Abstract
We investigated the effect of 17β-estradiol (E2) alone and separately vitamin E treatment on trace element status of rats following an ovariectomic operation. Forty rats were equally divided into four groups: Group 1, control, non-ovariectomized rats; Group 2, (OVX) rats, ovariectomized under general anesthesia; Group 3, (OVX+E2) rats, the group received a 40 µg kg−1 subcutan dose of E2 per day after ovariectomy; and Group 4, (OVX + E2 + vitamin E) rats, received the same E2 treatment, but with an additional 100 mg kg−1 intraperitoneal dose of vitamin E per day after ovariectomy. At the end of the 30-day experiment, the rats were sacrificed and their blood was collected for the measurement of zinc, copper, iron, phosphorus, selenium, magnesium, calcium, manganese, and chromium; copper–zinc superoxide dismutase (SOD); manganese-superoxide dismutase (Mn-SOD); glutathione peroxidase (Se-GSH-Px); and catalase (CAT). The levels of zinc, copper, iron, phosphorus, selenium, calcium, chromium, and manganese and activities of SOD, Mn-SOD, Se-GSH-Px, and CAT were lower in the OVX than in the control group, but magnesium level was unaffected. However, zinc, copper, iron, phosphorus, selenium, calcium, chromium, and manganese levels and SOD, Mn-SOD, Se-GSH-Px, and CAT activities were higher under separate E2 and E2 + vitamin E treatments. The level of magnesium in the treated-OVX groups was not different than in the OVX group. In conclusion, E2 treatment has an ameliorating effect on the trace element status in OVX, and this effect may be enhanced with the addition of vitamin E.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trace elements are essential in human and animal metabolism, growth, and tissue repair [1]. The risk of nutritional disturbances, particularly, trace elements deficiencies, are remarkable during menopause [2]. Most of the menopause-related pathologies (cardiovascular complications, osteoporosis, and non-insulin-dependent diabetes mellitus) involve variations in trace mineral status [3]. Trace elements in turn are active in a number of essential metabolic functions and therefore their importance in biochemical research has grown. Zinc and copper act as cofactors for several enzymes necessary for bone mineralization and formation of collagenous structure of bone, and thus being a part of connective tissue metabolism [4]. Fe requirement in vertebrates arises since it is an essential component of proteins that play redox or non-redox roles in many critical cellular functions, including respiration and cell division [5]. The other element chromium, as an insulin regulator, is involved in glucose and lipid metabolism. It improves insulin sensitivity and protects against glucose intolerance. Chromium also has a mission in corticosteroid metabolism postulated to contain losses in bone density and to maintain lean body mass which decrease with age [6]. The tissue most responsive to dietary intake of manganese plays an important role in a number of physiologic processes as a constituent of some enzymes and an activator of other enzymes. Manganese deficiency results in abnormal skeletal development in a number of animal species. Also, manganese seems to work with iron and is therefore necessary for proper iron metabolism [7]. The significance of magnesium in bone metabolism and in the development of metabolic bone disease has yet to be illuminated. Magnesium acts as a surrogate for calcium in transport and mineralization processes [2, 3]. An association between bone formation and phosphorus metabolism via bone mineralization or collagen synthesis has been reported [8].
Ovariectomized (OVX) animals are used to investigate the mechanisms responsible for menopause-related complications in humans. Recent studies have found that OVX rats experience an increased incidence of osteoporosis, cardiovascular complications and diabetes mellitus compared with normal rats. However, E2-administrated OVX rats seem to be protected [9, 10]. Though some interrelations among minerals and female hormones are known, mechanisms of the beneficial effects of E2 treatment are not fully understood. Trace minerals can be active in production and regulation of hormones while female hormones can influence trace mineral status.
Among the trace elements, selenium is an essential component of the glutathione peroxidase (GSH-Px) as a family of primary antioxidant enzymes, while copper and zinc are structural components of copper–zinc superoxide dismutase (SOD), zinc being also part of catalase (CAT) [11]. Declines of these enzymes lead to an imbalanced antioxidant defense system and may be associated with an increase of oxidative stress involved in degenerative diseases [3].
Vitamins such as vitamin E have major roles in lipid membrane protection. Determination of the numerous individual antioxidants is almost impossible [10]. A previous study has reported a correlation between vitamin E and E2. It has been found that E2 regenerates vitamin E radicals in vitro. E2, like vitamin E, possess ROS-scavenging chain-breaking antioxidant activity as hydrogen donors from their phenol–hydroxyl ring. However, E2 can induce antioxidant enzyme expression by stimulating the antioxidant defense system and inhibit the formation of lipid peroxides in various tissues in vitro [9, 10].
The present study is designed to investigate the potential role of E2 per se and the vitamin E treatment on the body status of serum trace element. Our findings showed that ovariectomy induces trace element deficiency. E2 alone and also E2 + vitamin E treatments enhance the status of trace element, as evidenced by a significant increase in serum of OVX rats.
Materials and Methods
Animal Model
Female Wistar Albino rats (10–12 weeks old, 200 g) purchased from the Laboratory Animal Production Unit of Firat University (Elazig/Turkey) were used in the study. They were hosted in an environment with controlled temperature (22−24°C), humidity (40–70%), and photoperiod (12-h light:12-h dark cycle) for 1 week before the start of the experiment. Animals were housed individually in stainless-steel cages in a pathogen-free University Laboratory Animal Research facility. The animals had free access to standard laboratory chow and water ad libitum. All procedures applied were in strict accord with the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals, and the approval of the ethic committee of school of Medicine, Firat University was obtained prior to the study.
Surgical Procedures
The rats in Groups 2, 3, and 4 were anesthetized with ketamine (Ketalar, Eczacibaşi Pharmaceutical Co.) and underwent a bilateral ovariectomy via a 10 mm incision in lower part the abdomen. The skin was separated from the underlying muscles, muscle fibers incised, and the forceps placed at the boundary between the oviduct and the uterus. After removing the ovary and oviduct, the uterus was put back into the abdominal cavity and the incision was closed with clips [12].
Experimental Design
Forty rats were equally divided into four groups: Group 1, control, non-ovarictomized rats; Group 2, (OVX) rats; being ovariectomized under general anesthesia; Group 3, (OVX + E2) rats, which received a 40 µg kg−1 subcutan dose of E2 (17β-estradiol was obtained Sigma, St. Lois, MO, USA) per day after being ovariectomized under general anesthesia; and Group 4, (OVX + E2 + vitamin E) rats, which received the same E2 treatment but an additional 100 mg kg−1 intraperitoneal dose of vitamin E (dl-α-tocopheryl acetate was obtained from F. Hoffman La Roche, Istanbul, Turkey) per day after ovariectomy.
At the end of the fourth week, the study rats were sacrificed via the taking of blood by cardiac puncture. The rats were anesthetized with ether for 5 min and blood samples (6-ml) obtained by cardiac puncture into tubes without anticoagulation, 3-ml blood samples were drawn into tubes bearing 3.8 mmol/I ethylenediaminetetraacetic acid. The anticoagulated blood was separated into plasma and erythrocytes by centrifugation for 10 min at 1,000×g at 4°C. Plasma samples were stored (−20°C) for no more than 1 week for the analyses of antioxidant enzymes activity. The remaining non-anticoagulated blood samples were taken in trace element-free vacutainer tubes. Serum was separated using acid-washed pipettes, diluted with distilled water, and stored in acid-cleaned microcentrifuge tubes at 4°C. Serum zinc, copper, iron, phosphorus, selenium, calcium, chromium, magnesium, and manganese concentrations were determined using atomic absorption spectrophotometer (ASC-600, Shimatsu Co., Ltd., Kyoto).
SOD and Mn-SOD activities were measured spectrometrically (Shimatsu Co, Ltd, 2R/UV, Tokyo, Japan) [13] based on the ability of SOD to inhibit the auto-oxidation of adrenalin to adrenochorome at alkaline PH. GSH-Px activity was determined using glutathione reductase and NADPH. The method is based on the decrease in absorbance at 412 nm [14]. The methods of Goth [15] were used for determination of catalyse activities in plasma. The yellow complex of molybdate and hydrogen peroxide measured at 405 nm against blank using a spectrophotometer.
Statistical Analysis
Statistical analyses were performed on a personal computer with the use of SPSS software 15.0 (SPSS Inc., Chicago IL, USA). The results are expressed as mean ± standard deviation (S.D). Data were analyzed using one-way analysis of variance (ANOVA) followed by LSD test. P values of < 0.05 were considered to be statistically significant.
Results
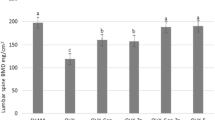
The zinc, copper, iron, phosphorus, selenium, magnesium, calcium, manganese, and chromium in the serum are shown in Table 1. Ovariectomy was associated with changes in trace element levels in serum. The zinc (p < 0.01), copper (p < 0.05), iron (p < 0.05), phosphorus (p < 0.05), selenium (p < 0.05), calcium (p < 0.05), manganese (p < 0.05), and chromium (p < 0.05) levels in serum were lower in the OVX than in the control group due to ovariectomy but magnesium level was unaffected. However, zinc (p < 0.01), copper (p < 0.05), iron (p < 0.05, p < 0.01), phosphorus (p < 0.05), selenium (p < 0.05), calcium (p < 0.05), and chromium (p < 0.05) levels in OVX rats were higher under separate E2 and E2 + vitamin E treatments although no significant change in magnesium level.
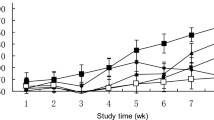
The SOD, Mn-SOD, Se-GSH-Px, and CAT activities in the serum are shown in Figs. 1, 2, 3, and 4. The SOD, Mn-SOD, Se-GSH-Px, and CAT activities in the serum were affected by ovariectomy. The SOD (p < 0.05), Mn-SOD (p < 0.05), Se-GSH-Px (p < 0.05), and CAT (p < 0.05) activities in the serum were significantly lower in the OVX group than in the control. On the other hand, E2 and E2 + vitamin E administration for 4 weeks have caused a significant decrease in SOD (p < 0.05), Mn-SOD (p < 0.05), Se-GSH-Px (p < 0.05), and CAT (p < 0.01) activities.
Discussion
The risk of nutritional disturbances, particularly trace element and vitamin deficiencies, is high during menopause [2]. Women with menopause have been found to have decreased serum zinc and copper levels, revealing a relationship between estrogen absence and trace element deficiency [16]. Besides, clinical studies have also showed that ovariectomy can lead to zinc and copper loss by causing lack of estrogens [17]. Our results showed that ovariectomy significantly decreased zinc and copper levels compared with control rats, while E2 alone and with the addition of vitamin E treatment augmented the serum zinc and copper levels. This result is in agreement with those of Bureau et al. [3] reporting that estrogen treatment increased the copper levels of blood of postmenopausal women. Also, estrogen insufficiency led to zinc deficiency. These results suggest that the absence of circulating E2 may well be responsible for the observed differences in zinc and copper levels in our OVX rats. Our results are supported by a recent study on animals showing that zinc level significantly increased vitamin E that were accompanied by the increase in serum and liver [18]. In addition, other studies on vitro reported that zinc stabilizes the red cell membrane against cellular changes caused by peroxidations and that zinc plays a role similar to that of vitamin E in reducing peroxidative damage on cellular membranes [3, 19].
Copper-deficiency leads to an important reduction of antioxidant enzymatic components, such as SOD [20]. Ovariectomy also induces an increase in lipid peroxidation and a decrease in enzymatic antioxidants like SOD and GSH-Px in rats [21, 22]. Furthermore, these antioxidants and their regenerative enzymes were rapidly normalized by a single dose of E2 and antioxidants (such as vitamin E and ascorbic acid). Furthermore, other major disturbances of antioxidant enzymes which participate in defense mechanisms occur in copper-deficiency. Indeed, a decreased activity of CAT and GSH-Px is associated with copper deprivation [23]. In the literature, protective effects of estrogens are widely described in both animals and humans [9]. Estrogens, like vitamin E, which possess a phenolic hydroxyl group, have an effective antioxidant action and inhibit lipid peroxidation in various models. These estrogens may be able to inhibit the generation of radicals reducing the peroxide levels [9, 10, 23]. Endogenous estrogens could thus offer some protection against the decrease of blood copper in animals through increased antioxidant status. This hypothesis is supported by the present data showing that E2 alone and with the addition of vitamin E treatment enhances the status of serum copper, as evidenced by a significant increase in serum of OVX rats.
The principal line of defense against the reactive oxygen species is the cellular antioxidant system, which includes cellular GSH-Px, followed by the extracellular antioxidants. Selenium deficiency has also been shown to reduce the cerebral GSH-Px activity after a few days in postmenopausal women [24]. This mechanism would have relevance in the postmenopausal population in which a decrease in estrogens level and the associated reduction in selenium status may accelerate oxidative damage after menopause [24, 25]. In addition, a relationship has been observed between the female reproductive hormone, estrogens, and selenium level, with blood selenium and GSH-Px activity coinciding with fluctuations in estrogens during the menstrual cycle. These findings suggest that the decrease in estrogens following menopause may cause a decrease in selenium level. Also, antioxidant role of estrogens may be the modulation of GSH-Px activity [26]. However, other studies have reported that selenium has important role in vitamin E metabolism. It is needed for absorption of lipids and vitamin E. Furthermore, selenium has roles to keep vitamin E within lipids [27]. The current study compared selenium levels and plasma GSH-Px activities in control group and OVX rats. OVX rats had significantly lower serum selenium concentrations and plasma GSH-Px activities compared to control group, possibly in response to oxidative processes associated with menopause. E2 alone and with the addition of vitamin E treatment in OVX rats significantly increased plasma GSH-Px activities and serum selenium levels.
Iron is essential for many biological processes and its deficiency or excess is involved in patho-physiological conditions. Previous studies have reported a correlation between ovariectomy or menopause and poor iron status [28]. Our results also showed that rats subjected to ovariectomy had a significant reduction in serum iron level. On the basis of previous findings, the reduction of serum iron levels after ovariectomy could also related to a lower iron uptake. In fact, it was showed that the increase of serum iron in OVX rats treated with E2 was due to a direct stimulating effect of the hormone in iron by duodenum and its transfer in to blood and liver [28]. However, our results showed that treatments of E2 alone/with vitamin E increased serum iron levels compared with OVX rats. This result is in agreement with those of Sullivan et al. [29] reporting that vitamin E causes iron release into the serum, thus increasing the serum iron concentration. Also, vitamin E is the most effective chain-breaking lipid soluble antioxidant in the biological membrane. It functions by stabilizing the biological membrane, protecting against oxidative stress induced by iron overload.
The participation of trace minerals in normal development and maintenance of the skeleton is related to their catalytic functions in organic bone matrix synthesis. Magnesium, phosphorus, and calcium, major components of bone matrix, have received considerable attention in the prevention and treatment of bone loss [30]. However, other minerals are also important to both bone structure and calcium metabolism. For instance, magnesium helps in transport and mineralization processes of calcium and like zinc, assists with calcium absorption [31]. It also exhibits estrogenic qualities, making it useful in the treatment of menopausal symptoms as well. Women with menopausal have been found to have decreased levels of calcium, zinc, phosphorus, and magnesium, suggesting the relationship between menopause and trace element deficiency [2]. As reported by [3], menopause is associated with increased renal excretion of calcium and decreased intestinal calcium absorption. The analyses in this paper show that ovariectomy significantly decreased calcium, magnesium, and phosphorus levels compared with control rats. These decreases were reversed by E2 alone and with the addition of vitamin E treatment.
Though animal and epidemiologic studies have demonstrated a positive correlation between serum manganese levels and bone density, few studies assessed manganese status in the enzymes that actively intervene in the development of the bone and cartilaginous matrix [17]. Manganese is a necessary co-factor for many enzymes involved in carbohydrate metabolism. It was reported that manganese level in serum is less in postmenopausal women with osteoporosis compared to the non-osteoporotic controls [32]. In another study, Rico et al. [17] studying ovariectomized rats observed that after ovariectomy, a greater osteoclastic activity, participating in the inhibition of bone resorption induced by free radicals, is observed. Manganese has been detected to exert greater influence as a free radicals sweeper than SOD [33]. In our study the manganese and Mn-SOD levels are significantly lower in the OVX group compared with the control group. However, no significant difference was found between OVX + E2 and OVX + E2 + vitamin E groups and OVX group in blood levels for manganese and Mn-SOD. Similarly, Bureau et al [3] reported on manganese status that there is no significant difference between postmenopausal women under hormone replacement therapy.
Chromium is an essential trace element required for normal glucose (as an insulin regulator) and lipid metabolism. It improves insulin sensitivity and protects against glucose intolerance [6]. Chromium also functions in lipid metabolism, postulated to decrease losses in bone density. All these variables are also distorted with menopause [6, 34]. Menopausal women are at risk of chromium deficiency since chromium status declines with age. Thus, alterations of chromium status could be involved in menopause-related pathologies such as osteoporosis, cardiovascular diseases, glucose intolerance, and diabetes [3]. However, women receiving hormonal replacement therapy seem to be protected [6]. Serum chromium levels in OVX rats receiving E2 alone/with vitamin E treatment was raised in OVX-treated rats compared with OVX.
In conclusion, our analyses support the hypothesis that trace element metabolism is altered in OVX rats. In OVX rats receiving E2 alone and with the addition of vitamin E treatment, the trace element status based upon serum levels has improved. The E2 alone and with the addition of vitamin E treatment could likely have some protective effects on patho-physiological conditions based on alterations in metabolism of trace elements.
References
Hostetler CE, Kincaid RL, Mirando MA (2003) The role of essential trace elements in embryonic and fetal development in livestock. Vet J 166:125–139
Zhang DW, Cheng Y, Wang XL, Zhang JC et al (2008) Synergistic effect of trace elements and flavonoids from Epimedium koreanum Nakai on primary osteoblasts. Chin Sci Bull 53:347–356
Bureau I, Anderson RA, Arnaud J et al (2002) Trace mineral status in post menopausal women: impact of hormonal replacement therapy. J Trace Elem Med Biol 16:9–13
Kim MH, Choi MK, Sung CJ (2007) Bone mineral density of Korean postmenopausal women is similar between vegetarians and nonvegetarians. Nutr Res 27:612–617
Wallander ML, Leibold EA, Eisenstein RS (2006) Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim Biophys Acta 1763:668–689
Roussel A-M, Bureau I, Favier M (2002) Beneficial effects of hormonal replacement therapy on chromium status and glucose and lipid metabolism in postmenopausal women. Maturitas 42:63–69
Rico H, Gomez-Raso N, Revilla M et al (2000) Effects on bone loss of manganese alone or with copper supplement in ovariectomized rats: a morphometric and densitometric study. Eur J Obstet Gynecol Reprod Biol 90:97–101
Pinheiro MM, Schuch NJ, Genaro PS et al (2009) Nutrient intakes related to osteoporotic fractures in men and women—the Brazilian Osteoporosis Study (BRAZOS). Nutr J 8:6
Ulas M, Cay M (2009) The effects of 17β-estradiol and vitamin E treatments on oxidative stress and antioxidant levels in brain cortex of diabetic ovariectomized rats. Acta Physiologica Hungarica (in press)
Ulas M, Cay M (2009) Effects of vitamin E and 17-β estradiol on plasma lipid and lipid peroxidation levels in ovariectomized and diabetic rats. Fırat University Veterinary Journal of Health Sciences 23(1):21–28, Turkish
Berger MM (2006) Antioxidant micronutrients in major trauma and burns: evidence and practice. Nutr Clin Prac 21:438
Robertson MC, Owens RE, Klindt J, Friesen HG (1984) Ovariectomy leads to a rapid increase in rat placental lactogen secretion. Endocrinology 114:1805–1811
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958
Beauchamp C, Fridovich I (1973) Isozymes of superoxide dismutase from wheat germ. Biochim Biopsy Acta 317:50–64
Goth LA (1991) Simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Di Gioacchino M, Forcucci R, Tiboni GM et al (2000) The influence of menopause and habitual smoking upon serum zinc, serum copper and the cardiovascular and immune parameters of women. Int J Immunopathol Pharmacol 13:91–97
Rico H, Gomez-Raso N, Revilla M et al (2000) Effects on bone loss of manganese alone or with copper supplement in ovariectomized rats: a morphometric and densitometric study. Eur J Obstet Gynecol Reprod Biol 90:97–101
Ozkaya MO, Naziroğlu M, Barak C, Berkkanoğlu M (2010) Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro-fertilization (IVF). Biol Trace Elem Res. doi:10.1007/s12011-010-8637-x
Faure P, Barclay D, Faure MJ, Halimi S (2007) Comparison of the effects of zinc alone and zinc associated with selenium and vitamin E on insulin sensitivity and oxidative stres in high-fructose-fed rats. J Trace Elem Med Biol 21:113–119
Bureau I, Gueux E, Mazur A et al (2003) Female rats are protected against oxidative stress during copper deficiency. J Am Coll Nutr 3:239–246
Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z (2007) Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem 295:45–52
Dilek M, Nazıroğlu M, Oral HB et al. (2010) Melatonin modulates hippocampus NMDA receptors, blood and brain oxidative stress levels in ovariectomized rats. J Membr Biol 233:135–142
Bureau I, Gueux E, Mazur A et al (2003) Female rats are protected against oxidative stress during copper deficiency. J Am Coll Nutr 3:239–246
Ha EJ, Smith AM (2003) Plasma selenium and plasma and erythrocyte glutathione peroxidase activity increase with estrogen during the menstrual cycle. J Am Coll Nutr 22:43–51
Cay M, Naziroglu M, Halis K (2009) Selenium and vitamin E modulates smoke exposure-induced oxidative stress in blood of rats. Biol Trace Elem Res 131(1):62–70
Miquel J, Ramirez Bosca A, Ramirez Bosca JV, Diaz-Alperi J (2006) Menopause: a review on the role of oxygen stress and favorable effects of dietary antioxidants. Arch Gerontol Geriatr 42:289–306
Burk RF, Hill KE, Nakayama A et al (2008) Selenium deficiency activates mouse liver Nrf2–ARE but vitamin E deficiency does not. Free Radic Biol Med 44:1617–1623
Raso GM, Irace C, Esposito E et al (2009) Ovariectomy and estrogen treatment modulate iron metabolism in rat adipose tissue. Biochem Pharmacol 78:1001–1007
O’Sullivan MG, Byrne DV, Nielsen JH, Andersen HJ, Martens M (2003) Sensory and chemical assessment of pork supplemented with iron and vitamin E. Meat Sci 64:175–189
Buchman AL, Moukarze A (2000) Metabolic bone disease associated with total parenteral nutrition. Clin Nutr 19(4):217–231
Gur A, Colpan L, Nas K et al (2002) The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and new effect of calcitonin. J Bone Miner Metab 20:39–43
CO, Li YV (2006) Zinc homeostasis and bone mineral density. Ohio Res Clin Rev 15:7–18
Reddi AR, Jensen LT, Naranuntarat A et al (2009) The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic Biol Med 46:154–162
Jain SK, Rogier K, Prouty L, Jain SK (2004) Protective effects of 17β-estradiol and trivalent chromium on interleukin-6 secretion, oxidative stress, and adhesion of monocytes: relevance to heart disease in postmenopausal women. Free Radic Biol Med 37:1730–1735
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulas, M., Cay, M. Effects of 17β-Estradiol and Vitamin E Treatments on Blood Trace Element and Antioxidant Enzyme Levels in Ovariectomized Rats. Biol Trace Elem Res 139, 347–355 (2011). https://doi.org/10.1007/s12011-010-8669-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8669-2