Abstract
This study was performed to determine the effects of copper proteinate on performance, blood chemistry, lipid peroxidation status, and organs as well as copper deposition in the liver and eggs of laying hens. Seventy-two 30-week-old Bovans laying hens were distributed into four groups with three replicates. Animals were fed basal diet containing at least 17% crude protein and 2,800 kcal/kg metabolizable energy supplemented with either 0, 150, 300, or 450 mg/kg copper as copper proteinate. Supplementation of 150 and 300 mg/kg copper increased egg production, whereas 450 mg/kg copper decreased (p < 0.001). Liver copper levels were elevated in 300 and 450 mg/kg copper-supplemented groups (p < 0.001). Egg copper contents increased in all treatment groups (p < 0.01). An increase in glucose (p < 0.001) and decreases in albumin (p < 0.01) and total cholesterol (p < 0.05) levels were determined with 300 and 450 mg/kg copper. Supplementation of 450 mg/kg copper increased alkaline phosphatase and gamma glutamyl transpeptidase activities (p < 0.05), malondialdehyde, and high-density lipoprotein levels (p < 0.01) but decreased alanine aminotransferase and lactate dehydrogenase activities (p < 0.01). No gross and microscopic changes were observed in the liver and kidneys. These results indicated that 150 and 300 mg/kg copper increased egg production without having marked adverse effects, but 450 mg/kg copper altered some blood chemistry variables and reduced egg production in laying hens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) is an essential trace element found in small amounts in a variety of cells and tissues [1]. Its role has been defined for many physiological functions related mostly to its presence in the active sites of cupro-enzymes [2]. Copper is either a cofactor or an integral part of many important enzymes including cytochrome c oxidase, tyrosinase, p-hydroxyphenyl pyruvate hydrolase, dopamine beta hydroxylase, lysyl oxidase, and copper-zinc superoxide dismutase (Cu-Zn SOD). These enzymes are involved in a number of vital biological processes related to growth, development, maintenance, and production [1, 2].
Copper is routinely added to poultry diets at prophylactic concentrations because of its antimicrobial activity and growth-promoting effects [3]. Common copper sources used in poultry diets are inorganic copper salts, primarily the sulfate form, because of their low cost and commercial availability. The influence of supplemental inorganic copper salts on performance of poultry species has been well documented [4–9].
Inorganic copper compounds have been added to poultry diets at excess levels (100–250 mg/kg) of the requirements of broilers (8 mg/kg) recommended by National Research Council. (NRC) [10]. However, copper requirement of the laying hens was not specified in the NRC [10]. When included at higher levels to diets, the so-called pharmacological levels, a high proportion of ingested copper is excreted through feces [2, 11]. Therefore, there is an increasing concern that feeding high levels of copper to poultry will have a negative impact on the environment. With increasing concern for environmental pollution from minerals in excreta, there is an interest in the poultry industry to find alternative sources of copper to improve the biological availability by using organic trace minerals in animal diet [12]. Most of the organic minerals are classified as complexes, chelates, or proteinates [13]. Chelated products or proteinates have superior bioavailability over inorganic copper sources [14–16]. The purpose of a chelated product is to protect the compound of interest from digestion or complex formation thereby enabling the protected compound to reach its target site [17]; thus, lower dietary inclusion levels are required due to the better absorption [16], which enhances the efficiency. Although, animals vary by species in their sensitivity, poultry can tolerate many times their usual daily intakes of copper [1, 2]. However, various levels of organic copper used in diet should be studied for safety because exposure to elevated concentration of copper is damaging. Furthermore, determination of copper residue in the egg may be of importance for health concern of humans. Therefore, this study was performed to determine the effects of three levels of copper proteinate on body weight, egg production, egg weight, food consumption, food efficiency, and serum glucose, proteins, lipids, enzymes, minerals, and malondialdehyde (MDA), which is an indicator of lipid peroxidation status, liver and kidney histology, and copper content in liver and egg.
Materials and Methods
Animals, Diets, and Management
Seventy-two 30-week-old Bovans laying hens were used in this study. Hens were weighed to provide the equal live weight in all groups in the beginning of the study and distributed to four groups with three replicates containing six hens in each after 10 days of acclimation. The control group was fed commercial basal diet containing average 17% crude protein and 2,800 kcal/kg metabolizable energy (ME); treatment groups were fed basal diet supplemented with 150, 300, and 450 mg/kg Cu as copper proteinate (Bioplex Copper, Alltech, Nicholasville, KY, USA) for 4 weeks (Table 1). Hens were allowed ad libitum access to food and water. Animals were reared at 18–20°C at17 h lighting schedule.
Measurements of Performance
The body weight of animals was recorded in the beginning and in the end of the study. Egg production was recorded daily, and food consumption and egg weight were recorded at weekly intervals. Feed efficiency was calculated by determination of the amount of food consumed for 1 kg of egg.
Sample Collection and Analysis
In the end of the study, 12 blood samples (four per replicate) were collected from each group for biochemical analysis. The sera were separated by centrifugation at 1,300×g after 1 h incubation at room temperature and stored at −20°C until the analysis. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT) activities and triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), total protein, glucose, calcium (Ca), inorganic phosphorous (Pi), and magnesium (Mg) concentrations were determined with commercially available kits (Beckman, Ireland) by an auto-analyzer (Beckman LX-20 Coulter, Ireland). Serum MDA level, which is an end product of thiobarbituric-acid-reactive substances, was determined according to the method described by Moreno et al. [18].
Nine animals (three per replicate) from each group were neck dislocated, and liver and kidney samples were collected at the end of the feeding trial. Livers were wiped with paper tissues after removal of gall bladders. Liver and kidneys were weighed, and weights were recorded as grams per 100 g body weight.
Parts of liver samples were frozen individually in sealed plastic bags for copper analysis. Remaining parts of liver samples and kidney samples were preserved in buffered formalin for histopathological processing. Tissue samples were embedded in paraffin, sectioned in 5–6 μm, stained with hematoxylin–eosin (HE), and examined under light microscope [19].
Chemical composition of diet was analyzed by the methods of Association of Official Analytical Chemists (AOAC) [20]. Copper levels in tissue and food samples were determined with atomic absorption spectrophotometer (Perkin Elmer, Analyst 700) after microwave digestion by the method described in AOAC [21].
Statistical Analysis
Statistical analyses of data were performed by SPSS 10.0 version for Windows. One-way analysis of variance was used for the differences between groups. When the F values were significant, Duncan’s multiple range test was performed. All data were expressed as means ± SEMs.
Results
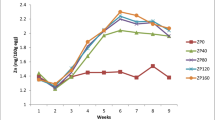
No differences were found in body weight, ratio of liver and kidney to body weight (Table 2), food consumption, feed efficiency, and egg weight. Supplementation of 150 and 300 mg/kg Cu increased, whereas 450 mg/kg Cu decreased egg production (p < 0.001; Table 3).
Liver copper contents were elevated in 300 and 450 mg/kg Cu supplemented groups (p < 0.001). Egg copper contents were increased by all supplemental copper levels (p < 0.01; Table 4). There were no differences between all groups with regard to serum lipase and AST activity, total protein, TG, Ca, Pi, and Mg levels. Copper supplementation to diet at the levels of 150 and 300 mg/kg had no effects on serum ALT, ALP, GGT, LDH, MDA, and HDL levels. Serum glucose levels were increased (p < 0.001), whereas albumin (p < 0.01) and TC levels were decreased (p < 0.05) by the supplemental levels of 300 and 450 mg/kg Cu. However, only 450 mg/kg Cu supplementation increased ALP and GGT activities (p < 0.05) and MDA and HDL levels (p < 0.01), and this level decreased ALT and LDH activities (p < 0.01; Table 5).
No gross and microscopic changes were observed by the histological examinations of liver and kidneys (Figs. 1 and 2).
Discussion
Copper has become a very popular feed additive in poultry diets because of its possible growth-promoting and antimicrobial effects with the moving away of antibiotics from diets [3]. Inorganic copper compounds are often fed to poultry at the levels of 100–250 mg/kg diet, which are quite above the recommended level of 8 mg/kg for broiler requirements by NRC [10]. Organic products have superior bioavailability over inorganic copper compounds [14, 16]. Although the enhanced bioavailability may relate to less susceptibility of organic compounds to various physiochemical factors which adversely affect the absorption of inorganic minerals [2], different organic copper complexes have different bioavailability [15]. To the author’s knowledge, there are very few studies investigating the effects of organic copper compounds in laying hens. Controversies exist in these studies concerning the levels of organic copper complexes recommended for laying hens without having deleterious effects on performance and metabolism [17, 22, 23]. Determination of possible changes due to copper proteinate, which is one of the organic copper compounds at high levels, in performance and metabolism as well as its accumulation in the liver and residue in the egg yolk may be of importance for health concern in both animals and humans. Therefore, in this study, as low as 150 mg/kg and as high as 450 mg/kg Cu were supplemented to diet of laying hens.
In the present study, supplementation of all levels of copper proteinate had no effects on body weight [5, 17], food consumption [5, 7, 24–26], feed efficiency [24, 26], and egg weight [7, 9, 17, 22, 24, 26] as indicated in previous studies in which layer diets had been supplemented with different sources and levels of organic and inorganic copper compounds. In addition, any level of supplemental copper did not influence serum total protein and major minerals (Ca, Pi, Mg) as reported by Mondal et al. [27].
It was suggested that copper improved the activities of digestive enzymes such as total protease, amylase, and lipase [28]. However, in the present study, similar effect of copper proteinate on serum lipase was not found. Elevated serum ALP and GGT activities were partly consistent with the result of Almansour [29], who found increases in ALP, AST, and ALT activities. The activity of ALT enzyme can be influenced by food restriction [30]. A reduction found in ALT activity confirming the findings of Pearce et al. [4] may be due to the slightly reduced food consumption, which is also confirmed by the slightly reduced body weight. Furthermore, the reduced albumin concentrations by 300 and 450 mg/kg Cu supplementations, consistent with the decreased ALT activity, may suggest an alteration in protein synthesis [30]. Changes in blood chemistry variables occur before the physiological and morphological lesions. Although gross and histopathological examinations revealed no pathological lesion in the kidneys and liver, consistent with no changes in the ratio of kidney and liver weights to the body weight, presence of alterations in the serum enzyme activities, especially in the animals fed diet supplemented with 450 mg/kg Cu, may suggest that when the animals are exposed to this level and higher amounts of copper for prolonged period, potential hazardous effects may arise.
Supplementation of 150 mg/kg Cu did not increase the copper content of liver as in the studies that used similar copper levels from different sources in poultry [16, 23, 31]. A remarkable increase in copper content in liver of hens supplemented with 300 and 450 mg/kg Cu confirmed the results of previous studies [11, 16, 32, 33] that used similar or higher copper levels. The liver copper content is the indicator of the copper status of the animal [2]. Increasing liver copper content consistent with the dietary copper level reflects a dose-dependent manner of copper accumulation in the liver. However, even at the highest dietary copper supplementation, liver copper concentration reached up to 19.63 mg/kg, which is quite a low level than the European Union limit of 80 mg/kg as reported by Skrivan et al. [11]. Lim and Paik [23] found no significant influence of 100 mg/kg dietary copper as copper chelates on the copper content of egg yolk. In contrast, in the present study, a linear increase in egg yolk copper content in all copper-supplemented groups confirmed the results of Pesti and Bakalli [7], who reported increased egg yolk copper content with 125 and 250 mg/kg Cu, and also the results of Skrivan et al. [11], who used a range of copper from 25 up to 240 mg/kg in the form of copper sulfate. Idowu et al. [22] found higher accumulation of inorganic copper than organic form in liver and egg yolk. The copper concentration of liver and egg yolk may depend on the sources and levels of copper used as supplement.
In some previous studies, no effects of copper sulfate from 50 up to 250 mg/kg on egg production were reported [9, 24, 26]. On the other hand, Al Ankari et al. [5] reported reduced egg production by the supplementation of diet with 250 mg/kg Cu as sulfate or acetate. However, in the studies with organic copper compounds, increases in egg production were reported with 100 mg/kg Cu in the form of methionine chelates [23] and with 125 and 250 mg/kg Cu in the form of copper proteinate [22]. Similarly, Pesti and Bakalli [7] reported positive effect of copper sulfate at a level of 250 mg/kg on egg production. In the present study, egg production was increased with the supplementation of copper proteinate at levels of 150 and 300 mg/kg consistent with the results of the studies in which similar levels and sources of copper were used [22, 23].
In this study, improvements in egg production were determined by 150 and 300 mg/kg copper supplementation. However, the reduced egg production by 450 mg/kg copper supplementation, which was consistent with reductions in the serum triglycerides and total cholesterol, may result from the lowered circulating level of 17 beta-estradiol, hence reproductive physiology due to the slightly reduced food consumption resulting in depressed hepatic lipid synthesis as found in the previous studies. In those studies, the serum triglycerides [5, 22, 27] and total cholesterol [5, 6, 9, 22, 25, 27] were also reduced by copper supplementation. In the present study, the reduced plasma cholesterol by supplemental copper may be due to the high liver Cu concentration that regulates cholesterol biosynthesis by reducing hepatic reduced glutathione concentration, which in turn reduces the activity of 3-hydoxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of cholesterol biosynthesis [5, 27, 33]. The decreases in total cholesterol may also be associated with the increased HDL synthesis. Although in the present study, the low-density lipoprotein (LDL) level could not be determined, the increased blood HDL concentration may result from the synthesis of HDL from LDL by modulation of HMG-CoA reductase activity by copper [27]. The elevated HDL concentration confirmed findings of Lien et al. [9], who fed the copper-sulfate-supplemented diet to laying hens, and Mondal et al. [27], who used copper proteinate and copper sulfate in broiler diets at the levels of 200 and 400 mg/kg.
Although normally bound to proteins, copper may be released and become free to catalyze the formation of highly reactive hydroxyl radicals. Data obtained from in vitro and cell culture studies are largely supportive of capacity of copper to initiate oxidative damage and interfere with important cellular events. It has been suggested that oxidative damage has been linked to chronic copper overload and/or exposure to excess copper [1, 34, 35]. The remarkable increase in copper content in liver of hens supplemented with 300 and 450 mg/kg Cu may reflect higher absorption of copper proteinate, thus animals may be exposed to excess copper. The increased glucose level confirmed the results of Almansour [29] who found the elevated serum glucose in quail fed diet supplemented with copper sulfate above the level of 750 mg/kg. It has been suggested that the increases in lipid peroxidation is associated with the elevated plasma glucose [36]. The increased MDA level in the present study might be resulted from the auto-oxidation of glucose [36] as well as copper itself [34].
The results of this study have shown that supplementation of 150 and 300 mg/kg Cu in the form of copper proteinate increased egg production without having marked adverse effects on the investigated parameters. However, alterations in some blood chemistry variables and reduction in egg production may suggest an adverse effect of copper proteinate at the supplemental level of 450 mg/kg in laying hens. Therefore, further more detailed and prolonged investigations with different organic copper compounds at various levels are needed to better understand the effects of this essential, but potentially toxic, trace element.
References
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicol 189:147–163
McDowell LR (1992) Copper and molybdenum, minerals in animal and human nutrition. Academic, San Diego, pp 176–204
Pesti GM, Bakalli RI (1996) Studies on the feeding of cupric sulfate pentahydrate and cupric citrate to broiler chickens. Poultry Sci 75(9):1086–1091
Pearce J, Jackson N, Stevenson MH (1983) The effects of dietary intake and of dietary concentration of copper sulphate on the laying domestic fowl: effects on some aspects of lipid, carbohydrate and amino acid metabolism. Br Poult Sci 24(3):337–348
Al Ankari A, Najib H, Hozab A (1998) Yolk and serum cholesterol and production traits, as affected by incorporating a supraoptimal amount of copper in the diet of the leghorn hen. Br Poult Sci 39:393–397
Konjufca VH, Pesti GM, Bakalli RI (1997) Modulation of cholesterol levels in broiler meat by dietary garlic and copper. Poultry Sci 76:1264–1271
Pesti GM, Bakalli RI (1998) Studies on the effect of feeding cupric sulfate pentahydrate to laying hens on egg cholesterol content. Poultry Sci 77:1540–1545
Skrivan M, Sevcikova S, Tumova E, Skrivanova V, Marounek M (2002) Effect of copper sulphate supplementation on performance of broiler chickens, cholesterol content and fatty acid profile of meat. Czech J Anim Sci 47(7):275–280
Lien TF, Chen KL, Wu CP, Lu JJ (2004) Effects of supplemental copper and chromium on the serum and egg traits of laying hens. Br Poult Sci 45(4):535–539
National Research Council (1994) Nutrient requirements of poultry, 9th edn. Natl. Acad. Press, Washington, DC
Skrivan M, Skrivanova V, Marounek M (2006) Effect of various copper supplements to feed of laying hens on Cu content in eggs, liver, excreta, soil, and herbage. Arch Environ Contam Toxicol 50:280–283
Ferket PR, van Heugten E, van Kempen TATG, Angel R (2002) Nutritional strategies to reduce environmental emission from nonruminants. J Anim Sci 80(E. Suppl. 2):168–182
Spears JW (1996) Organic trace minerals in ruminant nutrition. Anim Feed Sci Technol 58(1):151–163
Ledoux DR, Li YC, Broomhead JN, Greenwood MW, Bermudez AJ (2000) Effects of copper source on performance and liver and bile copper content of broiler chicks fed dietary treatments from day 1 to 42. Poultry Sci 79(1):28
Guo R, Henry PR, Holwerda RA, Cao J, Littell RC, Miles RD, Ammerman CB (2001) Chemical characteristics and relavite bioavailability of supplemental organic copper sources for poultry. J Anim Sci 79(5):1132–1141
Wang Z, Cerate S, Coto C, Yan F, Waldroup PW (2007) Evaluation of mintrex copper as a source of copper in broiler diets. Int J Poult Sci 6(5):308–313
Banks KM, Thompson KL, Rush JK, Applegate TJ (2004) Effects of copper source on phosphorus retention in broiler chickens and laying hens. Poultry Sci 83(6):990–996
Moreno IM, Mate A, Repetto G, Vazquez CM, Camean AM (2003) Influence of microcystin-LR on the activity of membrane enzymes in rat intestinal mucosa. J Physiol Biochem 59(4):293–300
Luna LG (1968) Manual of histologic staining methods of the armed forces institute of pathology, 3rd edn. McGraw-Hill, New York
Official Methods of Analysis of the Association of Official Analytical Chemists (A.O.A.C.), 14th ed. Inc Arlington, Virginia. (1984)
Official Methods of Analysis of the Association of Official Analytical Chemists (A.O.A.C.), Official Method 999.10, Lead, Cadmium, Zinc, Copper and Iron in Foods-Atomic Absorption Spectrophotometry after Microwave Digestion, AOAC, 18 th ed. (2005)
Idowu OMO, Laniyan TF, Kuye OA, Oladele-Ojo VO, Eruvbetine D (2006) Effect of copper salts on performance, cholesterol, residues in liver, eggs and excreta of laying hens. Arch Zootec 55(212):327–338
Lim HS, Paik IK (2006) effects of dietary supplementation of copper chelates in the form of methionine, chitosan and yeast in laying hens. Asian-Australas J Anim Sci 19(8):1174–1178
Balevi T, Coskun B (2004) Effects of dietary copper on production and egg cholesterol content in laying hens. Br Poult Sci 45(4):530–534
Lim KS, You SJ, An BK, Kang CW (2006) Effects of dietary garlic and copper on cholesterol content and quality characteristics of chicken eggs. Asian-Australas J Anim Sci 19(4):582–586
Azman MA, Yılmaz M (2006) The effects of dietary supplementation of copper sulphate on performance and some blood parameters of laying hens, Lalahan Hay. Araşt Derg 46(2):33–38
Mondal MK, Das TK, Biswas P, Samanta CC, Bairagi B (2007) Influence of dietary inorganic and organic copper salt and level of soybean oil on plasma lipids, metabolites and mineral balance of broiler chickens. Anim Feed Sci Technol 139:212–233
Luo XG, Dove CR (1996) Effect of dietary copper and fat on nutrition utilization, digestive enzyme activities, and tissue mineral levels in weanling pigs. J Anim Sci 74:1888–1896
Almansour MI (2006) Biochemical effects of copper sulfate, after chronic treatment in quail. J Biol Sci 6(6):1077–1082
Turgut K (2000) Veteriner Klinik Laboratuvar Teşhis, Bahçıvanlar Basım Sanayi AŞ., Konya, pp.222–224
Arias VJ, Koutsos EA (2006) Effects of copper source and level on intestinal physiology and growth of broiler chickens. Poultry Sci 85:999–1007
Sevcikova S, Skrivan M, Skrivanova V, Tumova E, Koucky M (2003) Effect of supplementation of copper in copper sulphate and Cu-glycine on fatty acid profile in meat of broiler chickens, cholesterol content and oxidation stability of fat. Czech J Anim Sci 48(10):432–440
Chowdhury SD, Paik IK, Namkung H, Lim HS (2004) Responses of broiler chickens to organic copper fed in the form of copper-methionine chelate 115:281–293
Toplan S, Dariyerli N, Özçelik D, Akyolcu MC (2005) The effects of copper applications on oxidative and antioxidant systems in rats. Trace Elem Electrolytes 22(3):178–181
Cummins KA, Solaiman SG, Bergen WG (2008) The effect of dietary copper supplementation on fatty acid profile and oxidative stability of adipose depots in Boer X Spanish goats. J Anim Sci 86:390–396
Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau JM, Kerkeni A (2001) Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. Am Coll Nutr 20(3):212–218
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Güçlü, B.K., Kara, K., Beyaz, L. et al. Influence of Dietary Copper Proteinate on Performance, Selected Biochemical Parameters, Lipid Peroxidation, Liver, and Egg Copper Content in Laying Hens. Biol Trace Elem Res 125, 160–169 (2008). https://doi.org/10.1007/s12011-008-8164-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8164-1