°C. After germination, the solution was changed to one-fourth strength of complete nutrient solution. After 10 days, the uniform seedlings were transferred to vessels filled with full-strength nutrient solution containing macronutrients [1.0 mM Ca(NO3)2, 0.5 mM MgSO4, and 0.5 mM K2HPO4] and micronutrients (14.0 μM H3BO3, 3.0 μM MnSO4, 0.3 μM Na2MoO4, 0.5 μM CuSO4, 16.0 μM Fe-EDTA, and 0.3 μM ZnSO4). The pH of solution was adjusted to 6.0. Nutrient solutions were renewed every 3 days. Plants were grown in a controlled environment growth chamber with a 16-h, 25°C day and an 8-h, 20°C night regime, approximately 10 klx light intensity, and 60–70% relative humidity. The 40-day-old plants were subjected to transporter inhibitors and partial Cu, Fe, and Zn deficiency and Cu excess treatments. Each treatment was maintained with three replicates.
Metal Uptake Experiments and Content Analyses
The seedlings were treated with 50 μM 2,4-dinitropheno (DNP) (the uncoupler of oxidative phosphorylation) or 50 μM Na3VO4 (the P-type ATPase inhibitor) for 5 h in nutrient solutions containing 5 or 20 μM CuSO4. In addition, the seedlings were treated with 0.1 mM LaCl3 (the Ca2+ ion channel inhibitor) for 48 h in the nutrient solutions containing 5 or 20 μM CuSO4 .
When plants were 40 days old, Fe or Zn deficiency was induced, replacing the full nutrient solution with a nutrient solution without Fe or Zn for 6 days and then seedlings were treated by 5 or 20 μM CuSO4 under Fe or Zn deficiency and were subsequently harvested after 48 h uptake.
Cu deficiency was induced, replacing the full nutrient solution with a nutrient solution without Cu for 6 days, and then the seedlings were harvested for metal determination. In addition, seedlings were treated by 0.5 (control), 5, 20, 50, 100, and 200 μM CuSO4 for 15 days, and then samples of roots were taken for metal content analyses. Subsamples of 100 μM CuSO4-treated roots were used for SRXRF measurements.
The roots of harvested seedlings were desorbed with 5 mM Pb(NO3)2 for 15 min and then rinsed thoroughly with deionized water and dried at 80°C. Subsamples of ground tissue were weighed (0.5 g) and digested with HNO3 and HClO4 (HNO3:HClO4 = 4:1). The clear digest samples were then transferred quantitatively to 25 mL calibrated flasks with deionized water, and the metal concentrations were determined using atomic absorption spectrometer. Blanks and a standard reference material were analyzed concurrently for accuracy assurance. Each sample was in triplicate.
Metal Distribution Detected by SRXRF
The 100-μM CuSO4-treated roots were cut off from plant and then frozen by liquid nitrogen. A longitudinal section (50 μm thick) around meristem and cross sections (50 μm thick) in elongation tissue (around 0.5 cm above the root apex) and in non-growing tissue (around 5 cm above the root apex) were cut with a cryotome (HM505E, MICROM CO.) at a temperature of −20°C and subsequently freeze-dried.
The SRXRF experiments were performed at the XRF microprobe station in Beijing Synchrotron Radiation Facility (BSRF). The X-ray light source comes from the 4W1B beam line at BSRF. This beam line can provide multi-chromatic X-rays (white light), whose energy range from 3.5 to 35 keV. The electron energy in the storage ring is 2.2 GeV, with a current range from 70 to 140 mA. The XRF microprobe experimental station lies 25 m away from the point of the light source. Because the emission angles of the light in horizontal and vertical directions are only 1.0 and 0.1 mrad, respectively, this high collimation confers superior performance on the synchrotron radiation XRF microprobe. XRF spectra were collected using a PGT Si (Li) solid detector, positioned at 90° to the beam line, 7 mm from the target. The apparatus of SRXRF in BSRF was reported in detail by Huang et al. [25]. During the SRXRF scanning experiments, the spot size of the X-ray beam was adjusted to 30 × 30 μm2. The scanning dot moved according to the displacement ΔX = 50 μm, ΔY = 50 μm, and each dot had been scanned for 60 s. The scanning areas of sections from different root growth tissue are shown in Fig. 6. All the SRXRF spectra were analyzed by AXIL.
Statistical Analysis
The data were statistically analyzed with one-way analysis of variance (ANOVA) using the SPSS 16.0 for Windows. The results were expressed as means of three replicates. Bars in the figures indicated standard deviation (SD) of the data. A probability level of 0.05 was considered to be statistically significant.
Results
Cu Uptake by Root under Inhibitor or Fe and Zn Deficiency
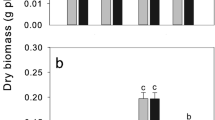
DNP is known as an uncoupler of oxidative phosphorylation, while vanadate is thought to inhibit P-type ATPases by blocking the shift in conformation states during the reaction cycle of these enzymes [26]. The influence of DNP and Na3VO4 on Cu uptake was shown in Fig. 1. Cu concentrations in roots decreased when adding DNP and Na3VO4 into solution containing 5 or 20 μM Cu (P < 0.05), which showed proper initiative Cu uptake in C. communis. The Ca2+-ATPases inhibitor LaCl3 did not inhibit Cu uptake in solution containing 5 or 20 μM Cu (Fig. 2), suggesting that Cu uptake by C. communis was not through Ca2+ ion channel.
Influence of the uncoupler and P-type ATPase inhibitor on the Cu uptake of C. communis. Seedlings were treated with 50 μM DNP or 50 μM Na3VO4 in nutrient solution containing 5 or 20 μM CuSO4. Plants were harvested after 5 h. Values are means ± SD, n = 3. Different letters (a, b, c...) in each histogram indicate a significant difference at the 0.05 level. The letters in this figure and those in Figs. 2, 3, 4 have the same meaning
Under micronutrient deficiency conditions, induction of high-affinity transporters occurs, whereas under toxic conditions, efflux pumps or sequestration into intracellular compartments serves to restrict the concentration in the cytoplasm [27]. Cu concentrations in Fe deficiency root were 2.9-fold or 3.7-fold higher than that in the control root, when the seedlings were treated with 5 or and 20 μM CuSO4, respectively (Fig. 3). Zn deficiency did not increase Cu concentration of the root under 5 μM Cu treatment (Fig. 3), but increased 1.8 times under 20 μM Cu treatment (P < 0.05), suggesting that Zn deficiency could induce Cu absorption in root under high excess Cu.
Metal Accumulation in Root Affected by Cu
Interactions between metals depend on Cu exposure. Cu deficiency and Cu excess differently alter these interactions and the balance among specific micronutrients. The effect of Cu deficiency on Fe, Mn, and Zn uptake was shown in Fig. 4. Fe concentrations in the Cu-deficient, treated roots were increased 150% compared with the control roots, while Mn and Zn concentrations were not promoted significantly by Cu deficiency (P < 0.05).
Cu concentrations of the root increased, while Fe, Mn, and Zn concentrations decreased with the Cu treatment from 0.5 to 200 μM (Fig. 5), indicating Cu, Fe, Mn, and Zn interactions in deposition by roots. Mn deposition in roots was more sensitive to Cu treatment than Fe and Zn depositions. Fe and Zn depositions in roots were not affected by Cu significantly until the Cu-treated concentrations increased to 50 μM. However, Mn concentrations in roots decreased 1.3 times when Cu treatment concentrations increased from 0.5 to 20 μM.
Metal Distribution in Root Growth Zones
Metal (Cu, Fe, Zn, Mn, and Ca) distribution in root growth zones of C. communis is shown in Fig. 6. From the longitudinal section around the meristem, the high, relative Cu concentrations were observed in the meristem and epidermis, and Cu content was lower in the cortex than in the vascular cylinder (Fig. 6a). Similar to Cu, the high contents of Zn and Ca were observed in the meristem, while Fe and Mn contents in the meristem were not high.
Metal distribution of C. communis root response to 100 μM Cu revealed by SRXRF. a Longitudinal section around the meristem and metal locations. b Cross section of elongation tissue and metal locations, c Cross- section of non-growing tissue and metal locations. The scale bar of sections represents 50 μm, and the metals include Cu, Fe, Zn, Mn, and Ca
In the cross section of elongation tissue, the Cu concentration decreased from the epidermis to the endodermis and reached the highest level in the vascular cylinder (Fig. 6b). In contrast, the highest Fe concentration was in the epidermis tissue and Fe concentration was higher in endodermis than in the vascular cylinder. Mn distribution and Zn distribution in the cross section of elongation tissue were more homogenous than that of Cu and Fe. Ca concentration was high in the epidermis and the vascular cylinder.
Compared with the elongation tissue, the obviously high concentrations of metals (including Cu, Fe, Mn, Zn, and Ca) were found in the epidermis of non-growing tissue (Fig. 6c). It was probably due to longer time of Cu treatment for non-growing tissue than that for elongation tissue. Metals were accumulated in the epidermis mostly by absorption and diffusion ands then transported to cortex by the symplast way and the apoplast way. However, the symplast way was blocked in the endodermis because the cells were thickened like horse hoof, which made the solution be chosen into the vascular cylinder. The fact that Cu concentration was higher in the vascular cylinder than in the cortex demonstrated that the root of C. communis could uptake Cu actively. The data show that Cu interacts with different metals such as Fe, Mn, and Zn.
Discussion
Acquisition of metals by plants is a complex phenomenon [28–30]. The concentration of free metal in the cytoplasm is very small and is highly regulated. At the root level, the regulation includes ionic and covalent binding in cell walls, metal transport across the plasma membrane of root cells, xylem loading and translocation, and detoxification and sequestration of metal in cellular organelles or root hairs.
The Cu uptake by roots of C. communis was inhibited by the uncoupler DNP and P-type ATPase inhibitor Na3VO4 (Fig. 1), suggesting that Cu could probably be assimilated actively by roots and be related with P-type ATPase. P-type heavy metal ATPases have been identified in a wide range of organisms and have been implicated in the transport of a range of essential and also potentially toxic metals across cell membranes [31, 32]. There were five Cu-transporting P-type ATPases, as has been reported in Arabidopsis. PAA1, PAA2, and HMA1 are localized in the chloroplast and involved in the transport of Cu into the organelle, whereas RAN1 is involved in the Cu delivery to the secretory pathway and HMA5 would function in Cu compartmentalization or detoxification [30]. Ca-ATPases is a kind of P-type ATPase. Lanthanum is thought to block the dephosphorylation of the phosphoenzyme in the reaction cycle and result in an increase in its net equilibrium level, a reduction in turnover of the Ca2+-ATPase and a reduction in calcium transport [26]. In this study, Cu uptake by the root was not inhibited by LaCl3 (Fig. 2), suggesting that Cu uptake by C. communis was not through Ca2+ ion channel.
Metal interactions affecting distribution have been noted in various species of plants with additional metals having both positive and negative effects on uptake and deposition of a given metal. In this study, we found that Fe or Zn deficiency could enhance Cu uptake, while high levels of Cu inhibited Fe, Zn, and Mn accumulation in the root (Figs. 3 and 4), suggesting Cu interactions with Fe, Zn, and Mn. It was reported that Fe deficiency in the growth medium increased Cu concentration in maize and pea. Peralta-Videa et al. [33] reported that the presence of Zn reduced Cu uptake in alfalfa plants (Medicago sativa). Chen et al. [34] reported that Fe deficiency induces Cu uptake and accumulation in C. communis. Bernal et al. [3] found that Cu accumulation was accompanied by Fe increase and Zn decrease in leaves treated with Cu, but the opposite was observed in leaves of plants treated by supplementing the growth medium with excess Cu. Metal interactions in roots have been reported to be mostly due to competition for the same membrane transporters [35]. The COPT (copper transporter) family plays a key role in Cu transport. COPT1 has been reported to be involved in Cu uptake [30]. There is no evidence that show whether COPT1 can mediate the transport of other metals. However, some studies suggest that IRT1 (iron-regulated transporter), a member of the ZIP (ZRT IRT-like protein) family, is able to mediate the transport of multiple metals, including Fe, Mn, Zn, Cd, and Cu [17, 36, 37].
SRXRF microprobe requires no sample pretreatment, which allows non-invasive examination of living materials, and can detect elemental abundances in the sub-microgram per gram range with a resolution of 10 μm or less, which was successfully completed in studies of metal localization in plants [23, 38]. The high levels of Cu were found in the meristem (Fig. 6a), indicating that vacuolar sequestration is not the primary detoxification mechanism in roots. The pattern is consistent with Cu being bound to the wall, since wall density is highest where cells are smallest, in the rapidly dividing zone, and crosswalls are progressively diluted by continuing cell expansion beyond the meristem. It was found that a large amount of Cu accumulated in E. splendens and was compartmentalized in the cell wall of roots [11, 39]. The higher Cu concentration in the vascular cylinder than in the endodermis (Fig. 6b, c) further proved the active Cu transport by roots. The Casparian strip and endodermis seem to provide selectivity against Cu, with reduced concentrations entering the stele [10]. Vesk [40] found an increasing Cu gradient from the epidermis through the root cortex of Eichhornia crassipes. However, Cu in the aquatic fern was found to decrease in the stele [41].
The results of metal localization in root growth zones revealed by SRXRF (Fig. 6) provided good information for us to further understand the metal interaction in roots, especially for Cu and Fe interaction. Under excess Cu stress, the highest levels of Fe were observed in epidermis tissue, and Fe levels were higher in the endodermis than in the vascular cylinder (Fig. 6), suggesting that Cu and Fe compete probably not only for the transporter (such as IRT1) in epidermal cells but also for the intercellular and intracellular transport in roots. Members of several different transport families have been implicated in the intracellular and intercellular metal transport including the Nramp (natural-resistance-associated macrophage protein) family and the YSL (yellow stripe-like) family [30].
References
Wong MH, Bradshaw AD (1982) A comparison of the toxicity of heavy metals, using root elongation of ryegrass, Loiium perenne. New Phytol 91:255–261
Silk WK, Bambic DG, O'Dell RE et al (2006) Seasonal and spatial patterns of metals at a restored copper mine site II. Copper in riparian soils and Bromus carinatus shoots. Environ Pollut 144:783–789
Bernal M, Cases R, Picorel R (2007) Foliar and root Cu supply affect differently Fe- and Zn-uptake and photosynthetic activity in soybean plants. Environ Exp Bot 60:145–150
Bringezu K, Lichtenberger O, Leopold I (1999) Heavy metal tolerance of Silene vulgaris. Plant Physiol 154:536–546
Küpper H, Zhao FJ, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–311
Küpper H, Lombi E, Zhao FJ et al (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 221:75–84
Lee J, Reeves RD, Brooks RR et al (1978) The relation between nickel and citric acid in some nickel-accumulating plants. Phytochemistry 17:1033–1035
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Nishizono H, Ichikawa S, Suziki S (1987) The role of the root cell wall in the heavy metal tolerance of Athyrium yokoscense. Plant Soil 101:15–20
MacFarlane GR, Burchett MD (2000) Cellular distribution of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.) Vierh. Aquat Bot 68:45–59
Ni CY, Chen YX, Lin Q (2005) Subcellular localization of copper in tolerant and non-tolerant plant. J Environ Sci 17:452–456
Murphy AM, Zhou J, Goldsbrough PB et al (1997) Purification and immunological identification of metallothioneins 1 and 2 from Arabidopsis thaliana. Plant Physiol 113:1293–1301
Schat H, Llugany M, Vooijs R et al (2002) The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and nonhyperaccumulator metallophytes. J Exp Bot 53:2381–2392
Weisa JS, Gloverb T, Weis T (2004) Interactions of metals affect their distribution in tissues of Phragmites australis. Environ Pollut 131:409–415
Panou-Filotheou H, Bosabalidis AM (2004) Root structural aspects associated with copper toxicity in oregano (Origanum vulgare hirtum). Plant Sci 166:1497–1504
Nenova V, Stoyanov I (1999) Physiological and biochemical changes in young maize plants under iron deficiency. 3. Concentration and distribution of some nutrient elements. J Plant Nutr 22:565–578
Cohen CK, Fox TC, Garvin DF (1998) The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol 116:1063–1072
Vedina O, Toma S (2000) Forms of microelements in apple leaves under different conditions of iron and zinc nutrition. J Plant Nutr 23:1135–1143
Lin S, Wu L (1994) Effects of copper concentration on mineral nutrient uptake and copper accumulation in protein of copper tolerant and nontolerant Lotus purshianus. Ecotoxicol Environ Saf 29:214–228
Luo Y, Rimmer DL (1995) Zinc–copper interaction affecting plant growth on metal-contaminated soil. Environ Pollut 88:79–83
Tang SR, Wilke BM, Huang CY (1999) The uptake of copper by plants dominantly growing on copper mining spoils along the Yangtze River, the People's Republic of China. Plant Soil 209:225–232
Tang SR, Wilke BM, Brooks RR (2001) Heavy-metal uptake by metal-tolerant Elsholtzia splendens and Commelina communis from China. Commun Soil Sci Plant Anal 32:895–905
Shi JY, Chen YX, Huang YY et al (2004) SRXRF as a technique for studying elements distribution in leaf of Elsholtzia splendens. Micron 35:557–564
Kang SX, Sun XJ, Huang YY et al (2002) Measurement and calculation of escape peak intensities in synchrotron radiation X-ray fluorescence analysis. Nucl Instrum Methods Phys Res B 192:365–369
Huang YY, Lu JX, He RG et al (2001) Study of human bone tumor slice by SRXRF microprobe. Nucl Instrum Methods Phys Res A 467(468):1301–1304
Evans DE, Williams LE (1998) P-type calcium ATPases in higher plants—biochemical, molecular and functional properties. Biochim Biophys Acta 1376:1–25
Grusak MA, Pearson JN, Marentes E (1999) The physiology of micronutrient homeostasis in field crops. Field Crops Res 60:41–56
Wintz H, Fox T, Wu YY et al (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278:47644–47653
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Grotz N, Guerinot ML (2006) Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta 1763:595–608
Palmgren MG, Harper JF (1999) Pumping with plant P-type ATPases. J Exp Bot 50:883–893
Williams LE, Pittman JK, Hall JL (2000) Emerging mechanisms for heavy metal transport in plants. Biochim Biophys Acta 1465:104–126
Peralta-Videa JR, Gardea-Torresdey JL, Walton J et al (2003) Effects of zinc upon tolerance and heavy metal uptake in alfalfa plants (Medicago sativa). Bull Environ Contam Toxicol 70:1036–1044
Chen YX, Shi JY, Tian GM et al (2004) Fe deficiency induces Cu uptake and accumulation in Commelina communis. Plant Sci 166:1371–1377
Pearson JN, Rengel Z, Jenner CF et al (1996) Manipulation of xylem transport affects Zn and Mn transport into developing wheat grains of cultured ears. Physiol Plant 98:229–234
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Plant Biol 3:153–162
Lombi E, Tearall KL, Howarth JR et al (2002) Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 128:1359–1367
Kim SA, Punshon T, Lanzirotti A et al (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314:1295–1298
Shi JY, Wu B, Yuan XF et al (2008) An X-ray absorption spectroscopy investigation of speciation and biotransformation of copper in Elsholtzia splendens. Plant Soil 302:163–174
Vesk PA (1998) Trace metal accumulation in the water hyacinth Eichhornia crassipes (Mart.) Kensington Pond, Centennial Park, Sydney. M.Sc. Thesis, University of Sydney
Sela M, Tel-Or E, Fritz E et al (1988) Localization and toxic effects of Cd, Cu, and U in Azolla. Plant Physiol 88:30–36
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (20777066) and the Natural Science Foundation of Zhejiang Province (Y2080844).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, J., Yuan, X., Chen, X. et al. Copper Uptake and Its Effect on Metal Distribution in Root Growth Zones of Commelina communis Revealed by SRXRF. Biol Trace Elem Res 141, 294–304 (2011). https://doi.org/10.1007/s12011-010-8710-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8710-5